Abstract
Leukotoxin (LtxA) from Aggregatibacter actinomycetemcomitans is known to target and lyse β2-integrin-expressing cells such as polymorphonuclear leukocytes and macrophages. LtxA is an important virulence factor that facilitates chronic inflammation and is strongly associated with a fast-progressing form of periodontitis caused by the JP2 clone of the bacterium. Here, we show that sialic acid residues are important for LtxA-induced cell lysis, regardless of whether the cell express β2-integrin or not. Clearly, removal of sialic acid groups significantly reduces a β2-integrin-specific LtxA-induced lysis. Moreover, sialic acid presented on alternative proteins, such as, for instance, on erythrocytes that do not express β2-integrin, also makes the cells more sensitive to LtxA. The data also illustrate the importance of the negative charge in order for the sialic acid to associate LtxA with the membrane. Removal of sialic acid is in itself sufficient to significantly reduce the negative charge on the erythrocytes. Moreover, we found that on human erythrocytes there is a positive association between the sensitivity to LtxA and the amount of negative charge caused by sialic acid. Interestingly, these features are not shared by all RTX toxins, since α-hemolysin from Escherichia coli induced cell lysis of both β2-integrin-expressing and nonexpressing cells and this lysis is independent of the presence of sialic acid residues. In conclusion, LtxA not only is cytotoxic to β2-integrin-expressing cells but can potentially initiate cell lysis in all cells that present a sufficient density of sialic acid groups on their plasma membrane.
INTRODUCTION
Aggregatibacter actinomycetemcomitans is a Gram-negative bacterium that frequently inhabits the human oral cavity, located primarily in dental plaques (1). The bacterium is associated mainly with periodontitis, one of mankind's most common inflammatory disorders (2–4), and is also known to be able to cause systemic diseases (5, 6). A. actinomycetemcomitans produces an ∼113-kDa protein, leukotoxin (LtxA) (7), and clinical data suggest that LtxA is a major virulence factor for the bacterium (2, 8, 9). A highly leukotoxic clone of the bacterium (the JP2 clone) is strongly associated with a fast-progressing form of periodontitis often affecting adolescents, termed localized aggressive periodontitis (2, 9). LtxA is a member of the repeat-in-toxin (RTX) family of bacterial exotoxins. The molecular characteristics of RTX toxins include the presence of glycine-rich nonapeptide repeats, which bind Ca2+ and play a role in the interaction with the host cell surface (10–12). While LtxA has been assumed to cause cell lysis by formation of hydrophilic membrane pores (13, 14), a recent study has argued against pore formation and suggested that LtxA may cause lysis through membrane destabilization (15). The toxin specifically kills leukocytes, particularly polymorphonuclear leukocytes and macrophages from humans, the great apes, and Old World monkeys (16–18). This allows the bacterium to colonize and invade by avoiding the local host-defensive immune mechanisms (19, 20). Before lysing phagocytes, LtxA induces degranulation and secretion of inflammatory cytokines from these cells, which may contribute largely to the tissue destruction associated with the infection (21–23). It is well established that the toxin uses β2-integrins on leukocytes as cell surface receptors (24–27). The family of β2-integrins includes four heterodimeric transmembrane glycoproteins, each containing a distinct α chain paired with the β2 chain: αLβ2 (CD11a/CD18, leukocyte function-associated antigen 1 [LFA-1]), αMβ2 (CD11b/CD18, complement receptor 3, Mac-1), αXβ2 (CD11c/CD18, complement receptor 4, p150/195), and αDβ2 (CD11d/CD18) (28, 29). Originally, it was shown that LtxA uses αLβ2-integrin as a receptor (25). A recent study, however, reports that in addition, αMβ2- and αXβ2-integrins also function as receptors for the toxin (27). Integrins are structurally labile and undergo major conformational changes in their ectodomains as part of the transition from the ligand-binding-inactive to -active state (30). It was suggested that LtxA specifically recognizes the ligand-binding-active conformation of the β2-integrin molecule (31) and that binding and lysis of the target cells depend on recognition of N-linked oligosaccharide chains of the receptors (26). Apparently, erythrocytes do not express β2-integrins (32, 33), which potentially could explain why LtxA from A. actinomycetemcomitans for a long time was not considered to be hemolytic (34, 35). In 2006, however, it was shown that LtxA was able lyse human, sheep, and horse erythrocytes (36). In a more recent study, gangliosides were suggested as receptors for LtxA in human erythrocytes based on reduction of hemolysis by addition of free gangliosides (37). That study did not find a preference for any ganglioside in particular, and despite the fact that gangliosides are all sialic acid-bearing glycosphingolipids, the critical subcomponent of these large moieties was not defined (37).
Recently, we documented that hemolysis induced by LtxA and another RTX toxin, α-hemolysin (HlyA), from Escherichia coli is not caused simply by the toxin pore in itself. Insertion of the toxins apparently triggers ATP release, which through activation of purinergic (P2) receptors amplifies cell lysis by increasing the overall permeability of the erythrocytes (38, 39). Moreover, interaction of the toxins with the plasma membrane of the erythrocyte causes a marked cell shrinkage, which precedes cell lysis (38–40).
Here, we investigated how LtxA and HlyA interact with the cell membranes of erythrocytes and β2-integrin-expressing K562 cells. We show that β2-integrins do not play any role in the hemolytic activity of LtxA, while, in sharp contrast, β2-integrins are required for the lysis of K562 cells. We demonstrate that glycans terminated with sialic acid residues are essential for the high sensitivity of LtxA regardless of whether it is β2-integrin or another protein which bears the sialic acid residue. Removal of sialic acid significantly reduces the negative surface charge on the erythrocytes, and in human erythrocytes there is an association between the sensitivity to LtxA and amount of negative surface charge caused by sialic acid. In contrast to the case for LtxA, we show that neither αLβ2 nor sialic acids function as receptors for HlyA. Thus, the present study reveals that it is the sugar structure and not the protein itself which constitutes the entity that associates LtxA with cell membranes for lysis to occur.
MATERIALS AND METHODS
Preparations of human, ovine, leporine, and murine erythrocytes.
Human blood samples were collected in EDTA-containing tubes by venipuncture from healthy volunteers. All individuals had given their written consent, and the Danish Scientific Ethics Committee had approved the sampling procedure (M201100217). The blood was washed three times in 0.9% (wt/vol) NaCl (twice at 1,162 × g for 3 min at 4°C and once at 581 × g for 2 min at 4°C), and the upper “buffy coat” containing the white blood cells was removed. The isolated erythrocytes were then washed once in HEPES-buffered isotonic saline (HBS) (1,162 × g for 3 min at 4°C) and kept at 4°C until experiments were conducted. A fresh blood sample was taken on the day of the experiment.
Defibrinated blood from sheep and rabbit was purchased from Statens Serum Institut (Copenhagen, Denmark). The ovine blood was prepared similarly to the human erythrocytes. Murine blood was obtained from C57BL6 and BALB/c mice of either sex after the mice were anesthetized (isoflurane, 1,500 cm3/min) and killed by decapitation. The mice were bred in-house according to the Danish animal welfare regulations. The murine and rabbit blood was washed three times in 0.9% (wt/vol) NaCl (once at 1,162 × g for 3 min at 4°C and twice at 581 × g for 2 min at 4°C) and twice in HBS (once at 581 × g for 2 min at 4°C and once at 1,162 × g for 3 min at 4°C).
K562 cell lines.
Human K562 erythroleukemia cells, unmodified or cotransfected with cDNA encoding the α and β subunits of αLβ2 (LFA-1), were a kind gift from T. A. Springer, Harvard Medical School, Boston (41). The recombinant expression was introduced by transfection protocols using the CD8M expression vector (42). The untransfected cells were grown in RPMI supplemented with 10% (vol/vol) fetal calf serum, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. The culture medium for the transfected cells was additionally supplemented with 4 μg ml−1 puromycin. Both cell types were kept at 37°C at 5% CO2 in atmospheric air.
