Abstract
Mice overexpressing the prion protein (PrP) sequence from various host species are widely used for measuring infectious titers in prion disease. However, the impact that the transgene expression level might have on the susceptibility to infection raises some concerns about the final biological relevance of these models. Here we report that endpoint titration of a sheep scrapie isolate in sheep and in mice overexpressing the ovine PrP results in similar estimates of the infectious titer.
TEXT
Bioassays play a pivotal role in transmissible spongiform encephalopathy (TSE) research. They are used to characterize the nature of the agent (strain typing) and/or to measure the infectious titer, which is a critical parameter for assessing the risk of disease transmission (1).
Historically, laboratory rodents have been used for titrating infectivity. However, the difficulties of transmitting certain TSE isolates, such as, for instance, sporadic Creutzfeld-Jakob disease (CJD), in conventional rodents remained a major limitation to their use. This “transmission barrier” phenomenon is mainly attributed to the amino acid divergences in the prion protein (PrP) sequences between the donor and the recipient (2).
The apparent abrogation of the transmission barrier in transgenic hosts that express an homologous PrP sequence that results in transmission to that of the donor species has led to the development of a variety of transgenic mouse models expressing the sheep, bovine, porcine, and human PrP. An inverse correlation between the survival time and expression level of the transgene in the brain has been noticed in mice transgenic for mouse, hamster, sheep, and bovine PrP (1). Whether the transgene expression level has an impact on the final susceptibility of the model to infection remains a subject of debate, and the final pertinence of infectious titers as measured in mice overexpressing PrP to the risk of transmitting the disease in the natural host species is uncertain (3).
In this study, we first produced a large batch of stock inoculum, using the brain stems from 70 ARQ/VRQ sheep clinically affected with scrapie. All these animals were born and raised in a flock naturally affected by scrapie (Langlade flock) and belonged to the 2005/2006 birth cohort (4). A 1/10 dilution series of the inoculum (starting from a 4% [wt/vol] tissue homogenate) was prepared in negative sheep brain homogenate, and aliquots were stored at −80°C.
Groups of (i) tg338 mice that are homozygotes for the sheep PrP VRQ variant and knock down for the mouse PrP (PrPKo) (5), (ii) tg338 mice cross bred with PrPKo mice, and (iii) tg338 mice cross bred with tgShpXI (that are homozygotes for the sheep PrP ARQ variant and PrPKo) were produced (1).
The expression levels in the brain of the tg338, tg338 × PrPKo, and tg338 × tgShpXI mice, as estimated by Western blot analysis, were approximately 8-fold, 4-fold, and 8-fold higher than in the brain of ARQ/VRQ TSE-free sheep (Fig. 1).
FIG 1.
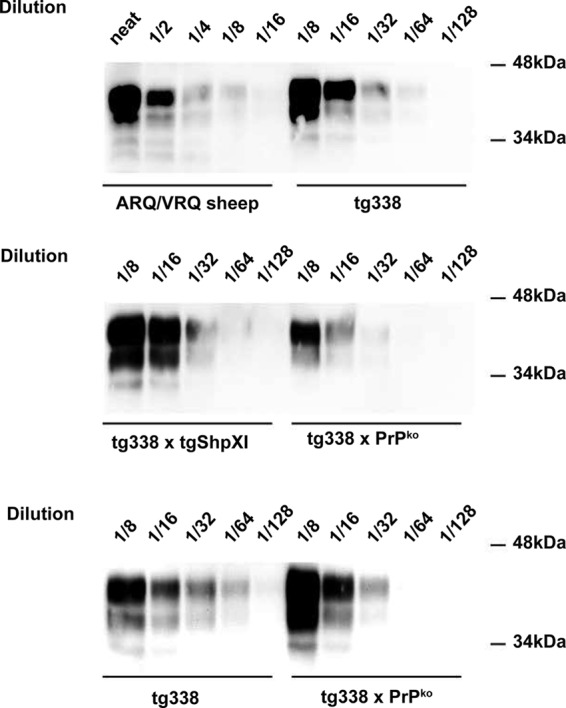
Cellular PrP expression level in the brain of ARQ/VRQ sheep and tg338, tg338 × PrPko, and tg338 × tgShpXI mice. tg338 and tgShpXI mice express the ovine VRQ and ARQ variants of PrP, respectively. For each of the three mouse lines and a control sheep brain (cerebral cortex), a 10% brain homogenate was prepared. After denaturation in Laemmli's buffer, samples (either neat or diluted in Laemmli's buffer) were run on a 12% acrylamide gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. PrPc was then detected using 8G8 antibody (epitope, 97SQWNKP102).
