ABSTRACT
Although significant clinical efficacy and safety of rotavirus vaccines were recently revealed in many countries, the mechanism of their attenuation is not well understood. We passaged serially a cell culture-adapted murine rotavirus EB strain in mouse pups or in cell cultures alternately and repeatedly and fully sequenced all 11 genes of 21 virus samples passaged in mice or in cell cultures. Sequence analysis revealed that mouse-passaged viruses that regained virulence almost consistently acquired four kinds of amino acid (aa) substitutions in VP4 and substitution in aa 37 (Val to Ala) in NSP4. In addition, they gained and invariably conserved the 3′ consensus sequence in NSP1. The molecular changes occurred along with the acquisition of virulence during passages in mice and then disappeared following passages in cell cultures. Intraperitoneal injection of recombinant NSP4 proteins confirmed the aa 37 site as important for its diarrheagenic activity in mice. These genome changes are likely to be correlated with rotavirus virulence.
IMPORTANCE Serial passage of a virulent wild-type virus in vitro often results in loss of virulence of the virus in an original animal host, while serial passage of a cell culture-adapted avirulent virus in vivo often gains virulence in an animal host. Actually, live attenuated virus vaccines were originally produced by serial passage in cell cultures. Although clinical efficacy and safety of rotavirus vaccines were recently revealed, the mechanism of their attenuation is not well understood. We passaged serially a murine rotavirus by alternating switch of host (mice or cell cultures) repeatedly and sequenced the eleven genes of the passaged viruses to identify mutations associated with the emergence or disappearance of virulence. Sequence analysis revealed that changes in three genes (VP4, NSP1, and NSP4) were associated with virulence in mice. Intraperitoneal injection of recombinant NSP4 proteins confirmed its diarrheagenic activity in mice. These genome changes are likely to be correlated with rotavirus virulence.
INTRODUCTION
Rotaviruses, which form one genus within the family Reoviridae, are divided into at least seven species/groups (A to G/H) (1, 2). Although group A to C rotaviruses have been detected in humans with diarrhea, group A rotavirus is the single most important etiologic agent causing severe diarrhea in infants and young children worldwide, resulting in approximately 453,00 deaths (37% of deaths attributable to diarrhea and 5% of all deaths) among children <5 years of age in 2008 (3). In the United States alone, rotavirus (RV) infections are estimated to cause approximately 20 to 30 deaths, 50,000 to 67,000 hospitalizations, 390,000 to 410,000 physician visits, and a more than $890 million to $1 billion societal cost annually (4, 5). Thus, the introduction of a RV vaccine capable of alleviating this enormous health burden has been an important global public health goal.
The RV genome consisting of 11 segments of double-stranded RNA (dsRNA) encodes six structural proteins (VP1 to VP4, VP6, and VP7) and six nonstructural proteins (NSP1-NSP6). The virion has three concentric protein layers, with the outer layer (outer capsid) formed by VP4 and VP7, the middle layer (inner capsid) formed by VP6, and the inner layer (core shell) formed by VP2. VP1 (the viral RNA-dependent RNA polymerase) and VP3 (RNA capping enzyme), as well as the 11-dsRNA genomes are located inside the core shell with VP2. Six nonstructural proteins (NSP1 to NSP6) participate in replication (1).
Previously, in a study involving a semihomologous system of gnotobiotic newborn pigs infected with a virulent porcine RV, an avirulent human RV, or their reassortants, Hoshino et al. demonstrated that (i) the third (VP3), fourth (VP4), ninth (VP7), and tenth (NSP4) porcine RV genes each played an important role in the virulence of RV infection in piglets and that (ii) all four of the porcine RV virulence-associated genes were required for the induction of diarrhea and the shedding of RV in piglets (6). These novel observations suggested a potential new strategy for attenuating wild-type human RV, with the prospect of new safe and effective vaccines.
Serial passage of a virulent wild-type virus in vitro often results in loss of virulence of the virus in an original animal host, while serial passage of a cell culture-adapted avirulent virus in vivo often gains virus virulence in an original animal host (7). Actually, live-attenuated virus vaccines (against measles, rubella, mumps, chickenpox, yellow fever, and RV diarrhea) were originally produced by serial passages of viruses in cell cultures. Although significant clinical efficacy and safety of two live-attenuated RV vaccines, i.e., Rotarix (monovalent human RV vaccine [Glaxo-SmithKline]) and RotaTeq (pentavalent human-bovine RV reassortant vaccine [Merck]), was experienced in many countries (8–12), the mechanism of their attenuation is not well understood. To analyze mechanisms underlying this phenomenon, we passaged serially a cell culture-adapted murine RV EB strain by an alternating the host (mice in vivo and cell cultures in vitro) repeatedly. We then harvested the mouse- and cell culture-passaged viruses and fully sequenced their 11 genes. We sought to determine a correlation of nucleotide changes with virulence or avirulence, and we tried to provide insight into the mechanisms of attenuation of RV vaccine.
MATERIALS AND METHODS
Viruses.
Murine RV EB strain (G16-P[16]-I7-R7-C7-M8-A7-N7-T10-E7-H9) was isolated from a suckling mouse with diarrhea in the United States in 1982 (13). At first, this strain was adapted to grow in primary African green monkey kidney (10 AGMK) cell roller tube cultures and was subsequently plaque purified on a secondary AGMK cell monolayer. After plaque purification, murine RV EB strain was passaged serially in MA104 cell roller tube cultures 10 to 20 times. We used this strain as an original virus (Po) in order to start this passage experiment with a completely cell culture-adapted murine RV strain.
Serial passages in mice and cell cultures.
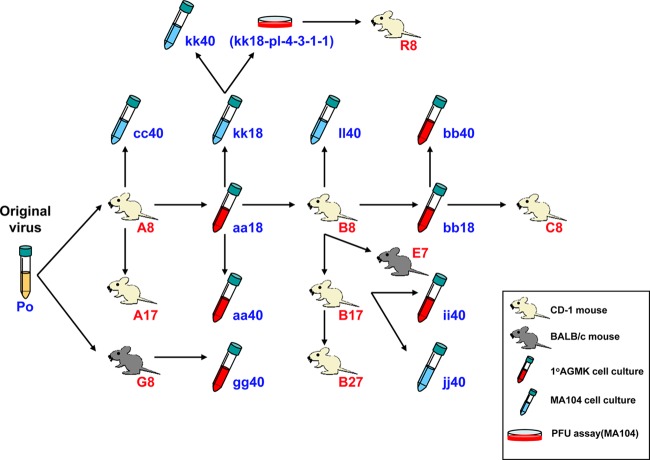
Figure 1 is a schematic diagram of serial passage history of the present study. Murine RV EB strain was passaged serially in 4- to 5-day-pld mouse pups (CD-1 or BALB/c; in vivo) or in cell cultures (10 AGMK cell or MA104 cell line; in vitro) alternately and repeatedly. A cell culture-adapted avirulent murine RV EB strain was passaged serially 7 to 27 times in 4- to 5-day-old mouse pups (CD-1 or BALB/c; in vivo) that were born to a dam free of RV-specific antibodies. Eight pups were inoculated orally and inspected daily for diarrhea by gentle palpation of their abdomen. When diarrhea was observed, the infected pups were euthanized. In cases of no diarrhea, the animals were euthanized at 96 h postinfection (14). Thereafter, the intestines of one selected mouse were homogenized, and 50 μl of 10% homogenates diluted with phosphate-buffered saline (PBS) was used as a next passage inoculum. Mouse experiments were approved by the Animal Care and Use Committee at the National Institute of Allergy and Infectious Diseases and performed according to Animal Biosafety Level 3 practice at the National Institutes of Health.
FIG 1.
Schematic diagram of serial passage history in the present study. Murine RV EB strain was passaged serially in 4- to 5-day-pld mouse pups (CD-1 or BALB/c; in vivo) or in cell cultures (10 AGMK cell or MA104 cell line; in vitro) alternately and repeatedly. With regard to in vivo passage, eight pups were inoculated orally and inspected daily for diarrhea by gentle palpation of their abdomens. When diarrhea was observed, infected pups were euthanized. In cases of no diarrhea, the animals were euthanized at 96 h postinfection. Thereafter, the intestines of a selected mouse were homogenized, and 50 μl of 10% homogenates diluted with PBS was used as a next-passage inoculum. With regard to in vitro passage, when ca. 75% of the infected cells displayed CPE within 3 to 5 days postinfection, the cultures were frozen and thawed once. Afterward, the lysates were passaged undiluted or diluted 10−1 to 10−5 (100-μl aliquots). Po indicates original murine RV EB strain. It was adapted to grow in 10 AGMK cell roller tube cultures and subsequently plaque purified on a secondary AGMK cell monolayer. After plaque purification, murine RV EB strain was passaged serially in MA104 cell roller tube cultures 10 to 20 times. Capital (A, B, C, E, G, and R) and lowercase (aa, bb, cc, gg, ii, jj, kk, and ll) letters indicate mouse- and cell culture-passaged viruses. Numbers (7, 8, 17, 18, 27, and 40) indicate the passage level. kk18-pl-4-3-1-1 was plaque purified kk18 consecutively four times in MA104 cells.
The mouse-adapted virulent murine RV EB strain was passaged serially 18 to 40 times in 10 AGMK cell or MA104 cell roller tube cultures (in vitro). When ca. 75% of the infected cells displayed cytopathic effects (CPE) within 3 to 5 days postinfection, the cultures were frozen and thawed once. Afterward, the lysates were passaged (i) undiluted or diluted 10−1 before the 20th passage and (ii) diluted arbitrarily from 10−1 to 10−5 (100-μl aliquots) after the 20th passage to avoid gene rearrangements.
In all, we selected 20 virus samples, i.e., five low mouse-passaged (A8, B8, C8, G8, and R8), four high mouse-passaged (A17, B17, B27, and E7), six AGMK cell culture-passaged (aa18, aa40, bb18, bb40, gg40, and ii40), and five MA104 cell culture-passaged (cc40, jj40, ll40, kk40, and kk18-pl-4-3-1-1) viruses (Fig. 1). All viruses, except for aa18 and bb18, were fully sequenced, including their 3′ and 5′ ends, and compared to the original virus (Po).
RNA extraction, RT-PCR, and nucleotide sequencing.
dsRNA was extracted with TRIzol (Invitrogen) from 10% intestinal homogenates or infected cell culture lysates. The primers used for the reverse transcription-PCR (RT-PCR) are listed in Table 1. The RT-PCR and nucleotide sequencing were performed using a procedure described previously (17). Briefly, the extracted RNA was added to 1 μl of dimethyl sulfoxide. The mixture was denatured at 94°C for 3 min and placed on ice. RT was performed at 45°C for 45 min using SuperScript II (Invitrogen), followed by 94°C for 3 min. PCR was performed using GoTaq DNA polymerase (Promega) under the following conditions: initial denaturation at 95°C for 15 min; 40 cycles at 94°C for 45 s, 42 or 50°C for 45 s, and 70°C for 2.5 min; and a final extension at 70°C for 7 min. The PCR amplicons were purified with the Wizard SV Gel and PCR Cleanup system (Promega) and sequenced directly with the BigDye Terminator v3.1 cycle sequencing reaction kit (Applied Biosystems) on an ABI 3730 DNA analyzer (Applied Biosystems). We confirmed mutations of virus samples (i) by amplifying one to three different PCR products and (ii) by sequencing them using two to eight different primers.