Antibodies.
Hybridoma cells producing monoclonal antibody KIM127, reacting with an epitope of human CD18 (43), were purchased from the American Type Culture Collection (Manassas, VA, USA), and the antibody was purified from culture supernatant as previously described (44). IgG1κ from murine myeloma was used as an unspecific control antibody.
Measurements of hemolytic activity.
The erythrocyte volume fraction of the final test solution was 1.25% in HEPES-buffered saline. LtxA was added in 96-well plates with or without antibody and placed in an incubation chamber for up to 120 min at 37°C with shaking (250 rpm). The 96-well plates were then centrifuged (1,162 × g for 3 min at 4°C), and the hemolytic activity was measured as the light absorbance of hemoglobin released from lysed erythrocytes using the optical density at a wavelength of 540 nm (PowerWave microplate spectrophotometer; Biotek Instruments, Winooski, VT, USA). Hemolytic activity was calculated as a percentage of the maximal hemolysis induced by deionized water. For all experiments, the value for a control without LtxA was subtracted from the sample values.
With regard to the neuraminidase experiments, the erythrocytes were preincubated with or without neuraminidase (60 mU ml−1) or with increasing concentrations of neuraminidase (up to 100 mU ml−1) for 60 min at 37°C with shaking (250 rpm). Subsequently, the erythrocyte suspension was washed three times in HBS (805 × g for 3 min at room temperature), added together with increasing concentrations of LtxA in 96-well plates, and placed in an incubation chamber for 60 min at 37°C with shaking (250 rpm). After this point, the protocol was identical to that described above.
Measurements of cytolytic activity.
K562 cells, unmodified or cotransfected with cDNA encoding the α and β subunits of αLβ2 (LFA-1), were incubated with calcein-AM (5 μM) in transfer tubes for 30 min at 37°C with shaking (250 rpm). Thereafter, the cells were washed twice in HBS (805 × g for 3 min at room temperature), and different concentrations of LtxA or HlyA were added. In the experiments testing the effect of KIM127, either KIM127 or the control antibody was added together with LtxA. The transfer tubes were then placed in an incubation chamber for 90 min at 37°C with shaking (250 rpm). Subsequently, the tubes were centrifuged (805 × g for 3 min at room temperature), and the supernatants were transferred to a 96-well plate. Calcein was excited at 485 nm, and the emission was collected at 535 nm. The cytolytic activity was measured as the fluorescence intensity of calcein released from lysed K562 cells, using a plate reader (Mithras LB 940 multimode microplate reader; Berthold Technologies, Bad Wildbad, Germany). Cytolytic activity was calculated as a percentage of the maximal lysis induced by Triton X-100. For all experiments, the value for a control without LtxA was subtracted from the sample values.
With regard to the neuraminidase experiments, the K562 cells were preincubated with neuraminidase (60 mU ml−1) together with calcein (5 μM) in transfer tubes for 60 min at 37°C with shaking (250 rpm). One unit of neuraminidase is defined as the amount that will liberate 1.0 μmol of N-acetylneuraminic acid per minute at pH 5.0 at 37°C. The cell suspension was then washed three times in HBS (805 × g for 3 min at room temperature), and the same protocol as described above was followed.
Measurements of zeta potential.
Erythrocytes were preincubated with or without neuraminidase (60 mU ml−1) for 60 min at 37°C with shaking (250 rpm). Subsequently, the erythrocyte suspension was washed three times in HBS (805 g for 3 min at room temperature) and diluted to a final volume fraction of 0.2% in HEPES-buffered saline. The electrophoretic mobility of each sample was measured by using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, Worcestershire, United Kingdom), and the ζ potential was calculated from electrophoretic mobility based on the Smoluchowski equation. A zeta transfer potential standard (silica colloids) of −50 ± 5 mV from Malvern Instruments was used for calibration. Approximately 750 μl of erythrocyte suspension was transferred to a disposable polystyrene cell (Malvern) with two electrodes. The cell was placed in the Zetasizer, which applied an alternating electric field for mobility measurement. Two measurements were performed for each sample at 25°C.
Data analysis and statistics.
Data are presented as the mean ± standard error of the mean (SEM). The n value indicates the number of individuals, mice, or cells (see the figure legends for details). For experiments on ovine and rabbit erythrocytes, the n value indicates the number of repeats from one blood sample. The data were tested for normal distribution by the Kolmogorov-Smirnov test. Normally distributed data were tested by Student's t test for single comparison and by one-way analysis of variance (ANOVA) for multiple comparisons to determine significant differences. In the few cases where the data did not meet the criteria of normal distribution, the Mann-Whitney Wilcoxon matched-pairs test for single comparison was used. A P value of less than 0.05 was considered statistically significant.
Solutions and materials.
The Ca2+-containing HEPES-buffered isotonic saline (HBS) contained the following (in mM): Na+, 132.0; Cl−, 126.9; K+, 5.3; Ca2+, 1.8; Mg2+, 0.8; SO42−, 0.8; HEPES, 14.0; and glucose, 5.6 (pH 7.4, 37°C). Triton X-100, neuraminidase, and IgG1κ (from murine myeloma) were obtained from Sigma-Aldrich. Calcein-AM was from Invitrogen (Taastrup, Denmark). All of the reagents were dissolved in HBS and pH adjusted to 7.4 at 37°C unless otherwise stated. Purification of LtxA from A. actinomycetemcomitans strain HK921 (JP2) was performed according to the methods described by Reinholdt et al. (27). HlyA from E. coli was purified according to the method described by Hyland et al. (45). Note that the HlyA preparation is given in vol/vol because the precise determination of the protein content is problematic due to the buffer composition.
RESULTS
LtxA induces lysis of human, ovine, and murine erythrocytes.
We have previously shown that LtxA readily lyses human erythrocytes (38). This finding opposes the notion of an obligatory requirement for β2-integrin, since human erythrocytes do not express this protein (32, 33). This sensitivity for LtxA is not restricted to human erythrocytes, since ovine erythrocytes were equally sensitive. Surprisingly, erythrocytes from mice showed a remarkably low susceptibility to LtxA. Figure 1 shows lysis of erythrocytes from all three species after 60 min at 37°C at increasing concentrations of LtxA. At a concentration of 100 μg ml−1, the toxin caused 100% lysis of human and ovine erythrocytes, whereas only ∼25% of the murine erythrocytes were lysed at that concentration (Fig. 1). This is surprising since murine erythrocytes are much more sensitive to HlyA than human erythrocytes (39). We used a highly purified preparation of LtxA with minimal lipopolysaccharide or other bacterial components (for details on the purification method, see reference 39), and thus attribute our findings to LtxA in itself. In addition, we have previously shown that LtxA-induced hemolysis was completely prevented by a specific antibody directed against LtxA (38), which substantiates that the hemolysis observed indeed was caused by LtxA.
FIG 1.
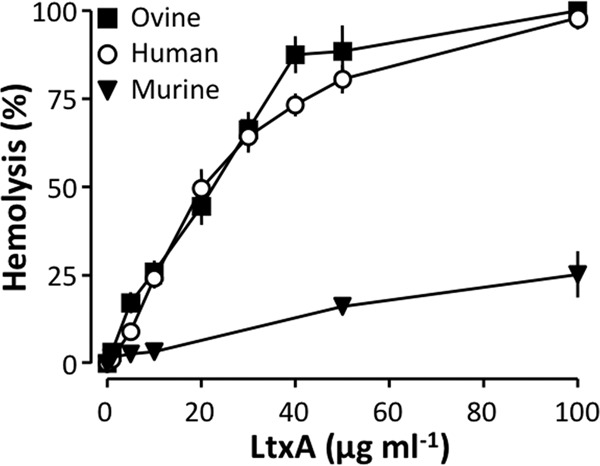
LtxA causes lysis of human, ovine, and murine erythrocytes. Human, ovine, and murine erythrocytes were incubated with increasing concentrations of LtxA from A. actinomycetemcomitans for 60 min at 37°C at 250 rpm. Values are given as mean ± SEM for human erythrocytes (n = 9), murine erythrocytes (n = 5), and ovine erythrocytes (n = 6).