Groups of six mice belonging to these three different mice lines were intracerebrally (IC) inoculated with the prepared dilution series. Similarly, groups of six ARQ/VRQ sheep were IC challenged using the same dilution series (Table 1). For each tested dilution, each of the mice and sheep received the same amount of brain material. Both mice and sheep were then monitored for clinical TSE occurrence. In clinically suspect animals, TSE transmission was confirmed by abnormal PrP (PrPSc) detection in the animals' tissues using Western blot analysis (Sha31 anti-PrP monoclonal antibody; epitope YEDRYYRE) (6).
TABLE 1.
Endpoint titration by the intracerebral route of a reference scrapie isolate in transgenic mice that express the ovine PrP and in ARQ/VRQ sheepa
| Dilution of the 4% (wt/vol) brain homogenate | Brain material per animal (g) | Transmission inb: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| tg338 mice |
tg338 × PrPKo mice |
tg338 × tgShpXI mice |
ARQ/VRQ sheep |
||||||
| No. of infection-positive mice/total no. of mice | Incubation period (days) (mean ± SD) | No. of infection-positive mice/total no. of mice | Incubation period (days) (mean ± SD) | No. of infection-positive mice/total no. of mice | Incubation period (days) (mean ± SD) | No. of infection-positive sheep/total no. of sheep | Incubation period (days) (mean ± SD) | ||
| 10−3 | 8 × 10−7 | 6/6 | 94 ± 6 | 6/6 | 103 ± 4 | 6/6 | 111 ± 9 | 6/6 | 212 ± 16 |
| 10−4 | 8 × 10−8 | 4/6 | 117 ± 12 | 4/6 | 128 ± 12 | 2/6 | 124, 154 | 3/6 | 242, 263, 275 |
| 10−5 | 8 × 10−9 | 0/6 | >250 | 0/6 | >250 | 0/6 | >250 | 0/6 | >650 |
| 10−6 | 8 × 10−10 | 0/6 | >250 | 0/6 | >250 | 0/6 | >250 | 0/6 | >650 |
| 10−7 | 8 × 10−11 | 0/6 | >250 | 0/6 | >250 | 0/6 | >250 | 0/6 | >650 |
Successive 1/10 dilutions of a 4% tissue homogenate prepared using the brainstem from 70 ARQ/VRQ scrapie affected sheep (Langlade flock) were inoculated into groups of tg338 mice (n = 6), tg338 × PrPKo, tg338 × tgShpXI and ARQ/VRQ sheep (n = 6). In camparison with ARQ/VRQ sheep, (i) tg338 mice express about 8-fold, (ii) Tg338 × PrPKo mice express about 4-fold, and (iii) tg338 × tgShpXI mice express about 8-fold the ovine PrPC in their brain (see Fig. 1). Animals were euthanized when they showed clinical signs of infection or after 250 days postinoculation (mice) or 650 days postinoculation (sheep). Animals were considered infected when PrPres deposition was detected in the brain by Western blot analysis using the Sha31 monoclonal antibody which recognizes amino acids 145 to 152 (YEDRYYRE) of the sheep PrP. Infectious titers were estimated by the Spearman-Karber method (7), and they are reported as the number of ID50 per gram of brain tissue.
The most likely value and the lower and upper values of the 95% confidence intervals (in parentheses) of the infectious titers (ID50 per gram of tissue) are as follows: for tg338 mice, 107.26 (106.88 to 107.65); for tg338 × PrPKo mice, 107.25 (106.88 to 107.65); for tg338 × tgShpXI mice, 106.93 (106.55 to 107.32); and for ARQ/VRQ sheep, 107.1 (106.69 to 107.51).
On the basis of these results (Table 1), the infectious titer in sheep was estimated to be 107.1 50% infective doses (ID50) per gram (95% confidence interval [CI 95%], 106.69 to 107.51) by the Spearman-Karber method (7). In both tg338 and tg338 × PrPKo mice, the infectious titer was 107.26 ID50 per gram (CI 95%, 106.88 to 107.65). In tg338 × tgShpXI mice, the inoculum displayed an infectious titer of 106.93 ID50 per gram (CI 95%, 106.55 to 107.32). According to these results, the infectious titers measured in the different animal models were not statistically different.
