Abstract
Polymicrobial infections involving Staphylococcus aureus exhibit enhanced disease severity and morbidity. We reviewed the nature of polymicrobial interactions between S. aureus and other bacterial, fungal, and viral cocolonizers. Microbes that were frequently recovered from the infection site with S. aureus are Haemophilus influenzae, Enterococcus faecalis, Pseudomonas aeruginosa, Streptococcus pneumoniae, Corynebacterium sp., Lactobacillus sp., Candida albicans, and influenza virus. Detailed analyses of several in vitro and in vivo observations demonstrate that S. aureus exhibits cooperative relations with C. albicans, E. faecalis, H. influenzae, and influenza virus and competitive relations with P. aeruginosa, Streptococcus pneumoniae, Lactobacillus sp., and Corynebacterium sp. Interactions of both types influence changes in S. aureus that alter its characteristics in terms of colony formation, protein expression, pathogenicity, and antibiotic susceptibility.
INTRODUCTION
Staphylococcus aureus is an opportunistic and resilient human pathogen that colonizes the mucosal surfaces. It is the causative agent of many serious acute and chronic infections. The anterior nares are the primary reservoirs of S. aureus. Asymptomatic colonization occurs in approximately 20% of the normal population, and 60% are transiently colonized, while 20% appear to be rarely or never colonized (1). Extranasal colonization of S. aureus also takes place in several locations, including the skin, rectum, axillae, vagina, pharynx, and gastrointestinal tract (2).
S. aureus causes numerous infections, including skin infections (boils, furuncles, styes, impetigo), surgical and trauma wounds, urinary tract infections, gastrointestinal tract infections, pneumonia, osteomyelitis, endocarditis, thrombophlebitis, mastitis, meningitis, infections on indwelling medical devices, toxic shock syndrome (TSS), and septicemia (3, 4). The factors contributing to the rise of this organism as a formidable pathogen involve multiple mechanisms of virulence. These include the evolution of strategies to resist antibiotics and evade host defenses, as well as the production of an arsenal of virulence factors such as capsule, coagulase, lipase, hyaluronidase, protein A, fibrinogen binding proteins, fibronectin binding proteins, and secreted toxins such as secreted enterotoxins (SEs), toxic shock syndrome toxin-1 (TSST-1), Panton-Valentine leucocidin (PVL), hemolysins, and phenol-soluble modulins (PSM) (5–9).
Several studies have confirmed S. aureus as one of the coinfecting microbes in many patients with polymicrobial infections (10). The interactions between S. aureus and the coexisting microbes are either cooperative, as with Candida albicans (11–14), Enterococcus faecalis (15, 16), Haemophilus influenzae (17–19), and influenza virus (20, 21), or competitive, as with Pseudomonas aeruginosa, Streptococcus pneumoniae (18, 19), Lactobacillus sp. (22–27), and Corynebacterium sp. (17, 28–30). Irrespective of whether the interactions are cooperative (Fig. 1) or competitive (Fig. 2), S. aureus within a community behaves differently with respect to its monomicrobial growth. This article focuses on reviewing the significance of interactions between S. aureus and other microorganisms and its effect on disease progression and outcome.
FIG 1.
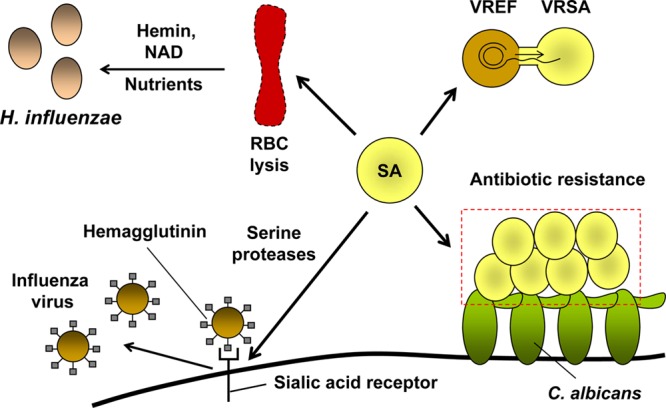
Cooperative interactions between S. aureus and other microbes. S. aureus can cocolonize with H. influenzae, E. faecalis, C. albicans, and influenza virus. S. aureus-induced lysis of red blood cells (RBC) leads to the release of hemin and NAD, which act as nutrients and support the growth of H. influenzae. S. aureus secretes proteases that cleave the host sialic acid receptor and increase the infectivity of influenza virus by releasing the virus from the host cell surface. S. aureus gained vancomycin resistance from E. faecalis due to horizontal gene transfer and became more resistant to antibiotics during coinfection with C. albicans. Symbols: SA, S. aureus; VRSA, vancomycin-resistant S. aureus; VREF, vancomycin-resistant E. faecalis.