Role of the β2-integrin, αLβ2, in LtxA- and HlyA-induced lysis.
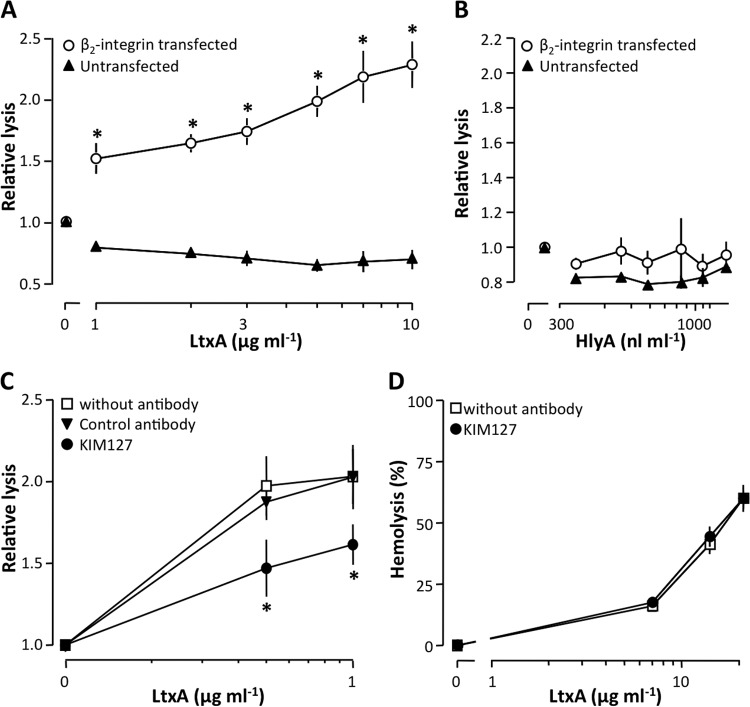
K562 cells, unmodified or cotransfected with cDNA encoding the integrin, αLβ2, were used to confirm previous studies, which have shown that LtxA uses β2-integrins as cell surface receptors (24–27). Unmodified K562 cells do not express β2-integrins and were resistant to the lytic effects of LtxA (Fig. 2A). The αLβ2-integrin-expressing K562 cells, however, were lysed by the toxin in a concentration-dependent manner. This confirms that αLβ2-integrin is important for LtxA-mediated cytotoxicity (Fig. 2A). Surprisingly, both unmodified and transfected K562 cells were resistant to HlyA-induced lysis at concentrations ∼14 times higher than what is needed to lyse human erythrocytes (Fig. 2B). These findings suggest that HlyA does not require β2-integrin to associate with cell membranes. To further substantiate this finding, we tested whether a monoclonal antibody directed against β2-integrin (KIM127) influenced the LtxA-mediated cytotoxicity. Figure 2C shows that KIM127 significantly inhibited LtxA-induced lysis of the transfected K562 cells, which suggests β2-integrin as a cell surface receptor for the toxin. At the molecular level, several alternative mechanisms may account for this inhibitory effect of KIM127 on LtxA-mediated lysis. For instance, nonspecific steric hindrance and/or antibody-toxin interactions could prevent LtxA from binding to the cell surface. To address this issue we performed experiments with a control antibody (IgG1κ from murine myeloma), which did not affect the LtxA-induced lysis (Fig. 2C). Moreover, we investigated whether KIM127 would also impede LtxA-mediated hemolysis, as would be the case if KIM127 binds LtxA. However, KIM127 did not affect the LtxA-induced hemolysis (Fig. 2D), which also supports the notion that LtxA attaches to the erythrocyte membrane via a different mechanism and indirectly argues that human erythrocytes do not express β2-integrin.
FIG 2.
Role of αLβ2 in LtxA- and HlyA-induced lysis. (A and B) Effects of LtxA (A) and HlyA (B) on unmodified (wild type) and αLβ2-integrin-transfected K562 cells. (C) The β2-integrin antibody KIM127 decreases LtxA-induced lysis of αLβ2-integrin-transfected K562 cells. (D) KIM127 has no effect on LtxA-induced hemolysis. The human erythrocytes and K562 cells were incubated for 60 and 90 min, respectively, at 37°C with shaking (250 rpm). Values are normalized to a control without toxin showing ∼0% lysis, and data are shown as mean ± SEM (n = 3 to 7). *, statistical difference from the control (P < 0.05).
Sialic acid residues are required for LtxA-induced lysis of human erythrocytes.
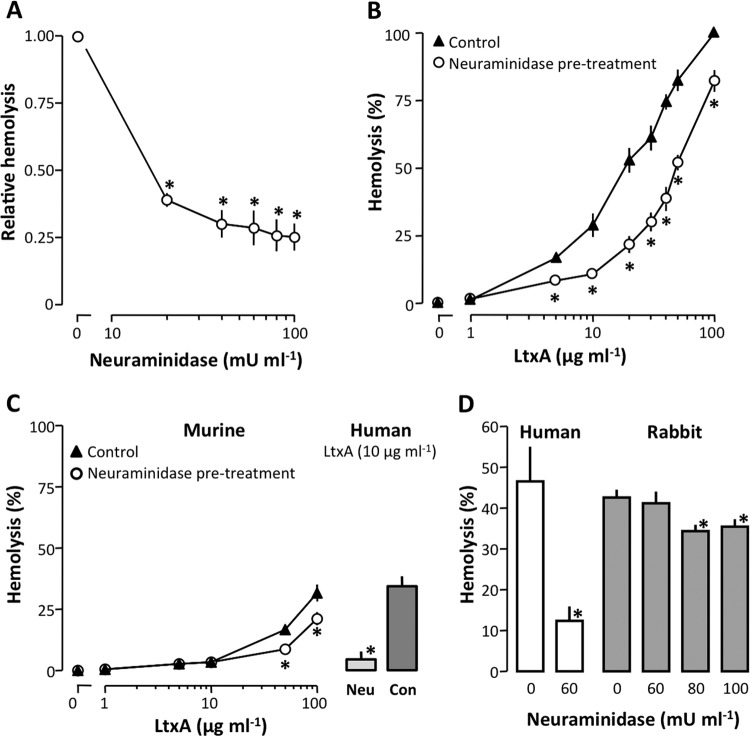
LtxA readily lyses erythrocytes independent of β2-integrin, which suggests that LtxA recognizes a different binding site on the erythrocytes. It is reasonable to assume that this protein, which associates LtxA with the erythrocyte membrane, has a moiety similar to the binding site for LtxA on β2-integrin. We therefore speculated that these receptors may share a similar sugar structure. Given the strong positive charge of LtxA, sialic acid is a likely candidate for a common binding site, particularly because it is known to serve as a component of binding sites for various pathogens and toxins (46–48). Therefore, we tested whether neuraminidase, which catalyzes the hydrolysis of terminal sialic acid residues, would affect the LtxA-induced cell lysis. Preincubation of human erythrocytes with neuraminidase reduced LtxA-mediated hemolysis in a concentration-dependent manner. At a concentration of 40 mU ml−1, neuraminidase treatment inhibited the LtxA-induced hemolysis by approximately 70% (Fig. 3A). In addition, Fig. 3B shows that treatment of human erythrocytes with neuraminidase (60 mU ml−1) significantly reduced LtxA-mediated hemolysis even at high toxin concentrations. As mentioned above, we observed that murine erythrocytes had a remarkably low susceptibility to LtxA compared to human erythrocytes. The difference in sensitivity between the two species suggests that LtxA interacts differently with human and murine erythrocyte membranes. Therefore, we investigated how neuraminidase would affect the LtxA-induced hemolysis of murine erythrocytes. At an LtxA concentration of 100 μg ml−1 (resulting in 32% hemolysis), removal of sialic acid reduced the lysis by 34% (Fig. 3C). To compare this effect of neuraminidase to the one observed in human erythrocytes, we conducted experiments with the same solution of neuraminidase (60 mU ml−1) in parallel on both human and murine erythrocytes. At an LtxA concentration (10 μg ml−1) causing 34% lysis of untreated human erythrocytes, removal of sialic acid resulted in an 85% reduction of hemolysis (Fig. 3C, bars). Thus, it seems that sialic acid acts as a binding site for LtxA