The incubation periods observed in tg338 mice were significantly (Student's test; P < 0.05) shorter than in tg338 × PrPKo and tg338 × tgShpXI mice (10−3 dose; see Table 1). However, no statistical difference was observed between incubation periods in tg338 × PrPKo and tg338 × tgShpXI mice. These results were unexpected, since the TSE incubation period is supposed to be shorter in hosts expressing a higher level of PrPC. The results suggest that the coexpression of ARQ and VRQ PrPC in the mice somehow interfered with the dynamics of the TSE agent propagation. Further experiments are ongoing to clarify the mechanism underlying this phenomenon.
In parallel to the IC endpoint titration experiment, newborn ARQ/VRQ lambs were orally challenged (natural suckling) with decreasing amounts of infectious material. Lambs (n = 50) were separated from their mothers within the first 6 h following birth, and each received a single dose of inoculum corresponding to 102.7, 103.7, 104.7, or 105.7 IC ID50 in sheep (natural suckling). The experimental challenge was performed within the first 48 h of the life of the lamb. A group of animals was kept unchallenged (Table 2).
TABLE 2.
Oral inoculation of ARQ/VRQ lambs with decreasing amounts of a reference endpoint titrated (IC route in ARQ/VRQ sheep) scrapie isolatea
| Equivalent brain material amount per lamb | No. of ID50 IC units in ARQ/VRQ sheep | Transmission in orally challenged ARQ/VRQ sheep |
|
|---|---|---|---|
| No. of affected sheep/total no. of sheep | Incubation period (days) (mean ± SD) | ||
| 2 g | 107.4 | 10/10 | 257 ± 18 |
| 200 mg | 106.4 | 10/10 | 279 ± 37 |
| 20 mg | 105.4 | 8/8b | 305 ± 44 |
| 2 mg | 104.4 | 6/9b | 545 ± 61 |
| Noninoculated control | 0/10 | >1,200 | |
Groups of 10 ARQ/VRQ lambs were orally challenged with decreasing amounts of a reference scrapie isolate. This isolate had previously been endpoint titrated by the intracerebral route in ARQ/VRQ (Table 1). Lambs were challenged within their first 48 h of life by natural suckling.
Some of the challenged animals died from intercurrent disease within the first months of life. At death, to confirm the scrapie infection status, PrPSc deposition in lymphoid tissues and central nervous system was assessed in each animal by Western blot analysis.
No disease transmission or PrPSc accumulation was observed in lymphoid tissues (spleen, tonsil, mesenteric lymph node) or the central nervous system of the unchallenged control sheep. A 100% attack rate of disease was observed in sheep challenged with inocula containing 107.4 to 10 5.4 IC ID50 units in sheep. In the animals that were challenged with 104.4 IC ID50 units in sheep, transmission was observed in 6 of the 9 animals. Clinical suspicions were confirmed by the presence of PrPSc in the animals' tissues using Western blot analysis.
Together, these results indicate that in the investigated scrapie isolate, the infectious titers as measured by intracerebral endpoint titration in the different ovine PrP-expressing mice and in the ARQ/VRQ sheep were not statistically different. These findings are in agreement with data reported by Peretz et al. in hamster and transgenic mice overexpressing the hamster PrP and by Thackray et al. in conventional mice and transgenic mice overexpressing the mouse PrP (8, 9). They contradict the view that animal models that overexpress PrP have an intrinsic higher susceptibility to TSE agent infection than the natural host. They also strongly support the contention that the infectious titers measured by intracerebral endpoint titration in the transgenic mouse model and in the natural host are equally relevant for elaboration of TSE transmission risks.
Data reported in some mouse models (10) and bovine spongiform encephalopathy (BSE) in cattle (11) indicated that 1 ID50 administered by the oral route is approximately equivalent to 105.5 to 105.6 IC ID50 units. This range of values was the one we used to design our experiment. The results we obtained, using a sheep scrapie isolate and ARQ/VRQ sheep, indicated that 1 ID50 oral unit in our paradigm is equivalent to less than 104.4 IC ID50 units.