TABLE 1.
Summary of primers used for amplification and sequencing of MRV-EB strain cDNAsa
| Gene | Use | Primerb | Sense | Position | Sequence (5′–3′) |
|---|---|---|---|---|---|
| VP1 | RT-PCR | VP1-1 | + | 1–22 | GGC TAT TAA AGC TGT ACA ATG G |
| VP1-M1 | + | 503–523 | TTT ATA AGA GAC GGT TAG ACC | ||
| VP1-M7 | + | 1807–1827 | AAA GCG GCA AAC TCA ATT GCT | ||
| VP1-M5 | − | 915–896 | CCC TGC TTT ACT CAT AGC AA | ||
| VP1-M2 | − | 2055–2038 | TTT CGC TAT TTC GAT CCC | ||
| VP1-1-4-R | − | 3302–3282 | GGT CAC ATC TAA GCG CTC TAA | ||
| FLAC | VP1-M15 | + | 2909–2933 | CTT TAG GAG TGC CAA AAA TAG ACG C | |
| VP1-M23 | + | 3079–3098 | CCT TTT AAA GGG AAG ATA CC | ||
| VP1-M21 | − | 288–269 | GGC TAG CTT AGT TTC GAC GG | ||
| VP1-542m | − | 542–520 | GAC GCC ACG GTG ATG AAT AGG TC | ||
| Seq | VP1-M12 | + | 269–288 | CCG TCG AAA CTA AGC TAG CC | |
| VP1-M24 | + | 551–571 | ATA AGT ACG GTG TTC CAA GAC | ||
| VP1-M4 | + | 896–915 | TTG CTA TGA GTA AAG CAG GG | ||
| VP1-M18 | + | 1467–1492 | CGC AAA GTC AAC TAG AGA ATA TGC CG | ||
| VP1-M9 | + | 2349–2365 | GTC AGA TGA TGA AGT TT | ||
| VP1-M10 | + | 2955–2973 | GGT CTA TGC GCA AGA TAA A | ||
| VP1-M17 | − | 1045–1022 | CCG CAT CCA CCA TTT TCT GTT TTC | ||
| VP1-M14 | − | 1375–1354 | CCC TCC GAC CTA ACG GAA TTG G | ||
| VP1-M11 | − | 1646–1626 | TCT AAC CCC ATG ATG ATA CCC | ||
| VP1-M19 | − | 1940–1917 | GAG TTA AAT TGA AGC ACA GCG TAG | ||
| VP1-M8 | − | 2157–2138 | CAG CAC TGC AGC TTG GTC CC | ||
| VP1-2517m | − | 2517–2497 | CTG TGC TAT TCC TCG TGA CAC | ||
| VP1-M26 | − | 2943–2920 | GTA AGT GTC TGC GTC TAT TTT TGG | ||
| VP1-M25 | − | 3286–3267 | TCT AAT CTT GAA AGA AAC TGG | ||
| VP2 | RT-PCR | VP2-1 | + | 1–20 | GGC TAT TAA AGG CTC AAT GG |
| VP2-5m | + | 1337–1356 | CCA CGA ATG CAT TAC AGA AA | ||
| VP2-N2 | + | 1740–1760 | AAT TAA CTT CAG TCA CGT CCC | ||
| VP2-N1 | − | 1633–1611 | CCC TAA TCG ATT CGA AAG CAA AA | ||
| VP2-6m | − | 1991–1966 | GGT CAT CCG GAA CAC GTG CAA CAT CG | ||
| VP2-8 | − | 2681–2662 | GGT CAT ATC TCC ACA GTG GG | ||
| FLAC | VP2-N4 | + | 2440–2462 | TTT GGT AGC GAA TTA TGA CTG GG | |
| VP2-N17 | + | 2477–2499 | ACT AAG GTG TAC AAA CAA ATT CC | ||
| VP2-N16 | − | 244–221 | AGT CTT GAG AAC TTC GAG TAG CTG | ||
| VP2-N15 | − | 282–259 | AAA ATT TCA TAC TGT ACT TCT CGC | ||
| Seq | VP2-N8 | + | 465–484 | AAG ACA CAC TGC CAG ATG GT | |
| VP2-N14 | + | 800–823 | CCG CTG AAC AAC GAC ATA ATA TTC | ||
| VP2-N10 | + | 1364–1382 | CCT CAG ACT CCG TTC CAA A | ||
| VP2-N13 | + | 1785–1808 | CAG TCA TTC CAA GTC CAC AAA CGC | ||
| VP2-7m | + | 1966–1991 | CGA TGT TGC ACG TGT TCC GGA TGA CC | ||
| VP2-N11 | − | 679–656 | CCT TCT GAC CAC GCC TTC GGT CTC | ||
| VP2-N9 | − | 1165–1144 | TGC CGC AAT TAC TGT CTT AAA A | ||
| VP2-N3 | − | 2256–2234 | TTT GTC ACT TGA CCA TAG TCT CC | ||
| VP2-N18 | − | 2520–2501 | GCT CTG AAA TCA AAT TGT TG | ||
| VP3 | RT-PCR | VP3-1 | + | 1–21 | GGC TTT TAA AGC AGT ACC AGT |
| VP3-M17 | + | 281–305 | GCC AAT TTC ACC TAC AAT TTT GAG G | ||
| VP3-M5 | + | 976–992 | TTT ACC CAC GCC ATT TT | ||
| VP3-M4m | + | 1373–1397 | GTA TTT ATT CAA AAG CCA TTT AAA G | ||
| VP3-M12 | + | 2087–2111 | GAA ATA GAG AAA TAC ATT AAT ACG G | ||
| VP3-495m | − | 495–471 | GTC GCA GCG TTT TGG CAA GTG AAA G | ||
| VP3-M7 | − | 1110–1085 | CTC CAT TTT TGC CAA TCA ATG TTT CC | ||
| VP3-M2 | − | 1647–1628 | CCA AAC ATG CCC AGG CAG CC | ||
| VP3-M11 | − | 2279–2255 | GCG CTT TGA AAC TTT TAA TGA CGT C | ||
| EB-VP3-End | − | 2591–2572 | GGT CAC ATA GTG ACT GAT GT | ||
| FLAC | VP3-M10 | + | 2255–2279 | GAC GTC ATT AAA AGT TTC AAA GCG C | |
| VP3-M24 | + | 2375–2398 | AAA CTA TAC AAC GCA TTT TAC AAG | ||
| VP3-M23 | − | 307–282 | GTC CTC AAA ATT GTA GGT GAA ATT GG | ||
| VP3-495m | − | Above | Above | ||
| Seq | VP3-M1m | + | 391–416 | CAC AGA TTA TAT ATT TCC AGG CTG GG | |
| VP3-M8 | + | 1915–1942 | CAA TTA TTC GTT CGA CCT AAA GAG ATG G | ||
| VP3-M15 | − | 603–580 | CTG GCG ACT GGA AGT GCT GTA GTC | ||
| VP3-M13 | − | 1397–1373 | CTT TAA ATG GCT TTT GAA TAA ATA C | ||
| VP3-M9 | − | 1942–1915 | CCA TCT CTT TAG GTC GAA CGA ATA ATT G | ||
| VP3-2580 | − | 2580–2561 | GAC TAG TGT GTT AAG TTT TT | ||
| VP4 | RT-PCR | VP4-Fsm | + | 1–20 | GGC TAT AAA ATG GCT TCA CT |
| VP4-14 | + | 994–1019 | GGC GGT TCG CTA CCC ACT GAC TTC GG | ||
| VP4-8 | + | 1671–1690 | AGG ACT GGC CGC ATC AGT TT | ||
| VP4-9m | − | 1155–1139 | CCC TCC AGT GCA TTC GA | ||
| VP4-15 | − | 1794–1774 | CGA TGA AAC GTC TGT CCA AGC | ||
| VP4-Rsm | − | 2359–2343 | GGT CAC ATC CTC TAG AC | ||
| FLAC | VP4-7 | + | 1951–1969 | GCA CAA ATT GCA CCG AAC A | |
| VP4-22 | + | 2063–2086 | CTG ACG GAC GTT TCT TCG CAT ACC | ||
| VP4-21 | − | 217–196 | GGT ATG GTC CAT CGA GCA CTG G | ||
| VP4-6m | − | 270–251 | GGT CGG TGA GAG TAG AAT AT | ||
| Seq | VP4-16 | + | 505–528 | GCA GTG GCA AAG CAC ACA GAT CGC | |
| VP4-3 | + | 601–620 | TTA ACC GCA CAC TGC GAT TT | ||
| VP4-18 | + | 1341–1365 | GGG GCT ATA CGG TTT GCC GGC TGC G | ||
| VP4-10 | + | 1489–1513 | GAG AGA CAA CTA GGC GAA CTA CGC G | ||
| VP4-11 | − | 695–671 | GTA TTT TGG ATT GGC GGT AAT CCG G | ||
| VP4-12 | − | 1365–1341 | CGC AGC CGG CAA ACC GTA TAG CCC C | ||
| VP6 | RT-PCR | VP6-F | + | 1–20 | GGC TTT TAA ACG AAG TCT TC |
| VP6-R | − | 1356–1337 | GGT CAC ATC CTC TCA CTA TG | ||
| FLAC | VP6-1 | + | 1161–1181 | GAC AAC CTA CAA CGC GTA TTT | |
| VP6-7 | + | 1195–1216 | TTA GAA GCA TGT TGG TAA AGT G | ||
| VP6-3 | − | 268–243 | CTG GCT GAC TCA ACA TAA TTT GCG TC | ||
| VP6-6 | − | 346–323 | CCA TTT CTT TGT GAC TCT CGC ACC | ||
| Seq | VP6-4 | + | 498–521 | CCA TAT TCA GCA TCA TTC ACA CTG | |
| VP6-9 | − | 913–890 | GGT CTC ATC AAT TGA AAC GAA AGG | ||
| VP7 | RT-PCR | Beg9s* | + | 1–18 | GGC TTT AAA AGA GAG AAT |
| End9s* | − | 1062–1049 | GGT CAC ATC ATA CA | ||
| FLAC | EB-VP7-1 | + | 838–854 | GGC GGC TCA GAT GTA AT | |
| EB-VP7-4 | + | 871–891 | CCA ACA ACT GCA CCA CAA ACC | ||
| EB-VP7-3 | − | 304–281 | GAT AGT AAA GGC ATA GAG TGG AAG | ||
| EB-VP7-2 | − | 390–373 | CCC TGT TGG CCA TCC TTT | ||
| NSP1 | RT-PCR | EB-NSP1-1 | + | 1–21 | GGC TTT TTT TAT GAA AAG TCT T |
| EB-NSP1-2 | − | 1605–1581 | GGT TCA CAT TTT TTG CCG GCT AGC G | ||
| FLAC | NSP1-N3 | + | 1318–1338 | CCA TTT ACT CTC AGC TGT AAA | |
| NSP1-N9 | + | 1360–1381 | GCA TTA GTA GAG AGA TGG TAT G | ||
| NSP1-N8 | − | 223–201 | ATT GAC AGA CAT GAT GAA GTG AG | ||
| NSP1-N2 | − | 251–230 | AAA AAG CAT CTA CCA TAC TGT A | ||
| Seq | NSP1-N1 | + | 350–371 | CAA TCA ATC ATT CAG TTG TAA A | |
| NSP1-N5 | + | 716–740 | CCG TGA AGA CAC TGA TTA ACT CTG G | ||
| NSP1-N7 | + | 972–997 | CCA ACC AGC ATC TAA AGT ACG CTG CC | ||
| NSP1-N10 | − | 547–523 | GGT ACG GTA AAT TAG ATT GAT TTG C | ||
| NSP1-N6 | − | 898–874 | GAT TTA AAT TGT GCG TGA GAA ATG G | ||
| NSP1-N11 | − | 1215–1193 | AAA ACA GTG AAA TAG AAA GTG AG | ||
| NSP1-N4 | − | 1439–1420 | TCA ATT AAG CGG TTG GTT GG | ||
| NSP2 | RT-PCR | NSP2-1 | + | 1–28 | GGC TTT TAA AGC GTC TCA GTC GCC GTT T |
| NSP2-2 | − | 1056–1032 | GGT CAC ATA AGC GCT TTC TAT TCT T | ||