in erythrocytes, and this may partially explain the difference in sensitivity between human and murine erythrocytes. It is worth noting that murine erythrocytes contain mainly O-acetylated sialic acid on the membrane, whereas humans have mainly N-acetylated sialic acid (49). Interestingly, rabbit erythrocytes have previously been shown to have a low level of membrane sialic acid (50), and therefore we tested how LtxA affects rabbit erythrocytes compared to human erythrocytes. Figure 3D shows that at an LtxA concentration causing 47% lysis of human erythrocytes, 43% of the rabbit erythrocytes were lysed. In addition, neuraminidase treatment (60 mU ml−1) of the erythrocytes markedly decreased lysis of human erythrocytes (to 12%), whereas no effect was observed for rabbit erythrocytes. Increasing the neuraminidase concentration (80 mU ml−1), however, significantly reduced the lysis of rabbit erythrocytes from 43% to 34%. These data support that rabbit erythrocytes do have a low content of sialic acid on the surface, which underscores that sialic acid is not obligatory for a high LtxA sensitivity. If sialic acid, as a residue, is instrumental in the affiliation of LtxA to biological membranes, it should be possible to reduce membrane binding of LtxA by adding free sialic acid. Figure 4 shows that adding 1 mM sialic acid at the same pH significantly reduces the LtxA-induced hemolysis. A change in osmolality of up to 5 mM does not affect the LtxA-induced hemolysis. Nevertheless, we did not increase the concentration of sialic acid beyond 1 mM to avoid any complications with regard to the composition of the solution. We thus cannot conclude that the binding of LtxA can be prevented by free sialic acid, but our data suggest that it is reduced.
FIG 3.
Sialic acids are required for LtxA-induced hemolysis. (A) Neuraminidase treatment of human erythrocytes decreases LtxA-induced hemolysis in a concentration-dependent manner. Values are normalized to a control without neuraminidase showing ∼50% lysis. (B) Neuraminidase treatment (60 mU ml−1) of human erythrocytes reduces hemolysis at increasing concentrations of LtxA. (C) Neuraminidase treatment (60 mU ml−1) of murine erythrocytes reduces hemolysis at increasing concentrations of LtxA. Bars display results from simultaneous experiments with human erythrocytes at an LtxA concentration of 10 μg ml−1. (D) Neuraminidase treatment of rabbit erythrocytes only slightly inhibits LtxA-induced hemolysis.
FIG 4.
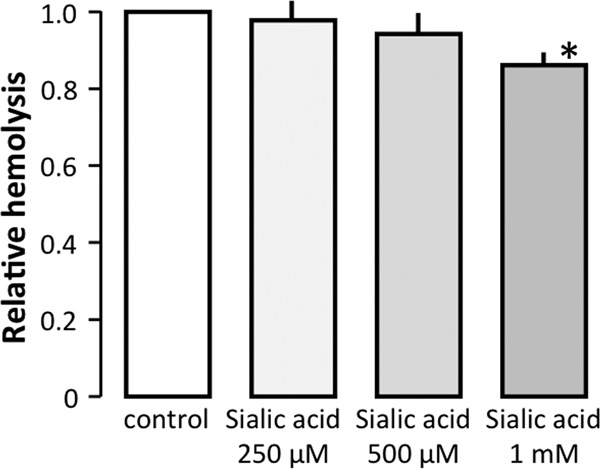
Effect of free sialic acids on LtxA-induced hemolysis. Human erythrocytes were incubated in the presence or absence of free sialic acid (N-acetylneuraminic acid) at 250 μM to 1 mM together with LtxA (15 μg ml−1) for 60 min at pH 7.4. The bars show mean ± SEM (n = 5).
To further investigate whether it is possible to define a common denominator for LtxA binding, we tested whether sialic acid was important for β2-integrin-dependent, LtxA-induced cell lysis. LtxA has been shown to recognize β2-integrin receptors through N-linked oligosaccharides in leukocytes (26). Figure 5A illustrates that removal of sialic acid with neuraminidase (60 mU ml−1) significantly reduced the LtxA-induced lysis of β2-integrin-transfected K562 cells at low concentrations of the toxin, whereas no effect was observed at high concentrations. Taken together, these data suggest that terminal sialic acid residues on cell membranes play a role in LtxA-mediated lysis of both erythrocytes and leukocytes. As shown in Fig. 2B, HlyA was unable to lyse K562 cells expressing β2-integrin, which suggests that HlyA does not have the same binding requirements as LtxA. This implies that sialic acid residues do not facilitate HlyA-induced cell lysis and that the HlyA-induced hemolysis should be insensitive to neuraminidase treatment. Consistent with this notion, preincubation of the human erythrocytes with neuraminidase did not affect the HlyA-induced hemolysis (Fig. 5B), which supports that sialic acids on the cell surface are not necessary for HlyA to lyse erythrocytes.
FIG 5.
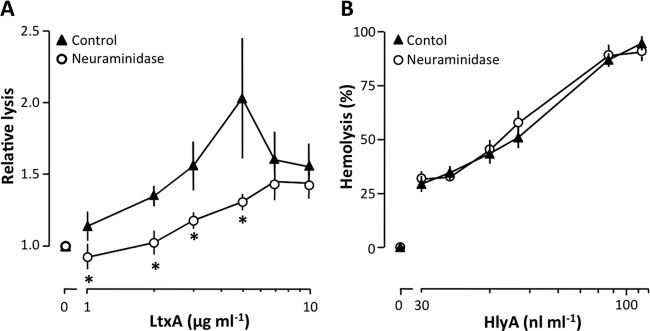
Sialic acids are required for LtxA-induced lysis of β2-integrin-expressing K562 cells. (A) Neuraminidase treatment (60 mU ml−1) of LFA-1-transfected K562 cells reduces lysis at increasing concentrations of LtxA. Values are normalized to a control without toxin showing ∼0% lysis. (B) Neuraminidase treatment (60 mU ml−1) of human erythrocytes has no effect on HlyA-induced hemolysis. The erythrocytes and K562 cells were preincubated with neuraminidase for 60 min and afterwards incubated for 60 and 90 min with the toxin, respectively. All experiments were conducted at 37°C with shaking (250 rpm). Data are shown as mean ± SEM (n = 3 to 7). *, statistical difference from the control (P < 0.05).
Role of electrostatic interactions in LtxA-induced hemolysis.