This discrepancy is a likely consequence of the complexity of the phenomena determining the capacity of TSE agents to transmit following peripheral exposure. Experimental evidence supports the view that both the nature of the TSE agent and the host species/genetic background have a direct impact on such transmission efficacy; whereas administration of 40,000 IC 50% lethal dose (LD50) units of 263K was shown to give l LD50 by the intraperitoneal (IP) route in hamsters (12), in CW mice, 1 IP LD50 of the 139A strain was equivalent on average to 430 IC LD50 units (13).
In any case, the data we report indicate that the minimal oral infectious dose enabling the oral transmission of certain TSE agents might be significantly lower than it is usually considered to be.
Footnotes
Published ahead of print 26 February 2014
REFERENCES
- 1.Groschup MH, Buschmann A. 2008. Rodent models for prion diseases. Vet. Res. 39:32. 10.1051/vetres:2008008 [DOI] [PubMed] [Google Scholar]
- 2.Béringue V, Vilotte JL, Laude H. 2008. Prion agent diversity and species barrier. Vet. Res. 39:47. 10.1051/vetres:2008024 [DOI] [PubMed] [Google Scholar]
- 3.BIOHAZ EPoBH. 2011. Joint scientific opinion on any possible epidemiological or molecular association between TSEs in animals and humans. EFSA J. 9:111 http://www.efsa.europa.eu/fr/efsajournal/pub/1945.htm [Google Scholar]
- 4.Elsen JM, Amigues Y, Schelcher F, Ducrocq V, Andreoletti O, Eychenne F, Khang JV, Poivey JP, Lantier F, Laplanche JL. 1999. Genetic susceptibility and transmission factors in scrapie: detailed analysis of an epidemic in a closed flock of Romanov. Arch. Virol. 144:431–445. 10.1007/s007050050516 [DOI] [PubMed] [Google Scholar]
- 5.Le Dur A, Beringue V, Andreoletti O, Reine F, Lai TL, Baron T, Bratberg B, Vilotte JL, Sarradin P, Benestad SL, Laude H. 2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc. Natl. Acad. Sci. U. S. A. 102:16031–16036. 10.1073/pnas.0502296102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Féraudet C, Morel N, Simon S, Volland H, Frobert Y, Créminon C, Vilette D, Lehmann S, Grassi J. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280:11247–11258. 10.1074/jbc.M407006200 [DOI] [PubMed] [Google Scholar]
- 7.Markus RA, Frank J, Groshen S, Azen SP. 1995. An alternative approach to the optimal design of an LD50 bioassay. Stat. Med. 14:841–852. 10.1002/sim.4780140812 [DOI] [PubMed] [Google Scholar]
- 8.Thackray AM, Klein MA, Aguzzi A, Bujdoso R. 2002. Chronic subclinical prion disease induced by low-dose inoculum. J. Virol. 76:2510–2517. 10.1128/jvi.76.5.2510-2517.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P, Safar JG, Glidden DV, McCulloch C, Nguyen HO, Scott M, Dearmond SJ, Prusiner SB. 2006. Inactivation of prions by acidic sodium dodecyl sulfate. J. Virol. 80:322–331. 10.1128/JVI.80.1.322-331.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimberlin RH, Walker CA. 1989. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 12:213–220. 10.1016/0168-1702(89)90040-3 [DOI] [PubMed] [Google Scholar]
- 11.Wells GA, Konold T, Arnold ME, Austin AR, Hawkins SA, Stack M, Simmons MM, Lee YH, Gavier-Widen D, Dawson M, Wilesmith JW. 2007. Bovine spongiform encephalopathy: the effect of oral exposure dose on attack rate and incubation period in cattle. J. Gen. Virol. 88:1363–1373. 10.1099/vir.0.82421-0 [DOI] [PubMed] [Google Scholar]
- 12.Kimberlin RH, Walker C. 1977. Characteristics of a short incubation model of scrapie in the golden hamster. J. Gen. Virol. 34:295–304. 10.1099/0022-1317-34-2-295 [DOI] [PubMed] [Google Scholar]
- 13.Kimberlin RH, Field HJ, Walker CA. 1983. Pathogenesis of mouse scrapie: evidence for spread of infection from central to peripheral nervous system. J. Gen. Virol. 64(Pt 3):713–716. 10.1099/0022-1317-64-3-713 [DOI] [PubMed] [Google Scholar]