FIG 2.
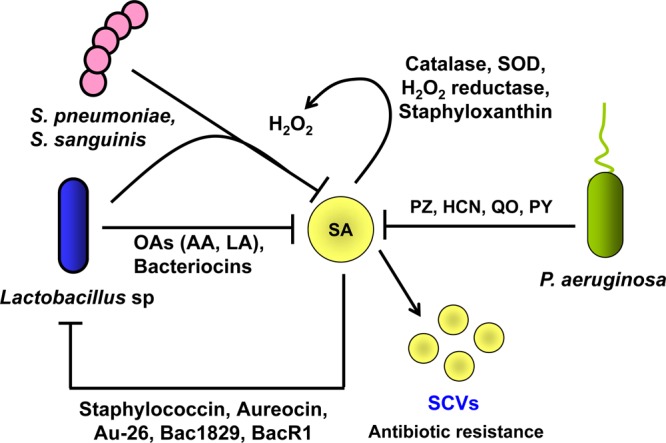
Competitive interactions between S. aureus and other microbes. S. aureus exhibits antagonism toward P. aeruginosa, Streptococcus sp., and Lactobacillus sp. P. aeruginosa produces phenazine (PZ), hydrogen cyanide (HCN), quinolone oxidase (QO), and pyocyanin (PY), resulting in the respiratory blockage of S. aureus, which in turn leads to the formation of small-colony variants (SCVs). SCVs are more persistent and are resistant to antibiotics. Lactobacillus sp. and Streptococcus sp. inhibit the growth of S. aureus by producing hydrogen peroxide (H2O2). S. aureus produces staphyloxanthin and catalase, which neutralize the toxic effects of H2O2. Additionally, Lactobacillus spp. produce organic acids and bacteriocins that limit the growth of S. aureus. Certain S. aureus strains also produce bacteriocins such as staphylococcin Au 26, which in turn inhibit the growth of lactobacilli. Blocked arrows indicate antagonism, and arrows indicate survival strategies of S. aureus.
Interactions with Candida.
Both Candida species and S. aureus usually exist as commensals and colonize human mucosal surfaces. Furthermore, they are opportunistic pathogens and cause a wide range of infections such as sepsis, pneumonia, denture stomatitis, and neonatal sepsis. Despite causing a number of infections independently, C. albicans and S. aureus can also be coisolated from several diseases such as cystic fibrosis, superinfection of burn wounds, urinary tract infections, and diabetic foot wounds and from the surfaces of various biomaterials, including dentures, voice prostheses, implants, endotracheal tubes, feeding tubes, and catheters (31–34).
Biofilm-embedded microbes are extremely resistant to both host clearance mechanisms and antimicrobial agents. S. aureus and C. albicans are often isolated concurrently from mixed bacterial-fungal biofilms on implanted medical devices (35). During biofilm-associated coinfections, C. albicans forms the base of the biofilm and facilitates the biofilm formation of S. aureus. C. albicans hyphal protein agglutinin-like sequence 3 (Als3p) mediates the binding of S. aureus with C. albicans hyphae (14, 36, 37). Within the polymicrobial biofilm, S. aureus exhibits enhanced resistance to vancomycin (13).