The fact that rabbit erythrocytes are lysed by LtxA in a sialic acid-independent manner suggests that sialic acid is not essential for a cell to be sensitive to LtxA. This means that the common denominator for LtxA binding to erythrocytes, if there is any, is unlikely to be sialic acid in itself. It could, however, be a distinct biophysical property of sialic acid. One could speculate that the interaction between LtxA and the erythrocyte membrane to some extent depends on electrostatic interactions. It is noteworthy that the negatively charged erythrocyte surface is primarily a consequence of terminal sialic acid residues of membrane glycoproteins and lipids (51). To clarify this issue, we measured the zeta potentials of human, mouse, and rabbit erythrocytes preincubated with or without neuraminidase (60 mU ml−1) (Fig. 6A). Neuraminidase treatment reduced the ζ potential of human erythrocytes from −13.2 mV to −5.9 mV (55% reduction), that of mouse erythrocytes from 13.6 mV to −12.3 mV (10% reduction), and that of rabbit erythrocytes from −7.1 mV to −4.9 mV (31% reduction). To test if the negative charge on sialic acid makes cells more sensitive to LtxA, we investigated whether the marked variability in LtxA sensitivity found in human erythrocytes is correlated with this charge. On the same day, we tested both the sensitivity to LtxA as the degree of hemolysis induced by 10 μg ml−1 LtxA and the ζ potential of the erythrocytes from the same individual with and without preincubation with neuraminidase (60 mU ml−1). These data are plotted in Fig. 6B, where the neuraminidase-sensitive potential denotes the ζ potential in the absence of neuraminidase subtracted from the ζ potential in the presence of neuraminidase. This curve clearly has two phases, one that shows a proportional association between the degree of LtxA-induced hemolysis and the neuraminidase-sensitive ζ potential (Fig. 6B and C). At very high neuraminidase-sensitive ζ potentials (around 8 mV), there is no longer an association between the two variables (Fig. 6B). This could possibly reflect that after a certain point more negative charge will not result in more LtxA binding to the membrane. Thus, it seems that the negative surface charge plays a role mainly in the interaction between LtxA and human erythrocytes, whereas murine erythrocytes hardly show any neuraminidase-sensitive changes in ζ potential. Neuraminidase decreased the ζ potential by on average 55% at a concentration (60 mU ml−1) inhibiting lysis of human erythrocytes by 71% (Fig. 3A). In murine erythrocytes, a reduction in zeta potential of 10% was observed at a neuraminidase concentration inhibiting the LtxA-induced hemolysis by 34 to 47% (Fig. 3C). With regard to rabbit erythrocytes, treatment with neuraminidase reduced the ζ potential with 31%. This neuraminidase concentration (60 mU ml−1), however, had no effect on the LtxA-induced hemolysis (Fig. 3D), indicating that electrostatic interactions are not involved in LtxA recognition of rabbit erythrocytes.
FIG 6.
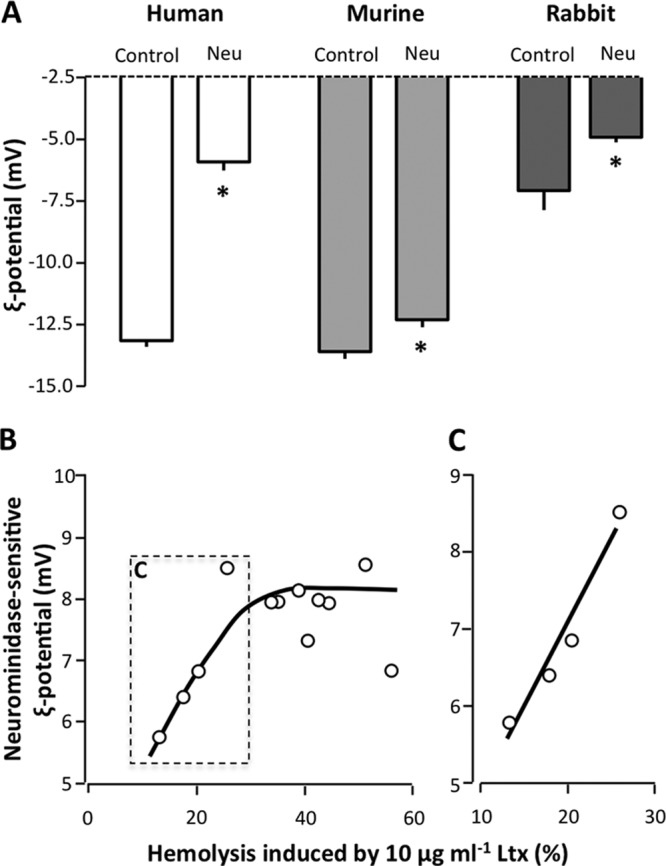
Effect of neuraminidase on the ζ potentials of human, murine, and rabbit erythrocytes. (A) Neuraminidase treatment of human, murine, and rabbit erythrocytes decreases the ζ potential. The erythrocytes were preincubated with or without (control) neuraminidase (60 mU ml−1) for 60 min at 37°C with shaking (250 rpm). (B) Association between the degree of LtxA-induced hemolysis (sensitivity to LtxA) and neuraminidase-sensitive ζ potentials (negative charge induced by sialic acid). In individuals where 10 μg ml−1 LtxA causes only ∼10 to 30% hemolysis, there is a proportional association between the degree of hemolysis and the neuraminidase-sensitive ζ potentials. (C) The initial values fitted to the line y = 0.217x − 12.8 (r = 0.96, P = 0.02). The ζ potential measurements were conducted at 25°C. *, statistical difference from the control (P < 0.05).
DISCUSSION
In the present study, we confirmed that LtxA can use the β2-integrin αLβ2 as a cell surface receptor (24–27). Overexpressing αLβ2-integrin in K562 cells, which natively lack these proteins, made them highly susceptible to LtxA-induced cell lysis, which was inhibited by antibody (KIM127) directed against β2-integrin. This requirement for β2-integrin for binding of bacterial toxins is not unique for LtxA. Adenylate cyclase toxin-hemolysin (CyaA) secreted by Bordetella pertussis has also been shown to depend upon β2-integrin (in combination with αM) to inflict cytolysis (52), and similar results have been reported for leukotoxin from Mannheimia hemolytica (53). These studies have led to the speculation that these bacterial toxins potentially inflict cell damage through cross-linkage and activation of the integrins. Here, we provide data showing that it is not the protein β2-integrin itself which is required for the association of LtxA with the plasma membrane. Degradation of the glucan structure by enzymatic cleavage of the sialic acid residues significantly reduces the LtxA susceptibility introduced by expression of β2-integrin. Moreover, we show that human erythrocytes, despite their lack of β2-integrin, are remarkably sensitive to LtxA. We could demonstrate that the sensitivity to LtxA also here was markedly enhanced by sialic acid. These data show that the binding of LtxA does not require a certain protein but is highly dependent on membrane protein glycosylation. The lack of an obligatory need for a specific protein also suggests that the cytotoxic effect of LtxA is not mediated by specific receptor activation but indicates that the LtxA interaction with the glycocalyx merely associates the toxin with the membrane for easier membrane insertion. Removal of sialic acid significantly reduces the negative charge on the erythrocytes, and in human erythrocytes there is a positive association between the sensitivity to LtxA and negative charge induced by sialic acid. Interestingly, the requirement for sialic acid cannot be extrapolated to other members of the RTX family. In contrast to the case for LtxA, we show that neither β2-integrin nor sialic acids function as binding sites for HlyA from E. coli. This was obvious since preincubation with neuraminidase had no effect on HlyA-induced hemolysis. In this context, it should be noted that the neuraminidase treatment does not change the cell volume or cause clustering of the erythrocytes (see Fig. S1 in the supplemental material).