| FLAC | NSP2-N3 | + | 837–859 | TTT GGC AAA ATT GGC ATG CAT TT | |
| NSP2-N5 | + | 877–897 | GGG TAA TAC ATT AGA TGT GTG | ||
| NSP2-N6 | − | 270–247 | TCC GTT TCG AAA TTC ATT CCT CGG | ||
| NSP2-N2 | − | 300–281 | CAC ACA AGC ATC GCC ACC TT | ||
| Seq | NSP2-N7 | + | 427–450 | CTC TAA AGA ACT ATT GCT AAA ATC | |
| NSP2-N4 | − | 733–710 | AAC TAC ACG ATA GTG GCC CTT CCC | ||
| NSP3 | RT-PCR | NSP3-1 | + | 1–25 | GGC TTT TAA TGC TTT TCA GTT GTT G |
| NSP3-2 | − | 1072–1046 | GGT CAC ATA ACG CCC CTA TAG CCA TTT | ||
| FLAC | NSP3-N3 | + | 811–837 | GGA ACA GCA ACT GAA TTC GAT TGA TTT | |
| NSP3-N7 | + | 860–883 | GAC GAC ATT GAA ACA TTA ATT CGG | ||
| NSP3-N6 | − | 254–231 | TGT TGT TTA GAG CCT GAT CTA TGG | ||
| NSP3-N2m | − | 295–270 | GTC GCA CAT CCA ATT TCT GTT CCT AA | ||
| Seq | NSP3-N1m | + | 273–293 | GGA ACA GAA ATT GGA TGT GCG | |
| NSP3-N5 | + | 569–595 | GAG GCA TCA AAA CAG AAA ACG ACA GAG | ||
| NSP3-N4 | − | 635–612 | CAT TAG TTT TCT TAG CTT TGG CGG | ||
| NSP4 | RT-PCR | 1UG10ad† | + | 1–19 | GGC TTT TAA AAG TTC TGT TC |
| EndG10s† | − | 750–735 | GGT CAC ATT AAG ACC A | ||
| FLAC | NSP4-M5 | + | 342–365 | GAG TGG TAA AAG AAT TAA GAC AGC | |
| NSP4-M7 | + | 500–524 | AAA ACT CTA CAT GAT TGG AAA AAC G | ||
| NSP4-M3 | − | 365–342 | GCT GTC TTA ATT CTT TTA CCA CTC | ||
| NSP4-M6 | − | 450–425 | CGT ACG ATC ATC ATA TCA TAT ATT CG | ||
| Seq | NSP4-M2 | + | 480–496 | AAA CTA ATC AAA AAG CG | |
| NSP4-M1 | − | 288–274 | CCA AGC CTC AGC AAA | ||
| NSP5 | RT-PCR | NSP5-5m | + | 1–20 | GGC TTT AAA AGC GCT ACA GT |
| NSP5-3 | − | 664–646 | GGT CAC AAA ACG GGA GTG G | ||
| FLAC | NSP5-M1 | + | 280–303 | GCT GGC GTG TCT ATG GAT TCA TGC | |
| NSP5-M6 | + | 396–420 | GGA CAC CAC AAG GTC AAA AAT TGC G | ||
| NSP5-M5 | − | 239–217 | CTG GTG AGT GGA TCG TTC GAA GC | ||
| NSP5-M2 | − | 354–331 | GGC GAG ATC CAC TTG ATT GCA TCC |
Determination of the 5′and 3′ terminal sequences.
To obtain the complete nucleotide sequence of all 11 genome segments, 5′ and 3′ terminal sequences of all 11 genome segments were determined using the full-length amplification of cDNA (FLAC) method described previously (17). Specific primers used for FLAC are given in Table 1.
Sequence analysis.
Sequence files were analyzed by using Sequencher 4.7 (Gene Codes Corp.) and MacVector 9.5.2 (Accelrys). Some virus samples were composed of mixed populations possessing original or mutated sequences. In such cases, the dominant virus was selected by signal intensity of chromatogram. If we could not find a difference between them, we described them as “original = mutated.” The nucleotide sequence data of murine RV EB strains reported in the present study have been submitted to the GenBank nucleotide sequence database and the accession numbers for each individual genome (VP1 to VP4, VP6, VP7, and NSP1 to NSP5/6) are listed in Table 2.
TABLE 2.
GenBank accession numbers of the murine rotavirus EB strains sequenced in this study
| Virus strain | Accession nos. (VP1 to VP4, VP6, VP7, and NSP1 to NSP5/6) |
|---|---|
| Original | |
| Po | JF309297-JF309307 |
| Mouse, low passage | |
| A8 | KJ477105-KJ477115 |
| B8 | KJ477116-KJ477126 |
| C8 | KJ477127-KJ477137 |
| G8 | KJ477138-KJ477148 |
| R8 | KJ477149-KJ477159 |
| Mouse, high passage | |
| A17 | KJ477160-KJ477170 |
| B17 | KJ477171-KJ477181 |
| B27 | KJ477182-KJ477192 |
| E7 | KJ477193-KJ477203 |
| AGMK cell passage | |
| aa18 | KJ477204-KJ477214 |
| aa40 | KJ477215-KJ477225 |
| bb18 | KJ477226-KJ477236 |
| bb40 | KJ477237-KJ477247 |
| gg40 | KJ477248-KJ477258 |
| ii40 | KJ477259-KJ477269 |
| MA104 cell passage | |
| cc40 | KJ477270-KJ477280 |
| jj40 | KJ477281-KJ477291 |
| ll40 | KJ477292-KJ477302 |
| kk40 | KJ477303-KJ477313 |
| kk18-pl-4-3-1-1 | KJ477314-KJ477324 |
Construction and characterization of recombinant baculoviruses expressing rotavirus NSP4 proteins.
The construction and characterization of recombinant baculoviruses expressing selected RV NSP4 proteins were performed using pBlueBac4.5/V5-His-TOPO vector (pBlueBac4.5/V5-His TOPO TA Cloning kit; Invitrogen) according to the manufacturer's instructions. The full-length NSP4 gene was amplified by RT-PCR, inserted into baculovirus transfer vector, and expressed in Spodoptera frugiperda 9 (Sf-9) insect cells. The His-tagged rNSP4 proteins were purified by using Ni-NTA agarose (Qiagen) according to the manufacturer's protocol and without removal of the His tags. A high-titer viral stock of the recombinant baculoviruses was prepared in Sf-9 cells, and its titer was determined using a plaque assay according to the manufacturer's instructions (Bac-N-Blue transfection kit; Invitrogen). A working viral stock of 107 PFU/ml was prepared using Grace's medium (Gibco). Western blot analysis was used to confirm the specific protein expression of each recombinant NSP4 (rNSP4) protein in Sf-9 cells. Hyperimmune guinea pig antiserum raised against NSP4 of murine RV EB strain diluted at 1:250 reacted with all rNSP4 proteins (18).
Diarrhea induction in neonatal mice.
Purified 1 nmol of the rNSP4 protein was diluted to 50 μl in sterile, endotoxin-free PBS and was injected intraperitoneally into 5- to 6-day-old CD-1 mouse pups by using a 30G needle. The neonatal mice were isolated and kept warm and humid. To determine the presence of diarrhea, we examined each neonatal mouse every 1 h for the first 8 h and then 12 and 24 h after inoculation by gently pressing its abdomen. The pups were monitored by one researcher. Watery diarrhea and loose yellow stool with some liquid were classified into the category of diarrhea. Loose yellow stool was not considered diarrhea. All pups were screened at 24 h after inoculation. Endotoxin-free PBS was used as a negative control. Statistical analysis was performed at the 5% level of significance using a chi-square test with Yates' correction.
Nucleotide sequence accession numbers.
The sequences newly determined in this study were deposited in GenBank under accession numbers KJ477105 to KJ477324 (Table 2).
RESULTS
Virulence of RV during serial passages in mice or in cell cultures.
Figure 1 is a schematic diagram of serial passage history in the present study. During the initial serial passages in mice from the original virus (Po) to A8, infected pups started developing signs of diarrhea after passage 4, and after four additional serial passages (for a total of eight passages), all infected pups developed diarrhea. Then, 10% intestinal homogenate from passage 8 in pups (A8) was inoculated onto 10 AGMK cells and passaged serially 18 times in cell cultures. Subsequently, cell lysate from passage 18 in 10 AGMK cells (aa18) was passaged serially in CD-1 mouse pups. Infected pups did not develop diarrhea until passage 5. After a subsequent three passages (a total of eight passages), all infected pups developed diarrhea (B8). During in vivo passage, infected pups started diarrhea after passage 4, and all of them developed diarrhea after passage 8. Additional passages were done using mice or cell cultures in the same manner.