Independent studies demonstrated that the interactions between S. aureus and C. albicans enhance disease severity in several ways (33, 38). Candidal infections cause physical damage to organ walls, allowing S. aureus to penetrate the internal organs more easily. S. aureus, on the other hand, secretes different proteases that help C. albicans to enhance its adhesion to the mucosal layer (12). During systemic infections, each organism helps the other to evade phagocytic killing mediated by polymorphonuclear leukocytes (PMNs). C. albicans secretes a proteinase that degrades the Fc portion of immunoglobulin G (IgG) and greatly reduces the opsonizing activity of human PMNs against S. aureus (39). On the other hand, S. aureus secretes coagulase and extracellular fibrinogen binding proteins (Efb) that protect Candida sp. from PMN-mediated phagocytosis. Coagulase activates prothrombin, which mediates the conversion of fibrinogen to fibrin. Formation of fibrin clots surrounding the candidal cells helps Candida spp. to evade phagocytic killing by granulocytes (40). Additionally, Efb binds to C3 complement and interferes with complement activation and C3-mediated opsonization (41). The cooperative infection of C. albicans and S. aureus represents a significant therapeutic challenge, and their coisolation from blood is an indication of a dire prognosis (42).
Competitive or antagonistic relationships between C. albicans and S. aureus have also been reported where the farnesol quorum-sensing molecule secreted by C. albicans inhibits the biofilm formation of S. aureus. Farnesol disrupts the S. aureus cell membrane integrity and thereby its viability. Additionally, in vitro results demonstrated that farnesol-treated S. aureus showed enhanced susceptibility to a variety of clinically important antibiotics (43). However, it is as yet unclear how much farnesol C. albicans secretes under in vivo conditions and whether the secreted concentrations are sufficient to inhibit the growth of S. aureus in vivo. Nevertheless, all available in vivo data suggest that S. aureus and C. albicans exist in synergy. Apart from Candida albicans, S. aureus was also isolated together with Candida tropicalis, Candida parapsiolosis, and Trichosporon asahii (44, 45).
Interactions with influenza virus.
The mechanisms of interaction of S. aureus with influenza virus are much more complex than the interactions between S. aureus and C. albicans. Superinfection of influenza virus and S. aureus is one of the major causes of severe influenza pneumonia, prolonged inflammation, and higher mortality rates. This represents the best-known model of bacterial-viral coinfection (20).
Influenza virus A infection promotes and enhances the nasopharyngeal adherence of S. aureus (46). On the other hand, S. aureus promotes the infectivity and spread of the influenza virus particles. Hemagglutinin (HA), a trimeric glycoprotein, present in multiple copies in the membrane envelope of influenza virus, is responsible for the attachment of the virus particle to sialic acid-containing receptors of the host ciliated columnar epithelial cells. Proteolytic cleavage of the hemagglutinin is an important prerequisite for the infectivity of the influenza virus and for the spread of the virus in the host organism and associated pathogenicity. Several strains of S. aureus have been found to secrete serine proteases that activate infectivity of influenza virus by proteolytic cleavage of the hemagglutinin (21).
Coinfections of S. aureus and influenza virus may lead to severe disease outcome, as influenza virus infection enhances the deleterious effects of staphylococcal enterotoxin B (SEB) and toxic shock syndrome toxin 1 (TSST-1) (47, 48). SEB and TSST-1 are superantigens that activate T cells in an uncontrolled manner and cause massive systemic release of cytokines. Concurrent S. aureus and influenza virus infection induces enterotoxin-mediated massive release of tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ). This results in fever, rash, hypotension, tissue injury, and shock. It has been hypothesized that the lethal synergism between concurrent influenza infection and S. aureus superantigen exposure may contribute to sudden and unexpected death from influenza virus infection (49).
Interactions with other bacteria.
The majority of the interactions between S. aureus and other bacterial species are competitive in nature, and only a few interactions are cooperative. Cooperative interactions involving S. aureus exist with H. influenzae and E. faecalis. Competitive interactions are observed between S. aureus and other bacteria, viz., Pseudomonas aeruginosa, Streptococcus pneumoniae, lactic acid bacteria (LAB), Corynebacterium sp., or S. epidermidis. That the interactions are competitive does not mean that these organisms completely inhibit the colonization of S. aureus; rather, S. aureus employs numerous defense strategies for its survival, counterattacking the competing bacteria and surviving in the same ecological niche. Cooperative or competitive interactions lead to the development of more-persistent S. aureus strains with altered colony morphology, antibiotic resistance, and increased virulence. The interactions of S. aureus with other bacterial species are listed below.