Moreover, both unmodified and αLβ2-integrin transfected K562 cells were resistant to HlyA-induced lysis. In addition to confirming that membrane insertion of HlyA is not facilitated by αLβ2-integrin, these data underscore previous findings that some cells are remarkable resistant to HlyA (54). A cellular resistance to HlyA could be explained by a variety of mechanisms, of which a lack of a receptor that associates the toxin with the membrane is the most simple. It is, however, generally accepted that HlyA does not require a membrane receptor to inflict cell lysis (55). Although there is little doubt that HlyA can insert spontaneously in artificial lipid bilayers, the idea that some proteins may facilitate HlyA insertion has been put forward (25). One study does, in contrast to our findings, show a differential sensitivity for HlyA in αLβ2-integrin-transfected K562 cells compared to controls (25). In contrast, and supported by our present findings, it has been documented that both the unmodified and αLβ2-integrin-transfected K562 cells were similarly susceptible to lysis by HlyA (55). It is, however, well established that HlyA is leukotoxic (56). We do not have an immediate explanation for these discrepancies, but our findings do suggest that HlyA does not have the same affinity for αLβ2-integrin as LtxA.
As mentioned above, our data show that removal of sialic acid residues significantly inhibited the LtxA-induced cytolysis, regardless of whether it is of αLβ2-integrin-expressing K562 cells or human erythrocytes. Sialic acid-bearing glycoconjugates are binding targets for many bacterial toxins (47, 57), and our findings are supported by a recent study which suggests that gangliosides (sialic acid-containing glycosphingolipids) may function as a receptor for LtxA in human erythrocytes (37). It should be noted, however, that neuraminidase did not abolish the LtxA-induced hemolysis but reduced it by ∼75%. As LtxA does not insert spontaneously into lipid bilayers (14), this finding could potentially suggest that there may be an alternative binding site in addition to sialic acid. These results have to be evaluated in the light of the one-hit model for toxin-induced hemolysis, which provides proof for a single toxin molecule being enough to lyse one erythrocyte (58). Neuraminidase will undoubtedly remove the majority of the tens of millions of sialic acid molecules typically expressed by cells (59). It is, however, equally likely that a few molecules remain, which in principle is enough to cause cell lysis according to the one-hit model. Removal of sialic acids reduces the net negative charge and hydrophilicity of the erythrocyte cell surface, and one could speculate that these modifications of the biophysical properties of the membrane are the ones actually responsible for the reduced susceptibility to LtxA. Thus, the interaction between LtxA and the erythrocyte membrane may well depend on electrostatic interactions, whereas specific linkages of sialic acids to a defined underlying sugar chain, protein, or lipid are less important. There is, however, evidence that the lipid composition is important for the embedding of LtxA in the membrane. The content of cholesterol in the membrane greatly improves the membrane binding, a feature that apparently requires the cholesterol recognition/amino acid consensus site CRAC336, which is conserved in other RTX toxins (60). This clearly shows that it is not only the immediate affiliation to the membrane that determines the cellular effects of the toxin. Therefore, differences in membrane composition (for instance, species differences) may also be of great importance in susceptibility to LtxA. It is intriguing that murine erythrocyte membranes apparently have a much lower cholesterol content (cholesterol/phospholipid molar ratio, 0.33) than those from humans (0.54) (61), where rabbit erythrocytes fall in between (0.47) (62).
In contrast to lysis of leukocytes, hemolysis was previously thought not to be species restricted, since human, sheep, and horse erythrocytes are lysed by LtxA at higher concentrations of LtxA than required for killing of leukocytes (36). Here, however, we document that murine erythrocytes show very low susceptibility to LtxA compared to human and sheep erythrocytes. Interestingly, it has been shown that the sialic acid content is much lower in murine erythrocytes than in human and sheep erythrocytes (50), suggesting that interspecies variations in the sialic acid content could be responsible for the different susceptibility to LtxA. In addition, we show that removal of sialic acids from αLβ2-integrin-transfected K562 cells also inhibited the LtxA-induced lysis. However, the effect of neuraminidase was lessened as the LtxA concentration increased. Thus, the interaction between LtxA and αLβ2-integrin involves terminal sialic acid residues. Apparently, it is possible partially to compensate for the neuraminidase treatment by increasing the LtxA concentration in the αLβ2-integrin-transfected K562 cells. This could, as mentioned above, reflect residual sialic acid residues, which have a higher chance of meeting a toxin as the concentration of the toxin increases. Alternatively, sialic acid could act as an important facilitator of a firmer interaction with other saccharides and/or the polypeptide chains of the αLβ2-integrin. This hypothesis also offers an attractive explanation for LtxA's ability to lyse erythrocytes with reduced efficiency. In any case, our results confirm a recent study which shows that LtxA recognizes αLβ2-integrin through N-linked oligosaccharides (26) and point to sialic acid as a main component in determining whether LtxA will cause damage to a certain cell type or not. In erythrocytes, LtxA may thus bind to the negatively charged sialic acid alone without the tighter association with αLβ2-integrin. Interestingly, recent evidence suggests that periodontal pathogens use sialic acid as a growth factor or as a sole carbon source (63), and targeting sialic acid-expressing cells could possibly provide nutrients for A. actinomycetemcomitans in order to grow and replicate.
In conclusion, we show that LtxA binds to human erythrocytes via a negative surface charge that may be provided by sialic acid moieties. These findings have several potential implications. Most importantly, knowledge of the mechanism of interaction between LtxA and target cells may open up possibilities for novel protection modalities against toxin-induced cell damage. Neutralization of negative surface charge, e.g., through reduction/blockage of sialic acids, may limit many of the clinical consequences of toxin attack and thus constrain LtxA's function as a virulence factor. Hemolysis is assumed to be an important physiological source of iron for A. actinomycetemcomitans, since the bacterium is unable to retrieve iron from human transferrin and lactoferrin (63, 64). Iron is an essential nutrient for most bacteria, and the ability to acquire iron is recognized as one of the key steps in the survival of a pathogen in its host (65). Therefore, targeting of host erythrocytes by A. actinomycetemcomitans may be a crucial step in the pathogenic process, and understanding this interaction could lead to novel therapeutic agents and strategies. Moreover, recent studies suggest that A. actinomycetemcomitans may also be associated with cardiovascular disease (66–69), further emphasizing the importance of understanding how this bacterium's virulence factors bind to host cells. Interestingly, LtxA is being developed as a targeted biologic agent for the treatment of white blood cell diseases such as hematologic malignancies and autoimmune/inflammatory diseases (7, 70). To better understand the clinical utility of LtxA as a therapeutic agent and to minimize adverse effects, it is essential to elucidate the mechanism of interaction between the toxin and cells of the body. Our present findings add new aspects to the mechanism of A. actinomycetemcomitans recognition of target cells and potentially make it possible to interfere with the LtxA-induced cell damage.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Novo Nordisk Foundation, the Danish Medical Research Council, and Aarhus University Research Foundation for financial support.
Footnotes
Published ahead of print 18 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01647-14.