At the onset, the original virus (Po) was considered to consist of a mixed population, because it had already been passaged serially in MA104 cells 10 to 20 times after plaque purification. Therefore, we could not conclude whether the mutations detected were newly induced mutations whenever hosts alternated or mutations selected from closely related, but slightly heterogeneous populations (quasispecies) that were possibly maintained in each inoculum. To investigate this question, we obtained kk18-pl-4-3-1-1 from cell culture-passaged kk18 virus after four consecutive plaque purifications, and considered this to be a single avirulent clone. After eight serial passages of kk18-pl-4-3-1-1 in mice, virulent virus, i.e., R8 appeared (Fig. 1); this implied the possibility of entire newly induced virulence-associated mutations. On the other hand, purification of mouse-passaged virulent virus from intestinal homogenate was difficult by plaque purification or limiting dilution. Therefore, we could not conduct the infection experiment with purified virulent virus clone. In the present study, we used 50 μl of 10% intestinal homogenates for in vivo inoculation. The virus inoculum was not always titrated every time before every in vivo passages; however, we measured the focus-forming unit (FFU) titer of selected virus inocula together afterward and confirmed the titer to be mostly 2 × 106 to 1 × 107 FFU/ml (data not shown).
Mutations and substitutions detected during serial passages in mice and/or in cell cultures.
Mutations and substitutions detected during serial passages in mice and/or in cell cultures in the present study are summarized in Table 3. At least five mutations were detected in each of all eleven genes. More than half of the mutations were nonsynonymous substitutions in the VP4 (24/31), VP7 (7/10), NSP2 (9/12), NSP4 (11/13), and NSP5/6 (4/5) genes. These five genes also had higher substitution rates (2.03 to 6.29%) than the other genes (0.36 to 0.83%).
TABLE 3.
Summary of mutations and substitutions detected during serial passages in mice and/or in cell cultures
| Genea | Total length (bp) | Total no. of mutations | Mutation rate (%)b | Total protein length (aa) | Total no. of substitutions | Substitution rate (%)c |
|---|---|---|---|---|---|---|
| VP1 | 3,302 | 27 | 0.82 | 1088 | 9 | 0.83 |
| VP2 | 2,681 | 15 | 0.56 | 878 | 5 | 0.57 |
| VP3 | 2,591 | 13 | 0.50 | 835 | 3 | 0.36 |
| VP4 | 2,359 | 31 | 1.31 | 775 | 24 | 3.10 |
| VP6 | 1,356 | 6 | 0.44 | 397 | 2 | 0.50 |
| VP7 | 1,062 | 10 | 0.94 | 326 | 7 | 2.15 |
| NSP1 | 1,605 | 10 | 0.62 | 493 | 3 | 0.61 |
| NSP2 | 1,056 | 12 | 1.14 | 316 | 9 | 2.85 |
| NSP3 | 1,072 | 11 | 1.03 | 310 | 2 | 0.65 |
| NSP4 | 750 | 13 | 1.73 | 175 | 11 | 6.29 |
| NSP5/6 | 664 | 5 | 0.75 | 197 | 4 | 2.03 |
Underlining indicates genome segments for which the mutation rate was far below the substitution rate.
The mutation rate was calculated as the total number of mutations/total length (bp).
The substitution rate was calculated as the total number of substitutions/total length (aa).
Summary of dominant mutations detected during serial passages in mice or in cell cultures.
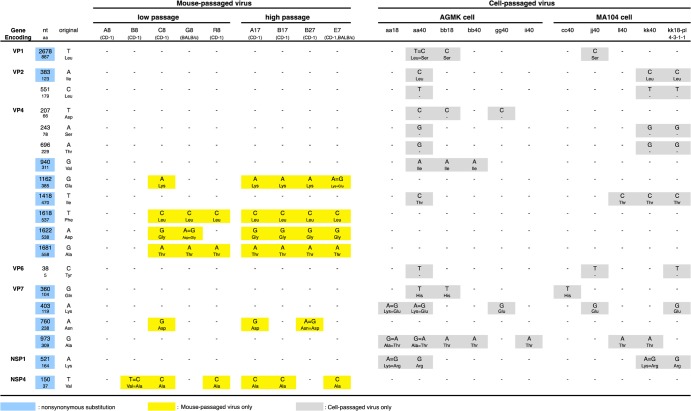
To determine the mutations that could be associated with virulence or lack of virulence, mutations detected exclusively in either mouse (virulent)- or cell culture (avirulent)-passaged viruses were collected. Mutations occurring in more than three virus samples are summarized in Fig. 2. Characteristic mutations of mouse-passaged viruses were four mutations in the VP4 gene and one mutation each in the VP7 and NSP4 genes. Especially noteworthy was that four mutations in the VP4 gene and one mutation in the NSP4 gene were detected in five to seven of nine mouse-passaged viruses. R8, which was obtained from serial passages in mice of plaque-purified avirulent virus (kk18-pl-4-3-1-1), also had these characteristic mutations (two in the VP4 gene and one in the NSP4 gene).
FIG 2.
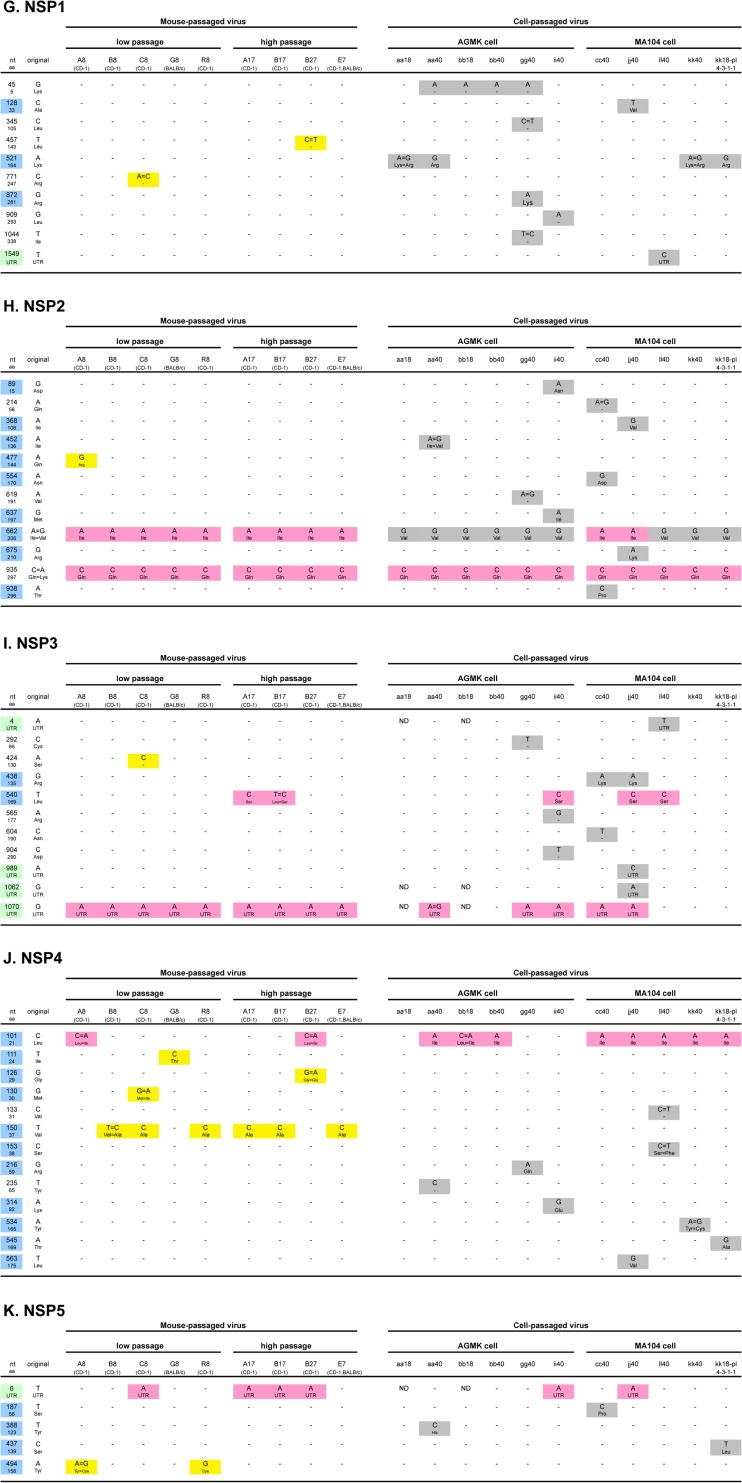
Summary of mutations detected in more than three virus samples during serial passages in mice or in cell cultures. “nt” and “aa” indicate nucleotides and amino acids, respectively. A blue background indicates nonsynonymous substitution. Yellow and gray backgrounds indicate mutations only detected in mouse-passaged virus and in cell culture-passaged virus, respectively. More than 10 mutations detected both in mice and cell cultures are not presented in this figure. All of the mutations detected in eleven genes are summarized in Fig. 3A to K, respectively.
Interestingly, these mutations disappeared again, as confirmed during the following cell cultures; five mutations in the VP4 or NSP4 genes, which were gained in the mouse-passaged B17 strain, were all lost in the subsequent cell culture-passaged viruses (ii40 and jj40). Similarly, three mutations in the VP4 gene in mouse-passaged G8 strain disappeared in the following cell culture-passaged virus (gg40). All mutations and substitutions detected in each of eleven genes during serial passages in mice and/or in cell cultures are summarized in Fig. 3A to K, respectively.