(i) Interactions with Haemophilus influenzae.
S. aureus and H. influenzae both colonize the nasopharynx and, in some instances, the conjunctivae and genital tract. H. influenzae reaches higher colony densities when the resident colonizer is S. aureus. The higher H. influenzae colony densities have been attributed to the availability of nutrients that S. aureus provides to facilitate its growth (19). S. aureus produces three major hemolysins (α, β, and γ) which lyse erythrocytes by compromising their membrane integrity (50). The hemolysis of erythrocytes by S. aureus-secreted hemolysins releases nutrients such as hemin and NAD, which are vital for the growth of H. influenzae (51–53). Margolis et al. demonstrated synergistic interactions of S. aureus and H. influenzae in the rat nasopharynx (19). However, Pettigrew et al. and van den Bergh et al. studied the compositions of nasal microflora among children and have reported antagonism or negative association between S. aureus and H. influenzae (54, 55). Both of those studies were designed to determine the microflora composition among children in the age group between 6 and 36 months.
(ii) Interactions with Pseudomonas aeruginosa.
The relation between S. aureus and P. aeruginosa is competitive in nature, although the two organisms are frequently found together in clinical settings. They have common niches within the host, for example, the lungs of cystic fibrosis (CF) patients, peritoneum of dialysis patients, catheters, diabetic foot wounds, and other type of wounds caused by skin injury or skin burn (44, 56). S. aureus is often reported as the primary pathogen infecting the lungs of the CF patients, followed by P. aeruginosa. Although coinfections of these pathogens are very common under in vivo conditions, several independent in vitro studies demonstrated that, when cocultured together, P. aeruginosa thrives better than S. aureus (57–59). The better survival of P. aeruginosa is attributed to its ability to produce respiratory toxins such as pyocyanin, hydrogen cyanide, and alkyl-hydroxyquinoline N-oxides that can block the electron transport pathway, thereby inhibiting the growth of S. aureus and other pathogenic staphylococci (57, 58).
Despite its sensitivity to respiratory inhibitors, S. aureus does not get completely cleared away by P. aeruginosa. To counter the effect of the respiratory toxins produced by P. aeruginosa, S. aureus forms electron transport-deficient small-colony variants (SCVs) that grow as tiny, nonpigmented colonies (57). Purified 4-hydroxy-2-heptylquinoline-N-oxide (HQNO) or pyocyanin produced by P. aeruginosa is sufficient to induce SCV selection in S. aureus (57, 59). These SCVs are auxotrophic to hemin or menadione and are resistant to antibiotics, especially aminoglycosides, trimethoprim-sulfamethoxazol (60), and the host antimicrobial peptide lactoferricin B (8). The resistance of SCVs is due in part to their severely decreased membrane potential as well as their reduced growth rate and metabolic processes. These SCVs also persist better than their normal counterparts.
P. aeruginosa also produces a 20-kDa endopeptidase, LasA, which selectively cleaves S. aureus peptidoglycan. LasA cleaves the glycyl-glycine and glycyl-alanine bonds of the pentaglycine interpeptide bridge in the S. aureus peptidoglycan and induces lysis (61, 62). Using the rat model of infection, Mashburn et al. showed that P. aeruginosa can lyse S. aureus cells and that the iron-containing proteins released from the lysed S. aureus cells serve as the source of iron, thereby increasing the pathogenic potential of P. aeruginosa (63, 64). However, this result is yet to be validated in clinical settings. P. aeruginosa exhibits a similar kind of antagonistic relationship with S. epidermidis, as well as with species representatives of S. haemolyticus, S. saprophyticus, S. hyicus, S. muscae, and S. lugdunensis (58).
(iii) Interactions with Streptococcus pneumoniae.