REFERENCES
- 1. Slots J, Ting M. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontology 2000 20:82–121. 10.1111/j.1600-0757.1999.tb00159.x [DOI] [PubMed] [Google Scholar]
- 2. Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. 2008. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 371:237–242. 10.1016/S0140-6736(08)60135-X [DOI] [PubMed] [Google Scholar]
- 3. Pihlstrom BL, Michalowicz BS, Johnson NW. 2005. Periodontal diseases. Lancet 366:1809–1820. 10.1016/S0140-6736(05)67728-8 [DOI] [PubMed] [Google Scholar]
- 4. Slots J, Reynolds HS, Genco RJ. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 29:1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. 2004. Actinobacillus actinomycetemcomitans endocarditis. Clin. Microbiol. Infect. 10:98–118. 10.1111/j.1469-0691.2004.00794.x [DOI] [PubMed] [Google Scholar]
- 6. van Winkelhoff AJ, Slots J. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontology 2000 20:122–135. 10.1111/j.1600-0757.1999.tb00160.x [DOI] [PubMed] [Google Scholar]
- 7. Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, Kaur M, Mei Y, Rao J. 2010. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leukemia Res. 34:777–785. 10.1016/j.leukres.2009.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haraszthy VI, Hariharan G, Tinoco EM, Cortelli JR, Lally ET, Davis E, Zambon JJ. 2000. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 71:912–922. 10.1902/jop.2000.71.6.912 [DOI] [PubMed] [Google Scholar]
- 9. Haubek D, Ennibi OK, Poulsen K, Poulsen S, Benzarti N, Kilian M. 2001. Early-onset periodontitis in Morocco is associated with the highly leukotoxic clone of Actinobacillus actinomycetemcomitans. J. Dent. Res. 80:1580–1583. 10.1177/00220345010800062001 [DOI] [PubMed] [Google Scholar]
- 10. Lally ET, Golub EE, Kieba IR. 1994. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J. Biol. Chem. 269:31289–31295 [PubMed] [Google Scholar]
- 11. Linhartova I, Bumba L, Masin J, Basler M, Osicka R, Kamanova J, Prochazkova K, Adkins I, Hejnova-Holubova J, Sadilkova L, Morova J, Sebo P. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34:1076–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez-Magraner L, Viguera AR, Garcia-Pacios M, Garcillan MP, Arrondo JL, de la Cruz F, Goni FM, Ostolaza H. 2007. The calcium-binding C-terminal domain of Escherichia coli α-hemolysin is a major determinant in the surface-active properties of the protein. J. Biol. Chem. 282:11827–11835. 10.1074/jbc.M700547200 [DOI] [PubMed] [Google Scholar]
- 13. Iwase M, Lally ET, Berthold P, Korchak HM, Taichman NS. 1990. Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 58:1782–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lear JD, Furblur UG, Lally ET, Tanaka JC. 1995. Actinobacillus actinomycetemcomitans leukotoxin forms large conductance, voltage-gated ion channels when incorporated into planar lipid bilayers. Biochim. Biophys. Acta 1238:34–41. 10.1016/0005-2736(95)00086-I [DOI] [PubMed] [Google Scholar]
- 15. Brown AC, Boesze-Battaglia K, Du Y, Stefano FP, Kieba IR, Epand RF, Kakalis L, Yeagle PL, Epand RM, Lally ET. 2012. Aggregatibacter actinomycetemcomitans leukotoxin cytotoxicity occurs through bilayer destabilization. Cell. Microbiol. 14:869–881. 10.1111/j.1462-5822.2012.01762.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taichman NS, Shenker BJ, Tsai CC, Glickman LT, Baehni PC, Stevens R, Hammond BF. 1984. Cytopathic effects of Actinobacillus actinomycetemcomitans on monkey blood leukocytes. J. Periodontal Res. 19:133–145. 10.1111/j.1600-0765.1984.tb00802.x [DOI] [PubMed] [Google Scholar]
- 17. Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J. 1987. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol. Immunol. 2:97–104. 10.1111/j.1399-302X.1987.tb00270.x [DOI] [PubMed] [Google Scholar]
- 18. Tsai CC, McArthur WP, Baehni PC, Hammond BF, Taichman NS. 1979. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect. Immun. 25:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson A, Sandstrom G, Claesson R, Hanstrom L, Kalfas S. 2000. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur. J. Oral Sci. 108:136–146. 10.1034/j.1600-0722.2000.00790.x [DOI] [PubMed] [Google Scholar]
- 20. Venketaraman V, Lin AK, Le A, Kachlany SC, Connell ND, Kaplan JB. 2008. Both leukotoxin and poly-N-acetylglucosamine surface polysaccharide protect Aggregatibacter actinomycetemcomitans cells from macrophage killing. Microb. Pathog. 45:173–180. 10.1016/j.micpath.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johansson A, Claesson R, Hanstrom L, Sandstrom G, Kalfas S. 2000. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal Res. 35:85–92. 10.1034/j.1600-0765.2000.035002085.x [DOI] [PubMed] [Google Scholar]
- 22. Kelk P, Abd H, Claesson R, Sandstrom G, Sjostedt A, Johansson A. 2011. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2:e126. 10.1038/cddis.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelk P, Claesson R, Chen C, Sjostedt A, Johansson A. 2008. IL-1beta secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int. J. Med. Microbiol. 298:529–541. 10.1016/j.ijmm.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 24. Kieba IR, Fong KP, Tang HY, Hoffman KE, Speicher DW, Klickstein LB, Lally ET. 2007. Aggregatibacter actinomycetemcomitans leukotoxin requires sheets 1 and 2 of the human CD11a propeller for cytotoxicity. Cell. Microbiol. 9:2689–2699. 10.1111/j.1462-5822.2007.00989.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, Wang JF, Shenker BJ, Ortlepp S, Robinson MK, Billings PC. 1997. RTX toxins recognize β2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463–30469. 10.1074/jbc.272.48.30463 [DOI] [PubMed] [Google Scholar]
- 26. Morova J, Osicka R, Masin J, Sebo P. 2008. RTX cytotoxins recognize β2 integrin receptors through N-linked oligosaccharides. Proc. Natl. Acad. Sci. U. S. A. 105:5355–5360. 10.1073/pnas.0711400105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reinholdt J, Poulsen K, Brinkmann CR, Hoffmann SV, Stapulionis R, Enghild JJ, Jensen UB, Boesen T, Vorup-Jensen T. 2013. Monodisperse and LPS-free Aggregatibacter actinomycetemcomitans leukotoxin: interactions with human β2 integrins and erythrocytes. Biochim. Biophys. Acta 1834:546–558. 10.1016/j.bbapap.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 28. Mazzone A, Ricevuti G. 1995. Leukocyte CD11/CD18 integrins: biological and clinical relevance. Haematologicaae 80:161–175 [PubMed] [Google Scholar]
- 29. Tan SM. 2012. The leucocyte beta2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci. Rep. 32:241–269. 10.1042/BSR20110101 [DOI] [PubMed] [Google Scholar]
- 30. Luo BH, Carman CV, Springer TA. 2007. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25:619–647. 10.1146/annurev.immunol.25.022106.141618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen TV, Fineza CD, Kachlany SC. 2012. Leukotoxin (Leukothera(R)) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 287:17618–17627. 10.1074/jbc.M111.314674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papayannopoulou T, Brice M. 1992. Integrin expression profiles during erythroid differentiation. Blood 79:1686–1694 [PubMed] [Google Scholar]
- 33. Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. 2006. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood 108:791–801. 10.1182/blood-2005-11-007799 [DOI] [PubMed] [Google Scholar]
- 34. Avila-Campos MJ. 1995. Haemolytic activity of Actinobacillus actinomycetemcomitans strains on different blood types. Rev. Inst. Med. Trop. Sao Paulo 37:215–217. 10.1590/S0036-46651995000300006 [DOI] [PubMed] [Google Scholar]
- 35. Bergley DH, Hiolt JG. 1994. Bergey's manual of determinative bacteriology. Lippincot, Williams & Wilkins, Baltimore, MD, USA [Google Scholar]