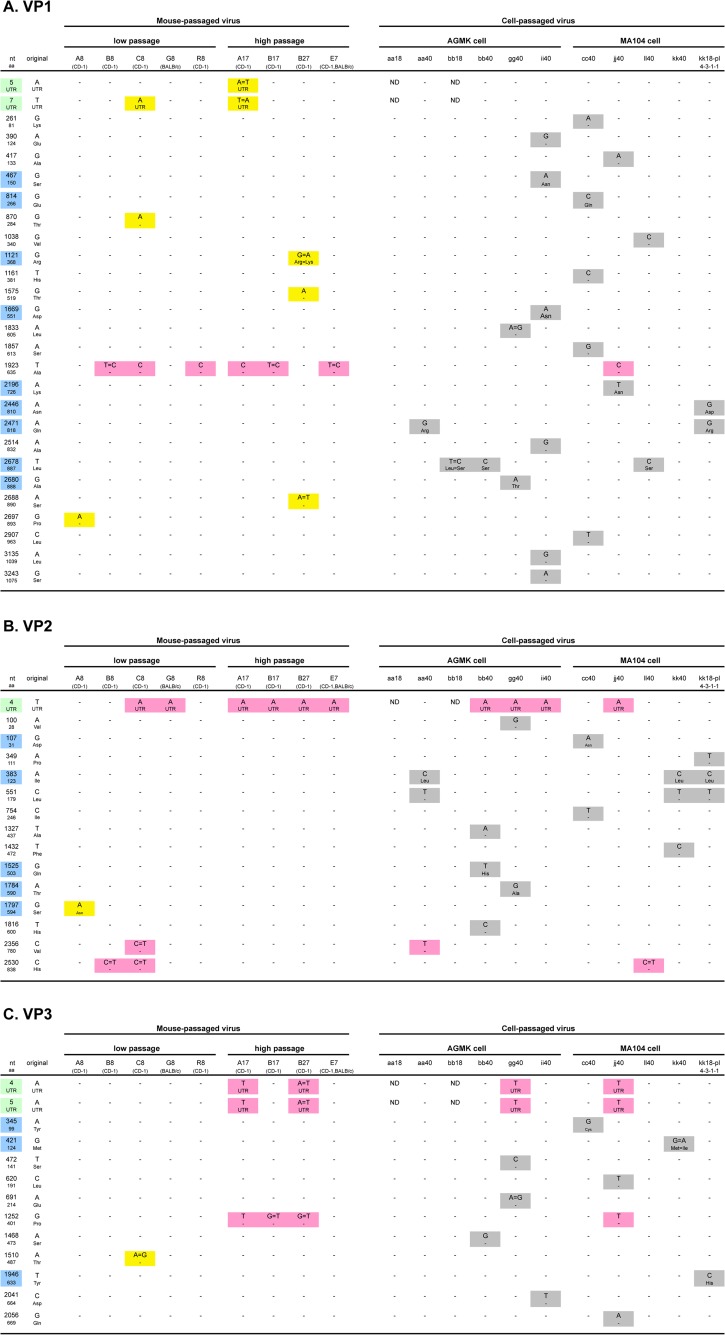
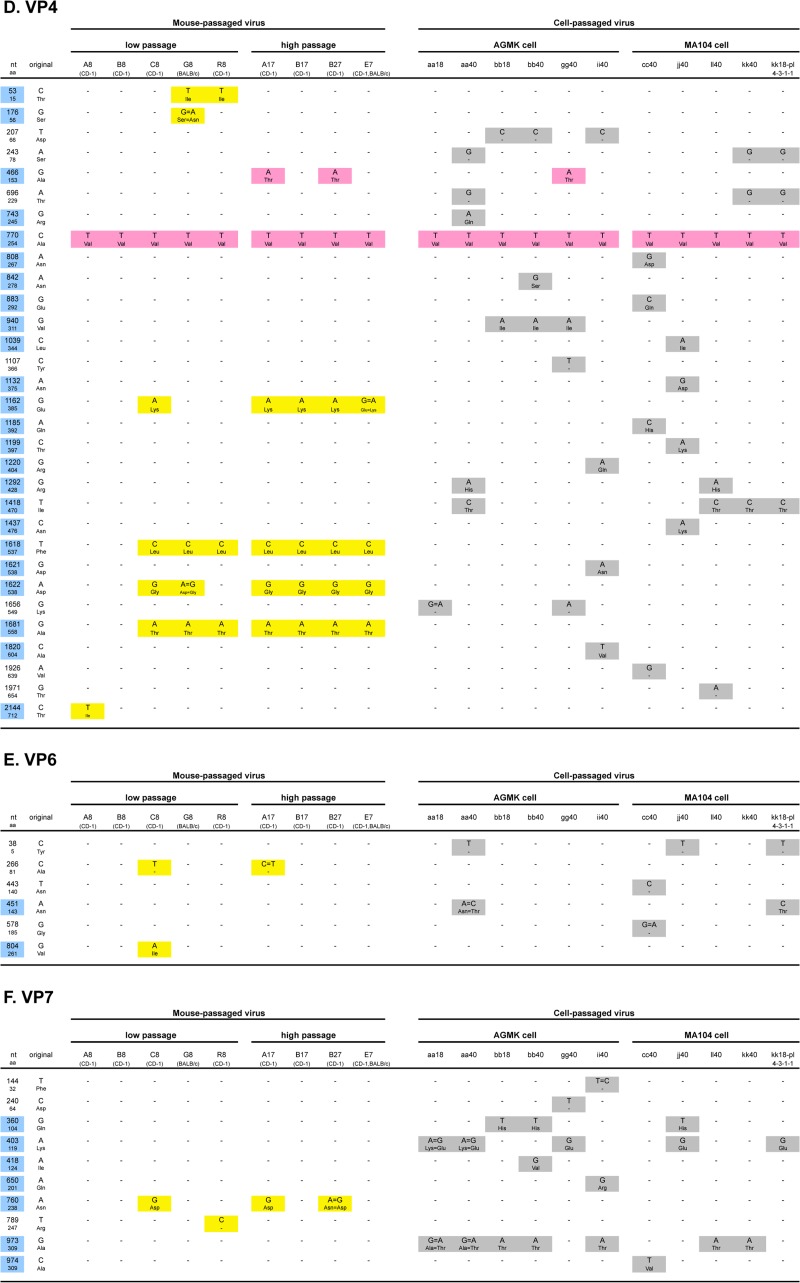
FIG 3.
Summary of mutations and substitutions detected in each of 11 genes (VP1 to VP4, VP6, VP7, and NSP1 to NSP5/6 [A to K, respectively]) during serial passages in mice and/or in cell cultures. “nt” and “aa” indicate nucleotides and amino acids, respectively. Blue and yellow-green backgrounds indicate nonsynonymous substitutions and untranslated regions, respectively. Yellow, gray, and pink backgrounds indicate mutations only detected in mouse-passaged virus, in cell culture-passaged virus, or both, respectively. ND, no sequencing data.
VP4 mutations and substitutions detected during serial passages in mice and/or in cell cultures.
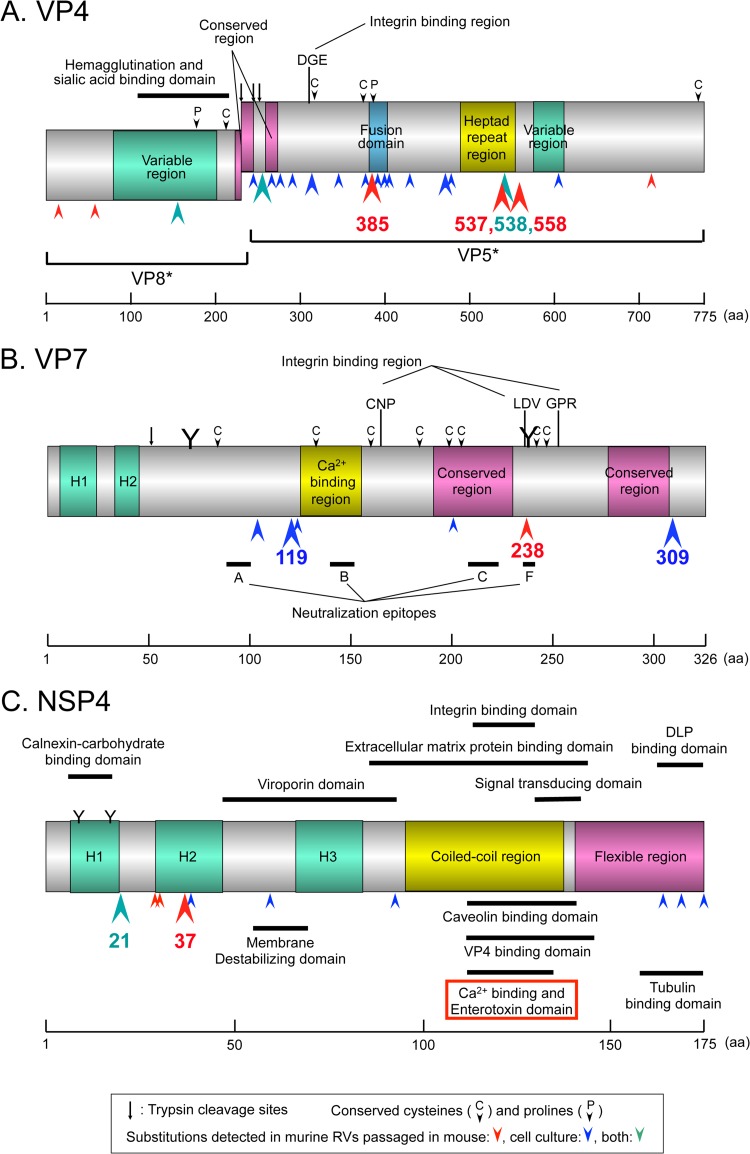
Figures 3D and 4A summarize the mutations and substitutions detected in the VP4 gene during serial passages in mice and/or in cell cultures. A total of 31 mutations were detected during these passages. Of the 31 substitutions, 24 were nonsynonymous. Among them, four mutations (substitutions) at nucleotide (nt) 1162 (amino acid [aa] 385), nt 1618 (aa 537), nt 1622 (aa 538), and nt 1681 (aa 558), were consistently detected in all four high mouse-passaged viruses. The substitution at aa 385 was located in the putative fusion domain (Fig. 4A).
FIG 4.
Schematic diagram of substitutions detected on VP4 (A), VP7 (B), and NSP4 (C). Numbers indicate the amino acid position on each of three genes (1, 22, 34–36). Red, blue, and green arrowheads indicate substitutions detected in murine RVs passaged in mouse, cell culture, or both, respectively. Big, middle, and small arrowheads indicate substitutions occurring, respectively, in >5, in 3 to 4, or in 1 to 2 strains detected during serial passages in mice and/or in cell cultures. (A) Substitution at aa 538 detected in both passages in mice and passages in cell cultures (see Fig. 3J).
VP7 mutations and substitutions detected during serial passages in mice and/or in cell cultures.
Figures 3F and 4B summarize the mutations and substitutions detected in the VP7 gene during serial passages in mice and/or in cell cultures. A total of ten mutations were detected during these passages. Seven of the ten substitutions were nonsynonymous. Among them, one mutation (substitution) at nt 760 (aa 238) was detected in two of four high mouse-passaged viruses. The substitution at aa 238 was located in an N-glycosylation site, an integrin-binding region, and neutralization epitope F (Fig. 4B).
NSP4 mutations and substitutions detected during serial passages in mice and/or in cell cultures.
Figures 3J and 4C summarize the mutations and substitutions detected in the NSP4 gene during serial passages in mice and/or in cell cultures. A total of thirteen mutations were detected during these passages. Eleven of these mutations were nonsynonymous. There were no substitutions detected in the enterotoxin domain (aa 114 to 135) in either group. On the other hand, the substitution at aa 37 (Val to Ala) was detected in six of nine mouse-passaged viruses (three of four high mouse-passaged viruses). This site corresponds to the H2 domain (Fig. 4C). In addition, substitution at aa 21 (Leu to Ile) was observed in eight of eleven cell culture-passaged viruses (Fig. 3J). This mutation was also observed in two of nine mouse-passaged viruses (A8 and B27); therefore, it was not presented in Fig. 2.
Each of three recombinant NSP4 proteins displayed different diarrhea-causing capabilities in mouse pups.
As mentioned above, the main substitutions in the NSP4 protein were observed at amino acid positions 21 and 37 (Fig. 3J and 4C). As a result, three representative NSP4 phenotypes were confirmed. The first was the NSP4 protein with Ile-21 and Val-37, which was observed in a part of the cell culture-passaged viruses (aa40, bb40, and cc40). The second was the NSP4 protein with Leu-21 and Val-37, which was confirmed in the original virus (Po) and one cell culture-passaged virus (aa18). The third was the NSP4 protein with Leu-21 and Ala-37, which was confirmed in the greater part of mouse-passaged viruses (R8, A17, B17, and E7).