The relation between S. pneumoniae and S. aureus is antagonistic. S. pneumoniae and S. aureus colonize the upper respiratory tract of children and compete with each other for the same niche (59, 65, 66). Various studies have shown that colonization of the upper airway by S. pneumoniae is negatively correlated with S. aureus colonization and that children who are vaccinated with pneumococcal conjugate vaccines are at major risk of S. aureus infections (18). This inverse relation suggests that one organism interferes with the colonization of the other. In vitro data demonstrate that hydrogen peroxide (H2O2), a byproduct of aerobic metabolism produced by S. pneumoniae, is responsible for the antagonistic relationship between these two pathogens (67). H2O2 production leads to the production of DNA-damaging hyperoxides through the Fenton reaction that induces the SOS response. The SOS response induces the resident prophages, resulting in the lysis of lysogenic staphylococci. Because the vast majority of S. aureus strains are lysogenic, the production of H2O2 is a very effective antistaphylococcal strategy of S. pneumoniae. H2O2, at concentrations typically produced by pneumococci, kills lysogenic but not nonlysogenic staphylococci (68). Pneumococci, however, are not SOS induced upon exposure to H2O2, as they are resistant to the DNA-damaging effects of the Fenton reaction (69).
It is interesting that S. aureus, which produces so many antioxidants and free radical scavengers, including catalase, alkyl hydroperoxide reductase, superoxide dismutase (SodA and SodM), and staphyloxanthin (16, 70), is susceptible to H2O2 produced by S. pneumoniae. A possible explanation could be that the amounts of free radical scavengers that S. aureus produces are not sufficient to neutralize all the H2O2 produced by S. pneumoniae. Regev-Yochay et al. demonstrated that staphylococcal species that secrete higher concentrations of catalase are resistant to S. pneumoniae (67).
However, other studies have offered hypotheses suggesting that the production of hydrogen peroxide may not be the main reason for the antagonistic relationship between these pathogens in vivo (71). Although both pathogens colonize the upper respiratory tract, their microniches are different. Therefore, direct antagonism mediated by H2O2 is an unlikely reason for their antagonism. Rather, the antibody response generated during S. pneumoniae infection, although ineffective in restricting this pathogen itself, is effective in providing cross-protection against S. aureus (71, 72).
(iv) Interactions with LAB.
The lactic acid bacteria (LAB) consist of a group of heterogeneous bacterial species comprising nonsporulating, Gram-positive cocci and bacilli that are able to ferment sugars predominantly into lactic acid. This leads to acidification of the environment down to a pH of 3.5. LAB colonize the gut and urogenital tract and contribute to defense against S. aureus-mediated food poisoning and genital infections. The antistaphylococcal activity of LAB strains is attributed to the production of H2O2, organic acids, antimicrobial proteins, biosurfactants, surface proteins, and quorum-sensing inhibitors. The most commonly studied members of intestinal and vaginal LAB include Lactobacillus acidophilus, L. casei, L. fermentum, L. salivarius, L. rhamnosus, L. gasseri, L. vaginalis, L. johnsonii, and L. delbrueckii (25, 73–75).
In similarity to the results seen with S. pneumoniae, LAB-produced hydrogen peroxide (H2O2) inhibits the growth of S. aureus (76, 77). Additionally, LAB secrete organic acids (lactic, acetic, formic, caproic, propionic, butyric, and valeric acids) that inhibit the growth of S. aureus (78). LAB-produced bacteriocins interfere with cell wall structure and biosynthesis and form pores in the S. aureus membrane (79). Among the bacteriocins produced by LAB, the most important are nisin, produced by Lactococcus lactis; pediocin, produced by Pediococcus acidilactici; and lacticin 3147, produced by Lactococcus lactis DPC 3147 (79, 80).
Apart from inhibiting the growth of S. aureus by the use of H2O2, organic acids, and bacteriocins, LAB compete with S. aureus for the host cell adhesion sites. Biosurfactants and surface proteins of LAB strains are involved in this competitive exclusion process. L. fermentum, L. acidophilus, L. crispatus CRL 1266, L. paracasei subsp. paracasei CRL 1289, L. salivarius CRL 1328, L. rhamnosus GG, Lactococcus lactis subsp. lactis, and Propionibacterium freudenreichii subsp. shermani were shown to disrupt the adherence of S. aureus to the intestinal and urogenital tract by competing for the same adhesion sites. Some LAB strains were also shown to displace previously adhered S. aureus from the vaginal epithelial cells (27). In a recent study, it was also shown that the small signaling molecule cyclic dipeptides cyclo(l-Tyr-LPro) and cyclo(l-Phe-l-Pro), produced by the human vaginal isolate L. reuteri RC-14, are able to interfere with the staphylococcal quorum-sensing system agr, a key regulator of virulence genes, and repress the expression of staphylococcal exotoxin TSST-1 (81).