- 36. Balashova NV, Crosby JA, Al Ghofaily L, Kachlany SC. 2006. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect. Immun. 74:2015–2021. 10.1128/IAI.74.4.2015-2021.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forman MS, Nishikubo JB, Han RK, Le A, Balashova NV, Kachlany SC. 2010. Gangliosides block Aggregatibacter actinomycetemcomitans leukotoxin (LtxA)-mediated hemolysis. Toxins (Basel) 2:2824–2836. 10.3390/toxins2122824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munksgaard P, Vorup-Jensen T, Reinholdt J, Soderstrom C, Poulsen K, Leipziger J, Praetorius H, Skals M. 2012. Leukotoxin from Aggregatibacter actinomycetemcomitans causes shrinkage and P2X receptor-dependent lysis of human erythrocytes. Cell. Microbiol. 4:1904–1920. 10.1111/cmi.12021 [DOI] [PubMed] [Google Scholar]
- 39. Skals MG, Jorgensen NR, Leipziger J, Praetorius HA. 2009. α-Hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc. Natl. Acad. Sci. U. S. A. 106:4030–4035. 10.1073/pnas.0807044106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skals M, Jensen UB, Ousingsawat J, Kunzelmann K, Leipziger J, Praetorius HA. 2010. Escherichia coli α-hemolysin triggers shrinkage of erythrocytes via KCa3.1 and TMEM16A channels with subsequent phosphatidylserine exposure. J. Biol. Chem. 285:15557–15565. 10.1074/jbc.M109.082578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu CF, Springer TA. 1997. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J. Immunol. 159:268–278 [PubMed] [Google Scholar]
- 42. Larson RS, Springer TA. 1990. Structure and function of leukocyte integrins. Immunol. Rev. 114:181–217. 10.1111/j.1600-065X.1990.tb00565.x [DOI] [PubMed] [Google Scholar]
- 43. Robinson MK, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher EC. 1992. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J. Immunol. 148:1080–1085 [PubMed] [Google Scholar]
- 44. Gjelstrup LC, Boesen T, Kragstrup TW, Jorgensen A, Klein NJ, Thiel S, Deleuran BW, Vorup-Jensen T. 2010. Shedding of large functionally active CD11/CD18 integrin complexes from leukocyte membranes during synovial inflammation distinguishes three types of arthritis through differential epitope exposure. J. Immunol. 185:4154–4168. 10.4049/jimmunol.1000952 [DOI] [PubMed] [Google Scholar]
- 45. Hyland C, Vuillard L, Hughes C, Koronakis V. 2001. Membrane interaction of Escherichia coli hemolysin: flotation and insertion-dependent labeling by phospholipid vesicles. J. Bacteriol. 183:5364–5370. 10.1128/JB.183.18.5364-5370.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ilver D, Johansson P, Miller-Podraza H, Nyholm PG, Teneberg S, Karlsson KA. 2003. Bacterium-host protein-carbohydrate interactions. Methods Enzymol. 363:134–157. 10.1016/S0076-6879(03)01049-8 [DOI] [PubMed] [Google Scholar]
- 47. Merritt EA, Hol WG. 1995. AB5 toxins. Curr. Opin. Struct. Biol. 5:165–171. 10.1016/0959-440X(95)80071-9 [DOI] [PubMed] [Google Scholar]
- 48. Varki A. 2007. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446:1023–1029. 10.1038/nature05816 [DOI] [PubMed] [Google Scholar]
- 49. Reuter G, Vliegenthart JF, Wember M, Schauer R, Howard RJ. 1980. Identification of 9-O-acetyl-N-acetylneuraminic acid on the surface of BALB/c mouse erythrocytes. Biochem. Biophys. Res. Commun. 94:567–572. 10.1016/0006-291X(80)91269-3 [DOI] [PubMed] [Google Scholar]
- 50. Ish C, Ong GL, Desai N, Mattes MJ. 1993. The specificity of alternative complement pathway-mediated lysis of erythrocytes: a survey of complement and target cells from 25 species. Scand. J. Immunol. 38:113–122. 10.1111/j.1365-3083.1993.tb01701.x [DOI] [PubMed] [Google Scholar]
- 51. Eylar EH, Madoff MA, Brody OV, Oncley JL. 1962. The contribution of sialic acid to the surface charge of the erythrocyte. J. Biol. Chem. 237:1992–2000 [PubMed] [Google Scholar]
- 52. Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (CD11b/CD18). J. Exp. Med. 193:1035–1044. 10.1084/jem.193.9.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fett T, Zecchinon L, Vanden Bergh P, Desmecht D. 2008. Mannheimia haemolytica leukotoxin-induced cytolysis of caprine (Capra hircus) leukocytes is mediated by the CD18 subunit of β2-integrins. Microb. Pathog. 45:337–342. 10.1016/j.micpath.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 54. Larsen CK, Skals M, Wang T, Cheema MU, Leipziger J, Praetorius HA. 2011. Python erythrocytes are resistant to α-hemolysin from Escherichia coli. J. Membr. Biol. 244:131–140. 10.1007/s00232-011-9406-2 [DOI] [PubMed] [Google Scholar]
- 55. Valeva A, Walev I, Kemmer H, Weis S, Siegel I, Boukhallouk F, Wassenaar TM, Chavakis T, Bhakdi S. 2005. Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J. Biol. Chem. 280:36657–36663. 10.1074/jbc.M507690200 [DOI] [PubMed] [Google Scholar]
- 56. Bhakdi S, Greulich S, Muhly M, Eberspacher B, Becker H, Thiele A, Hugo F. 1989. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J. Exp. Med. 169:737–754. 10.1084/jem.169.3.737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Imberty A, Varrot A. 2008. Microbial recognition of human cell surface glycoconjugates. Curr. Opin. Struct. Biol. 18:567–576. 10.1016/j.sbi.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 58. Jorgensen SE, Hammer RF, Wu GK. 1980. Effects of a single hit from the α hemolysin produced by Escherichia coli on the morphology of sheep erythrocytes. Infect. Immun. 27:988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. 2004. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl. Acad. Sci. U. S. A. 101:6104–6109. 10.1073/pnas.0400851101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown AC, Balashova NV, Epand RM, Epand RF, Bragin A, Kachlany SC, Walters MJ, Du Y, Boesze-Battaglia K, Lally ET. 2013. Aggregatibacter actinomycetemcomitans leukotoxin utilizes a cholesterol recognition/amino acid consensus site for membrane association. J. Biol. Chem. 288:23607–23621. 10.1074/jbc.M113.486654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ingraham LM, Burns CP, Boxer LA, Baehner RL, Haak RA. 1981. Fluidity properties and liquid composition of erythrocyte membranes in Chediak-Higashi syndrome. J. Cell Biol. 89:510–516. 10.1083/jcb.89.3.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bloom JA, Webb WW. 1983. Lipid diffusibility in the intact erythrocyte membrane. Biophys. J. 42:295–305. 10.1016/S0006-3495(83)84397-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stafford G, Roy S, Honma K, Sharma A. 2012. Sialic acid, periodontal pathogens and Tannerella forsythia: stick around and enjoy the feast! Mol. Oral Microbiol. 27:11–22. 10.1111/j.2041-1014.2011.00630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hayashida H, Poulsen K, Kilian M. 2002. Differences in iron acquisition from human haemoglobin among strains of Actinobacillus actinomycetemcomitans. Microbiology 148:3993–4001 [DOI] [PubMed] [Google Scholar]
- 65. Ratledge C, Dover LG. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881–941. 10.1146/annurev.micro.54.1.881 [DOI] [PubMed] [Google Scholar]
- 66. Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, Moss K, Elter J, Offenbacher S. 2005. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation 112:19–24. 10.1161/CIRCULATIONAHA.104.511998 [DOI] [PubMed] [Google Scholar]
- 67. Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554–1560. 10.1902/jop.2000.71.10.1554 [DOI] [PubMed] [Google Scholar]
- 68. Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 25:e17–e18. 10.1161/01.ATV.0000155018.67835.1a [DOI] [PubMed] [Google Scholar]
- 69. Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. 2003. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 23:1250–1254. 10.1161/01.ATV.0000072969.71452.87 [DOI] [PubMed] [Google Scholar]
- 70. Stenderup K, Rosada C, Dam TN, Salerno E, Belinka BA, Kachlany SC. 2011. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J. Invest. Dermatol. 131:2033–2039. 10.1038/jid.2011.161 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.