To test an enterotoxin activity of each NSP4 protein (19), we constructed rNSP4 proteins, which bore the above-mentioned three kinds of combinations of amino acid positions 21 and 37, using a baculovirus expression system (Table 4). Each of three rNSP4 proteins was injected into 5- to 6-day-old CD-1 mouse pups intraperitoneally. The first rNSP4 protein (Ile-21, Val-37) did not cause any diarrhea in twelve mouse pups. Only one of eight mouse pups inoculated with the second rNSP4 protein (Leu-21, Val-37) developed diarrhea. On the other hand, eleven of twelve mouse pups inoculated with the third rNSP4 protein (Leu-21, Ala-37) developed diarrhea. None of the twelve mouse pups inoculated endotoxin-free PBS had any diarrhea. As a result, Ala-37, which was found in most of mouse-passaged (virulent) viruses, was considered to be the critical substitution to exhibit NSP4 enterotoxin activity.
TABLE 4.
Comparison of diarrhea-causing ability in mouse pups given each of three recombinant NSP4 proteinsa
| Recombinant segment length (no. of nt, no. of aa) | Treatment group |
|||
|---|---|---|---|---|
| Control (PBS) | Attenuated | Original (Po) | Virulent | |
| 101, 21 | NA | A (Ile) | C (Leu) | C (Leu) |
| 150, 37 | NA | T (Val) | T (Val) | C (Ala) |
nt, nucleotides; aa, amino acids. Animals with diarrhea (number of mice with diarrhea/total number of mice) were as follows: control (PBS), 0/12; attenuated, 0/12; original (Po), 1/8; and virulent, 11/12. Statistical analysis was performed at a 5% level of significance using a chi-square test with Yates' correction: control/virulent, P < 0.01; attenuated/virulent, P < 0.01; and original/virulent, P < 0.01. NA, not applicable.
Alteration of 3′CSs of the NSP1 and NSP3 genes detected during serial passages in mice and/or in cell cultures.
Nine genome segments did not change their 3′ end sequences during serial passages in mice and cell cultures, the exceptions being the NSP1 and NSP3 genes. In the original virus (Po), the 3′ consensus sequence (3′CS; UGU GACC) in the NSP1 gene had already changed to UGU GAACC (Table 5) (20). However, 3′CS in the NSP1 gene of subsequent mouse-passaged viruses was regained and invariably conserved. On the other hand, it was lost and changed in all nine cell culture-passaged viruses (no data for aa18 and bb18). In short, it changed to UGU GAACC in seven strains (aa40, bb40, gg40, ii40, jj40, kk40, and kk18-pl-4-3-1-1), to UGU GAUCC in one strain (ll40), and to UGU GACACC in one strain (cc40).
TABLE 5.
Conservation of the 3′ consensus sequence (3′CS) in the NSP1 gene of RVA from GenBank data
| Straina | Host | Genotype |
3′CS (UGU GACC) | GenBank accession no. | Reference (PubMed ID) | |
|---|---|---|---|---|---|---|
| G/P | NSP1 | |||||
| Wild type | ||||||
| EB-mouse | Murine | G16P[16] | A7 | Conserved | This study | This study |
| B4106 | Human | G3P[14] | A9 | Conserved | AY740735 | 16571797 |
| DRC86 | Human | G8P[6] | A2 | Conserved | DQ005119 | 16672410 |
| DRC88 | Human | G8P[8] | A2 | Conserved | DQ005108 | 16672410 |
| B4633 | Human | G12P[8] | A1 | Conserved | DQ146644 | 17166908 |
| Dhaka12 | Human | G12P[6] | A1 | Conserved | DQ146666 | 17166908 |
| Dhaka16 | Human | G1P[8] | A1 | Conserved | DQ492675 | 17166908 |
| Dhaka25 | Human | G12P[8] | A1 | Conserved | DQ146655 | 17166908 |
| N26 | Human | G12P6] | A2 | Conserved | DQ146688 | 17166908 |
| RV161 | Human | G12P[6] | A2 | Conserved | DQ490540 | 17166908 |
| RV176 | Human | G12P[6] | A2 | Conserved | DQ490557 | 17166908 |
| Matlab13 | Human | G12P[6] | A1 | Conserved | DQ146677 | 17166908 |
| B3458 | Human | G9P[8] | A1 | Conserved | EF990709 | 18216098 |
| TB-Chen | Human | G2P[4] | A2 | Conserved | AY787647 | 18329063 |
| B1711 | Human | G6P[6] | A2 | Conserved | EF554088 | 18796733 |
| RC-18-08 | Antelope | G6P[14] | A11 | Conserved | FJ495130 | 19153225 |
| B383 | Bovine | G15P[11] | A13 | Conserved | FJ347117 | 19153225 |
| Chubut | Guanaco | G8P[14] | A3 | Conserved | FJ347106 | 19153225 |
| Rio_Negro | Guanaco | G8P[1] | A11 | Conserved | FJ347128 | 19153225 |
| GR10924/99 | Human | G9P[6] | A2 | Conserved | FJ183357 | 19264638 |
| PAH136 | Human | G3P[9] | A3 | Conserved | GU296410 | 20409385 |
| PAI58 | Human | G3P[9] | A3 | Conserved | GU296411 | 20409385 |
| BA222 | Feline | G3P[9] | A3 | Conserved | GU827412 | 21228122 |
| mani-265/07 | Human | G10P[6] | A3 | Conserved | HM348718 | 21884295 |
| MWI/1473 | Human | G8P[4] | A2 | Conserved | HQ657133 | 21915879 |
| 3133WC | Human | G12P[4] | A1 | Conserved | HQ657144 | 21915879 |
| 3176WC | Human | G12P[6] | A1 | Conserved | HQ657155 | 21915879 |
| 3203WC | Human | G2P[4] | A2 | Conserved | HQ657166 | 21915879 |
| 2371WC-B | Human | G9P[8] | A1 | Conserved | JN013975 | 22019521 |
| 2371WC-A | Human | G9P[8] | A2 | Conserved | JN013974 | 22019521 |
| 1603 | Bovine | G6P[5] | A3 | Conserved | JN831204 | 22541163 |
| 1604 | Bovine | G8P[1] | A3 | Conserved | JN831215 | 22541163 |
| 1605 | Bovine | G6P[5] | A3 | Conserved | JN831226 | 22541163 |
| E30 | Equine | G3P[12] | A10 | Conserved | JF712572 | 22190012 |
| E403 | Equine | G14P[12] | A10 | Conserved | JF712583 | 22190012 |
| E4040 | Equine | G14P[12] | A10 | Conserved | JN872871 | 22190012 |
| 04V2024 | Equine | G14P[12] | A10 | Conserved | JN903515 | 22190012 |
| EqRV-SA1 | Equine | G14P[12] | A10 | Conserved | JQ345493 | 22190012 |
| Tissue culture | ||||||
| EB-tc | Murine | G16P[16] | A7 | UGU GAACC | This study | This study |
| EB-tc-ll40 | Murine | G16P[16] | A7 | UGU GAUCC | This study | This study |
| EB-tc-cc40 | Murine | G16P[16] | A7 | UGU GACACC | This study | This study |
| KU | Human | G1P[8] | A1 | UGU GAACC | AB022769 | 10481750 |
| K9 | Canine | G3P[3] | A9 | UGU GAACC | AF111946 | 10481750 |
| RV198-95 | Canine | G3P[3] | A9 | UGU GAACC | HQ661140 | 21609783 |
| SA11-4F | Simian | G3P[2] | A5 | UGU GAACC | AF290883 | 11160712 |
| SA11-30-19 | Simian | G3P[2] | A5 | UGU GAACC | AF290881 | 11160712 |
| SA11-N2 | Simian | G3P[2] | A5 | UGU GAACC | JN827248 | 23263646 |
| SA11-N5 | Simian | G3P[2] | A5 | UGU GAACC | JQ688677 | 23263646 |
| L338 | Equine | G13P[18] | A6 | UGU GAACC | JF712561 | 22190012 |
| RRV-AUU | Simian | G3P[3] | A9 | UGA UUCC | AY117049 | 15372078 |
| RRV-CCUU | Simian | G3P[3] | A9 | UGCC UUCC | AY117050 | 15372078 |
| RRV-CUU | Simian | G3P[3] | A9 | UGC UUCC | AY117051 | 15372078 |
| RRV-U | Simian | G3P[3] | A9 | UG UUCC | AY117052 | 15372078 |
| RRV-UU | Simian | G3P[3] | A9 | UGU UUCC | AY117053 | 15372078 |
| 10V0112H5 | Avian | G23P[37] | A16 | UAU GACC | JX204817 | 23052396 |
| 03V0002E10 | Avian | G22P[35] | A16 | UAU GACC | JX204828 | 23052396 |
| RotaTeq-BrB-9 | Vaccine | G4P[5] | A3 | Conserved | GU565091 | 20451234 |
| RotaTeq-SC2-9 | Vaccine | G2P[5] | A3 | Conserved | GU565069 | 20451234 |
| RotaTeq-WI78-8 | Vaccine | G3P[5] | A3 | Conserved | GU565080 | 20451234 |
| RotaTeq-WI79-4 | Vaccine | G6P[8] | A3 | Conserved | GU565047 | 20451234 |
| RotaTeq-WI79-9 | Vaccine | G1P[5] | A3 | Conserved | GU565058 | 20451234 |
| B10 | Human | G3P[2] | A5 | Conserved | HM627559 | 21035567 |
| DS-1 | Human | G2P[4] | A2 | Conserved | HQ650120 | 21600242 |
| PA260-97 | Human | G3P[3] | A15 | Conserved | HQ661118 | 21609783 |
| RV52-96 | Canine | G3P[3] | A9 | Conserved | HQ661129 | 21609783 |
| 30-96 | Lapine | G3P[14] | A9 | Conserved | DQ205225 | 16571797 |
| RRV | Simian | G3P[3] | A9 | Conserved | AY117048 | 15372078 |
| PO-13 | Avian | G18P[17] | A4 | Conserved | AB009633 | 11325467 |
| RF | Bovine | G6P[1] | A3 | Conserved | M22308 | 2823457 |
| UK | Bovine | G6P[5] | A3 | Conserved | Not available | 15372078 |
The EB prefix indicates the murine RV EB strain used in this study. EB-mouse is the murine RV EB strain passaged by mouse pups. EB-tc is the murine RV EB strain passaged by cell cultures except for ll40 and cc40.
Also in the NSP3 gene of original virus (Po), 3′CS had already changed to UGU GGCC. However, 3′CS was regained in the following mouse-passaged virus and conserved in subsequent mouse- or cell culture-passaged viruses, except in four cell culture-passaged viruses (bb40, ll40, kk40, and kk18-pl-4-3-1-1) in which it returned to UGU GGCC.