To counter the detrimental effects of LAB species, S. aureus produces bacteriocins that have antibacterial activity against LAB. For example, S. aureus secretes bacteriocins such as staphylococcin Au-26, Bac1829, BacR1, aureocin A70, and aureocin A53 that inhibit the growth of lactobacilli (80).
(v) Interactions with Corynebacterium sp.
S. aureus and Corynebacterium sp. are two of the most important species infecting the skin and nasopharynx. Both organisms are associated with catheter-related infections. A lower incidence of S. aureus colonization has been observed in individuals heavily colonized by Corynebacterium sp. (C. accolens, C. pseudodiptheriticum, and C. tuberculostearicum). Corynebacterium spp. utilize competitive exclusion strategies similar to those of LAB in competing with S. aureus for the same adhesion site with host mucosal epithelial cells (30). No bacteriocin-like activity of Corynebacterium sp. against S. aureus has been reported. However, a number of bacteriocins secreted by S. aureus are active against Corynebacterium sp. These bacteriocins include Bac1829 (17), aureocin A70 (29), aureocin A53 (82) and staphylococcin 188 (28).
(vi) Interactions with S. epidermidis.
Besides these interactions, S. aureus is also known to interact with members of the same genus. Several reports indicate antagonistic relationships between S. aureus and S. epidermidis. Both S. aureus and S. epidermidis are opportunistic and nosocomial pathogens. Unlike S. aureus, which causes severe acute infections, S. epidermidis frequently causes chronic infections and has an exceptional capacity to attach to the indwelling medical devices during surgery and form biofilms. The presence of S. epidermidis in the nasal cavities has been reported to correlate with the absence of S. aureus (83). Similar to S. pneumoniae, this pathogen uses multiple strategies to inhibit S. aureus colonization. These include production of autoinducing peptide (AIP), phenol-soluble modulins (PSM), and bacteriocins. The production of virulence factors and other extracellular proteins in staphylococci is globally regulated by the accessory gene regulatory system (agr). agr encodes a two-component signaling pathway whose activating ligand is AIP, which is also encoded by agr (84). The AIPs can activate the agr response in the other members of the same group but show mutually inhibitory effects between members of different groups. Based on the agr loci present, S. aureus strains have been divided into 4 major groups, agr-1Sa to agr-4Sa, and S. epidermidis into 3 major groups, agr-1Se to agr-3Se (85). S. epidermidis AIP has been proven to inhibit the activity of agr-1Sa to agr-3Sa and thereby suppress the expression of virulence factors such as the alpha-toxin, β-toxin, δ-toxin, serine protease, DNase, fibrinolysin, enterotoxin B, and toxic shock syndrome toxin 1 in S. aureus. Among S. aureus AIPs, only agr-4Sa weakly inhibits the activity of agr-1Se (30, 86).
Additionally, S. epidermidis secretes an extracellular serine protease (Esp) that, alone or in combination with host β-defensin 2, eliminates S. aureus biofilms. Esp cleaves S. aureus major autolysin (Atl) protein and interferes with its function (87). Activity of Atl is necessary for DNA release and biofilm formation of S. aureus (88). Phenol-soluble modulins (PSMγ and PSMδ) and bacteriocins (Pep5, epidermin, epilancin K7, and epicidin 280) produced by S. epidermidis inhibit the growth of S. aureus. S. epidermidis-secreted PSM peptides cooperate with each other and with the host antimicrobial peptide, LL-37, to exert selective antimicrobial action against S. aureus (9, 89).
(vii) Interactions with Enterococcus faecalis.