DISCUSSION
In general, there are four patterns for genetic mutation—point mutation, reassortment, rearrangement, and recombination—of which point mutation and reassortment are important in RV vaccine development (7). Rotarix (monovalent human RV vaccine; Glaxo-SmithKline) was developed by accumulation of point mutations during serial passage in cell cultures, and RotaTeq (pentavalent human-bovine RV reassortant vaccine; Merck) was developed by reassortment; however, the mechanism of their attenuation is not well understood (8, 9, 21). To analyze mechanisms underlying this phenomenon, we passaged serially a murine RV EB strain in 4- to 5-day-old mouse pups (CD-1 or BALB/c; in vivo) or in cell cultures (10 AGMK cells or MA104 cell line; in vitro) alternately and repeatedly. We then fully sequenced all eleven genome segments of 21 passaged RV isolates and analyzed the correlation between point mutations and RV virulence. Using the reciprocal in vivo/in vitro system, we demonstrated the acquisition and subsequent loss of virulence-associated gene expression of murine RV.
In the present study, we showed that virulent mutations could be newly induced, e.g., plaque-purified avirulent kk18-pl-4-3-1-1 could produce virulent R8 RV after eight serial passages in mice (Fig. 1). On the other hand, we failed to isolate a single virulent clone population from mouse-passaged viruses (A8, B8, G8, and B17) either by plaque purification or by limiting dilution. Thus, we were unable to conduct the infection experiment with purified virulent virus. Therefore, we could not conclude whether the mutations detected were either newly induced mutations whenever hosts alternated or mutations selected from the quasispecies population that were possibly maintained in each inoculum. In any case, the viruses that acquired or preserved virulence-associated mutations could proliferate well and become predominant in mice, and eventually result in the onset of diarrhea. In contrast, the viruses lacking virulence-associated mutations might have some kind of growth advantage in cell cultures, becoming the dominant population.
Since the VP4 and VP7 gene products are located on the virus particle and induced neutralization antibody, it was interesting that we confirmed consistent or dominant substitutions in their fusion domain (aa 385 of VP4), glycosylation sites (aa 238 of VP7), or integrin-binding region (aa 238 of VP7) (Fig. 4A and B) (1, 22, 23). By using a genetic approach, previous studies showed that at least eight genome segments (i.e., genes encoding VP3, VP4, VP6, VP7, NSP1, NSP2, NSP3, and NSP4) were involved in the virulence of rotaviruses (6, 17, 24, 25). Our analysis of sequence data from mouse-passaged (virulent, in vivo) and cell culture-passaged viruses (attenuated, in vitro) was generally consistent with those of previous genetic approaches, although there were some disparities.
Sequencing analyses between parental (virulent) and serial cell culture-passaged (attenuated) human RV G1P[8] strains were performed for the VP4 gene (26–29). Substitution at aa 385 was found in two of four studies (27, 28). When the VP4 sequence of strain 89-12 (progenitor of Rotarix) was compared to that of the original strain, five substitutions were detected (aa 51, 167, 331, 385, and 695) (27). On the other hand, when strain CDC-9 (attenuated live vaccine candidate) was passaged many times, five substitutions (aa 51, 331, 364, 385, and 388) were detected in the VP4 gene (28). In addition to substitution at aa 385, substitution at aa 51 and 331 was confirmed in both studies. In the analysis of association between amino acid substitutions and the neutralization resistant mutants using monoclonal antibodies, substitution at aa 385 in the VP4 gene was also detected (30). The crystal structure analysis revealed that the region between aa 382 and 400 in the VP4 gene was a potential membrane interaction loop and was confirmed to interact with lipid bilayer directly (31, 32). Putting it all together, substitution at aa 385 in the VP4 gene appears to be essentially associated with RV virulence, especially from the aspect of viral attachment or penetration.
With regard to the VP7 gene, sequencing analysis of parental (virulent) and serial cell culture-passaged (attenuated) viruses was reported in a human RV study, although no substitutions were detected (28). On the other hand, in the study on neutralization resistant mutants by using monoclonal antibodies, substitution at aa 238 in the VP7 gene was confirmed, as we saw in our study (30). When aa 238 in the VP7 gene is Asn (N) or Asp (D), it creates either the N-X-T motif (238N) as a N-glycosylation site or the L-D-V motif (238D) as an integrin-binding region (Fig. 4B) (1, 22). In the present study, we showed that all cell culture-passaged viruses conserved the N-X-T motif (238N), whereas one of five low mouse-passaged (C8) and two of four high mouse-passaged viruses (A17 and B27) acquired the L-D-V motif (238D). Absolute preservation of the N-X-T motif (238N) as an N-glycosylation site in cell-cultured viruses implied its superiority for propagation in cell culture and agreed with the findings of Graham et al. (33) that simian RV SA11 strain, which conserved the N-X-T motif (238N) in the VP7 gene in cell culture, achieved a 10-fold-higher titer than the nonconserving strain. In contrast, the significance of our finding regarding the acquisition of the L-D-V motif (238D) as an integrin-binding region in the VP7 gene in mouse-passaged viruses remains to be elucidated.
NSP4 is a multifunctional protein and is essential for RV replication, transcription, and morphogenesis (19, 34). Apart from enterotoxin, a lot of NSP4 functions have been mapped to domains (Fig. 4C) (34–36). Sequencing analyses of the NSP4 gene between parental (virulent) and serial cell culture-passaged (attenuated) viruses were reported in four human group A rotavirus (RVA) studies, one murine RVA study, and one porcine RVA study (28, 37–41). All strains in human RV studies belonged to the G1P[8] genotype, and substitutions at aa 16 (Leu to Ser) and aa 34 (Pro to Leu) were detected commonly between parental and serial cell culture-passaged human RV Wa strains, although no clinical data were presented (40, 41). In a murine RV study, three murine RV strains (EW, EHP, and EC) were passaged serially in 10 AGMK and MA104 cell cultures (38). Substitution at aa 45 (Thr to Met) was detected commonly between parental and serial cell culture-passaged EW and EHP strains; however, its association with virulence was not demonstrated in vivo. In a porcine RV study, two porcine RV strains (OSU and Gottfried) were passaged serially in cell cultures (39), and substitutions at aa 135 (Val to Ala) and aa 138 (Pro to Ser) were commonly detected. Although aa 135 was within the enterotoxin domain (aa 114 to 135), no association of these substitutions with in vivo virulence was presented.
On the other hand, in rNSP4 protein study, we found that substitution at aa 37 (Val to Ala), which was observed dominantly in the mouse-passaged viruses, was evidently associated with virulence in mice (Table 4). Although aa 37 locates to one of three hydrophobic lesions (H2) and to a transmembrane domain, the function of this region, including virulence, remains unknown (Fig. 4C) (34–36). Previously, Zhang et al. reported that aa 138, which is also located outside the enterotoxin domain, was associated with virulence of porcine RV OSU strain based on the study in mice with rNSP4 proteins (39). Although the mechanism of rNSP4 virulence was unknown, the difference of substitution position to ours might be due to the different animal hosts used (i.e., a murine RV EB-homologous animal host versus a porcine RV OSU-heterologous animal host). In addition to these rNSP4 studies, comparative sequencing studies between parental (virulent) and serial cell culture-passaged (attenuated) viruses confirmed several mutations outside the enterotoxin domain (28, 37–41). On the other hand, no explanation has been offered as to how regions outside the enterotoxin domain regulate enterotoxin activity. For real insight into this question, future animal studies using a universally applicable and fully tractable reverse genetics system should be necessary.
All eleven genome segments of RV lack a polyadenylation signal and contain conserved consensus sequences at their 5′ and 3′ ends. Previously, the 3′CSs (UGU GACC) of eleven genome segments of RV were confirmed as an activator to promote dsRNA synthesis and translational enhancer to increase the expression of the viral proteins (20). Interestingly, in the present study, we found that the 3′CS of the NSP1 gene was completely conserved in mouse-passaged viruses but lost in all cell culture-passaged viruses. Previous studies reported that the loss of 3′CS of the NSP1 gene during serial passages in cell cultures was intricate and depended on the combination of RV strain, cell type, and passage conditions (20, 42). Therefore, we investigated 3′CS of the NSP1 gene in the GenBank database. We collected relevant information on 37 wild-type RVs and 29 tissue culture-adapted RVs (Table 5). Interestingly, 3′CSs of the NSP1 gene of 37 wild-type RVs were completely conserved, although their hosts and genotypes (VP7/VP4 and NSP1) varied greatly. On the other hand, those of almost half of the tissue culture-adapted RVs were lost and had changed diversely. From the aspect of RV virulence, it was recently revealed that RVs evaded the innate immune response, especially the interferon response through the NSP1 gene promoting the degradation of IRF3 and inhibition of NF-κB activity (43). Although no direct relationship between the 3′CS of the NSP1 gene and the virulence of RV has been described, the conservation of the 3′CS of the NSP1 gene must be associated with the expression of RV virulence not only in the mouse but also in other hosts.
Although not fully determined, it was strongly suggested that the consistent or dominant mutations confirmed here were at least partly associated with virulence, because they were exhibited along with the acquisition of virulence during passages in vivo (in an original animal host, i.e., mice) and disappeared during after passages in vitro (in cell cultures). In addition, enterotoxic activity of rNSP4 protein bearing particular substitutions was clearly presented. Currently, we are investigating (i) the presence of major and minor variant (quasispecies) within the original and passaged viruses by next-generation cDNA sequencing and (ii) whether the mutations seen in the present study (murine RV EB strain) are also consistently observed in human RV strains passaged serially in cell cultures. At the present stage, we do not have any helper virus-free, fully tractable reverse genetics system of RVs (44–48), therefore, the analysis of how RV virulence and point mutations are correlated could be very valuable for the next stage of RV vaccine development.
ACKNOWLEDGMENTS
This study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. There is no conflict of interest to declare.
We thank R. W. Jones for expert technical assistance, A. Z. Kapikian for reviews of the manuscript, and Y. Hoshino for continuing great support. We also thank P. Olley, University of Alberta, for English language advice.
Footnotes
Published ahead of print 5 March 2014
REFERENCES
- 1.Estes MK. 2001. Rotaviruses and their replication, p 1747–1786 In Knipe DM, Howley PM. (ed), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co, Philadelphia, PA [Google Scholar]
- 2.Matthijnssens J, Otto PH, Ciarlet M, Desselberger U, Van Ranst M, Johne R. 2012. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 157:1177–1182. 10.1007/s00705-012-1273-3 [DOI] [PubMed] [Google Scholar]
- 3.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2012. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 12:136–141. 10.1016/S1473-3099(11)70253-5 [DOI] [PubMed] [Google Scholar]
- 4.Tucker AW, Haddix AC, Bresee JS, Holman RC, Parashar UD, Glass RI. 1998. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA 279:1371–1376. 10.1001/jama.279.17.1371 [DOI] [PubMed] [Google Scholar]
- 5.Widdowson MA, Meltzer MI, Zhang X, Bresee JS, Parashar UD, Glass RI. 2007. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics 119:684–697. 10.1542/peds.2006-2876 [DOI] [PubMed] [Google Scholar]
- 6.Hoshino Y, Saif LJ, Kang SY, Sereno MM, Chen WK, Kapikian AZ. 1995. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology 209:274–280. 10.1006/viro.1995.1255 [DOI] [PubMed] [Google Scholar]
- 7.Hanley KA. 2011. The double-edged sword: how evolution can make or break a live-attenuated virus vaccine. Evolution 4:635–643. 10.1007/s12052-011-0365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M, Human Rotavirus Vaccine Study Group 2006. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 354:11–22. 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- 9.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM, Rotavirus Efficacy and Safety Trial (REST) Study Team 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 354:23–33. 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 10.O'Ryan M, Lucero Y, Linhares AC. 2011. Rotarix®: vaccine performance 6 years postlicensure. Expert Rev. Vaccines 10:1645–1659. 10.1586/erv.11.152 [DOI] [PubMed] [Google Scholar]
- 11.Tate JE, Patel MM, Steele AD, Gentsch JR, Payne DC, Cortese MM, Nakagomi O, Cunliffe NA, Jiang B, Neuzil KM, de Oliveira LH, Glass RI, Parashar UD. 2010. Global impact of rotavirus vaccines. Expert Rev. Vaccines 9:395–407. 10.1586/erv.10.17 [DOI] [PubMed] [Google Scholar]
- 12.Cortes JE, Curns AT, Tate JE, Cortese MM, Patel MM, Zhou F, Parashar UD. 2011. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N. Engl. J. Med. 365:1108–1117. 10.1056/NEJMoa1000446 [DOI] [PubMed] [Google Scholar]
- 13.Greenberg HB, Vo PT, Jones R. 1986. Cultivation and characterization of three strains of murine rotavirus. J. Virol. 57:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf JL, Cukor G, Blacklow NR, Dambrauskas R, Trier JS. 1981. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect. Immun. 33:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, Fang ZY. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horie Y, Masamune O, Nakagomi O. 1997. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J. Gen. Virol. 78:2341–2346 [DOI] [PubMed] [Google Scholar]
- 17.Tsugawa T, Hoshino Y. 2008. Whole genome sequence and phylogenetic analyses reveal human rotavirus G3P[3] strains Ro1845 and HCR3A are examples of direct virion transmission of canine/feline rotaviruses to humans. Virology 380:344–353. 10.1016/j.virol.2008.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan L, Honma S, Ishida S, Yan XY, Kapikian AZ, Hoshino Y. 2004. Species-specific but not genotype-specific primary and secondary isotype-specific NSP4 antibody responses in gnotobiotic calves and piglets infected with homologous host bovine (NSP4[A]) or porcine (NSP4[B]) rotavirus. Virology 330:92–104. 10.1016/j.virol.2004.09.021 [DOI] [PubMed] [Google Scholar]
- 19.Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101–104. 10.1126/science.272.5258.101 [DOI] [PubMed] [Google Scholar]
- 20.Kearney K, Chen D, Taraporewala ZF, Vende P, Hoshino Y, Tortorici MA, Baro M, Patton JT. 2004. Cell-line-induced mutation of the rotavirus genome alters expression of an IRF3-interacting protein. EMBO J. 23:4072–4081. 10.1038/sj.emboj.7600408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthijnssens J, Joelsson DB, Warakomski DJ, Zhou T, Mathis PK, van Maanen MH, Ranheim TS, Ciarlet M. 2010. Molecular and biological characterization of the 5 human-bovine rotavirus (WC3)-based reassortant strains of the pentavalent rotavirus vaccine, RotaTeq. Virology 403:111–127. 10.1016/j.virol.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 22.López S, Arias CF. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends. Microbiol. 12:271–278. 10.1016/j.tim.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Mackow ER, Shaw RD, Matsui SM, Vo PT, Dang MN, Greenberg HB. 1988. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc. Natl. Acad. Sci. U. S. A. 85:645–649. 10.1073/pnas.85.3.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104–107. 10.1126/science.272.5258.104 [DOI] [PubMed] [Google Scholar]
- 25.Kirkwood CD, Boniface K, Richardson S, Taraporewala ZF, Patton JT, Bishop RF. 2008. Nonstructural protein NSP2 induces heterotypic antibody responses during primary rotavirus infection and reinfection in children. J. Med. Virol. 80:1090–1098. 10.1002/jmv.21160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamoto N, Mattion NM, Estes MK. 1993. Alterations in the sequence of the gene 4 from a human rotavirus after multiple passages in HepG2 liver cells. Arch. Virol. 130:179–185. 10.1007/BF01319006 [DOI] [PubMed] [Google Scholar]
- 27.Ward RL, Kirkwood CD, Sander DS, Smith VE, Shao M, Bean JA, Sack DA, Bernstein DI. 2006. Reductions in cross-neutralizing antibody responses in infants after attenuation of the human rotavirus vaccine candidate 89-12. J. Infect. Dis. 194:1729–1736. 10.1086/509623 [DOI] [PubMed] [Google Scholar]
- 28.Esona MD, Foytich K, Wang Y, Shin G, Wei G, Gentsch JR, Glass RI, Jiang B. 2010. Molecular characterization of human rotavirus vaccine strain CDC-9 during sequential passages in Vero cells. Hum. Vaccines 6:247–253. 10.4161/hv.6.3.10409 [DOI] [PubMed] [Google Scholar]
- 29.Arora R, Dhale GS, Patil PR, Chitambar SD. 2011. Sequence analysis of VP4 genes of wild-type and culture adapted human rotavirus G1P[8] strains. Asian Pac. J. Trop. Med. 4:541–546. 10.1016/S1995-7645(11)60142-9 [DOI] [PubMed] [Google Scholar]
- 30.Kapikian AZ. 2001. Rotaviruses, p 1787–1833 In Knipe DM, Howley PM. (ed), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co, Philadelphia, PA [Google Scholar]
- 31.Dormitzer PR, Nason EB, Prasad BV, Harrison SC. 2004. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature 430:1053–1058. 10.1038/nature02836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trask SD, Kim IS, Harrison SC, Dormitzer PR. 2010. A rotavirus spike protein conformational intermediate binds lipid bilayers. J. Virol. 84:1764–1770. 10.1128/JVI.01682-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham KL, Fleming FE, Halasz P, Hewish MJ, Nagesha HS, Holmes IH, Takada Y, Coulson BS. 2005. Rotaviruses interact with α4β7 and α4β1 integrins by binding the same integrin domains as natural ligands. J. Gen. Virol. 86:3397–3408. 10.1099/vir.0.81102-0 [DOI] [PubMed] [Google Scholar]
- 34.Hu L, Crawford SE, Hyser JM, Estes MK, Prasad BV. 2012. Rotavirus nonstructural proteins: structure and function. Curr. Opin. Virol. 2:380–388. 10.1016/j.coviro.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estes MK, Kapikian AZ. 2007. Rotaviruses. p 1917–1974 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed. Lippincott/The Williams & Wilkins Co, Philadelphia, PA [Google Scholar]
- 36.Rajasekaran D, Sastri NP, Marathahalli JR, Indi SS, Pamidimukkala K, Suguna K, Rao CD. 2008. The flexible C terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties. J. Gen. Virol. 89:1485–1496. 10.1099/vir.0.83617-0 [DOI] [PubMed] [Google Scholar]
- 37.Ward RL, Mason BB, Bernstein DI, Sander DS, Smith VE, Zandle GA, Rappaport RS. 1997. Attenuation of a human rotavirus vaccine candidate did not correlate with mutations in the NSP4 protein gene. J. Virol. 71:6267–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angel J, Tang B, Feng N, Greenberg HB, Bass D. 1998. Studies of the role for NSP4 in the pathogenesis of homologous murine rotavirus diarrhea. J. Infect. Dis. 177:455–458. 10.1086/517374 [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Zeng CQ, Dong Y, Ball JM, Saif LJ, Morris AP, Estes MK. 1998. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J. Virol. 72:3666–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang KO, Kim YJ, Saif LJ. 1999. Comparisons of nucleotide and deduced amino acid sequences of NSP4 genes of virulent and attenuated pairs of group A and C rotaviruses. Virus Genes 18:229–233. 10.1023/A:1008068218966 [DOI] [PubMed] [Google Scholar]
- 41.Mohan KV, Atreya CD. 2000. Comparative sequence analysis identified mutations outside the NSP4 cytotoxic domain of tissue culture-adapted ATCC-Wa strain of human rotavirus and a novel interspecies variable domain in its C terminus. Arch. Virol. 145:1789–1799. 10.1007/s007050070056 [DOI] [PubMed] [Google Scholar]
- 42.Patton JT, Taraporewala ZF, Chen D, Chizhikov V, Jones M, Elhelu A, Collins M, Kearney K, Wagner M, Hoshino Y, Gouvea V. 2001. Effect of intragenic rearrangement and changes in the 3′ consensus sequence on NSP1 expression and rotavirus replication. J. Virol. 75:2076–2086. 10.1128/JVI.75.5.2076-2086.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angel J, Franco MA, Greenberg HB. 2012. Rotavirus immune responses and correlates of protection. Curr. Opin. Virol. 2:419–425. 10.1016/j.coviro.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komoto S, Taniguchi K. 2013. Genetic engineering of rotaviruses by reverse genetics. Microbiol. Immunol. 57:479–486 [DOI] [PubMed] [Google Scholar]
- 45.Richards JE, Desselberger U, Lever AM. 2013. Experimental pathways toward developing a rotavirus reverse genetics system: synthetic full-length rotavirus ssRNAs are neither infectious nor translated in permissive cells. PLoS One 8:e74328. 10.1371/journal.pone.0074328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komoto S, Sasaki J, Taniguchi K. 2006. Reverse genetics system for introduction of site-specific mutations into the double-stranded RNA genome of infectious rotavirus. Proc. Natl. Acad. Sci. U. S. A. 103:4646–4651. 10.1073/pnas.0509385103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trask SD, Taraporewala ZF, Boehme KW, Dermody TS, Patton JT. 2010. Dual selection mechanisms drive efficient single-gene reverse genetics for rotavirus. Proc. Natl. Acad. Sci. U. S. A. 107:18652–18657. 10.1073/pnas.1011948107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Troupin C, Dehée A, Schnuriger A, Vende P, Poncet D, Garbarg-Chenon A. 2010. Rearranged genomic RNA segments offer a new approach to the reverse genetics of rotaviruses. J. Virol. 84:6711–6719. 10.1128/JVI.00547-10 [DOI] [PMC free article] [PubMed] [Google Scholar]