The anterior nares are generally considered to be the primary site of colonization of S. aureus; however, low concentrations (≤105 CFU/g of feces) of this organism cocolonize the intestinal tracts together with E. faecalis in healthy humans. Both S. aureus and E. faecalis normally exist as commensals, but they can turn into opportunistic pathogens causing urinary tract infections, bacteremia, and infective endocarditis (15). Apart from the intestinal tract, E. faecalis and S. aureus are frequently isolated from the respiratory tract, urinary tract, and chronic foot ulcers and from diabetic foot wounds (44). The interaction between E. faecalis and S. aureus is neither truly synergistic nor antagonistic.
Many studies have focused on the mechanisms by which S. aureus acquired the vancomycin resistance gene from E. faecalis. Vancomycin-resistant S. aureus (VRSA) strains emerged due to horizontal transfer of a Tn1546 transposon containing the vanA gene from vancomycin-resistant E. faecalis (90–92). The transposon Tn1546 harboring the vanA gene present on the pAM830 plasmid is related to the Inc18 family of broad-host-range conjugative plasmids and is responsive to the cAM373 pheromone secreted by the plasmid-free (recipient) strains of E. faecalis. cAM373 triggers the process of conjugation, leading to the transfer of the vanA gene from the vancomycin-resistant E. faecalis (donor) strains to the vancomycin-susceptible E. faecalis (recipient) strains (93). S. aureus is also known to secrete a peptide, staph-cAM373 (amino acid sequence AIFILAA), with activity similar to that of E. faecalis cAM373 (amino acid sequence AIFILAS) that triggers the process of conjugation between vancomycin-resistant E. faecalis (donor) and S. aureus (recipient) (94). This conjugation results in the transfer of the vanA gene from E. faecalis to S. aureus. Genetic analysis of several vancomycin-resistant S. aureus (VRSA) strains showed that transposon Tn1546 harboring the vanA gene either jumped into a staphylococcal plasmid or integrated into the S. aureus chromosome (16, 91, 95). The acquisition of vanA by S. aureus resulted in incorporation of d-alanyl-d-lactate (d-Ala-d-Lac) precursors into the peptidoglycan instead of d-alanine-d-alanine (d-Ala-d-Ala). The E. faecalis and S. aureus cell wall harboring the d-Ala-d-Lac precursors has 1,000-fold less affinity for vancomycin, a drug that is considered the last-resort antibiotic to treat methicillin-resistant S. aureus (MRSA) infections (96). Interactions between these two bacteria have led to an increase in the numbers of multidrug-resistant staphylococci.
CONCLUSION
Most infections are polymicrobial in nature and can be seen in almost every niche in the human body, particularly in mucosal surfaces, where different species of microorganisms such as bacteria, fungi, and viruses coexist as communities. S. aureus is one of the most common pathogens found in polymicrobial infections. In polymicrobial infections, S. aureus differentially modulates host immune responses and disease severity and acquires altered growth and antibiotic susceptibility patterns. The altered immune response during polymicrobial infections could be beneficial or detrimental for S. aureus. For example, influenza virus infection inhibits Th17-mediated adaptive immune responses (97). Activated Th17 cells are necessary for protection against S. aureus infection, because this subset of T cells enhances neutrophil recruitment to sites of infection and kills S. aureus (98, 99). Therefore, Th17 cell-mediated immune activation is necessary to limit S. aureus infections. By inhibiting the Th17 cell-mediated immune response and subsequent neutrophil infiltration, influenza virus helps S. aureus to colonize and to cause severe secondary bacterial pneumonia (97, 100). In contrast to the immune suppression mediated by influenza virus that aids S. aureus, S. pneumoniae-mediated immune activation is detrimental to S. aureus. The antibody response generated during S. pneumoniae infection against its glyceraldehyde-3-phosphate dehydrogenase, although ineffective in inducing opsonophagocytic killing of S. pneumoniae, can cross-react with staphylococcal protein 1-pyrroline-5-carboxylate dehydrogenase and induce opsonophagocytic killing of S. aureus (71, 72). S. pneumoniae itself is protected from opsonophagocytic killing due to its antiopsonic polysaccharide capsule.
Additionally, S. aureus in polymicrobial infections displays enhanced persistence and antibiotic tolerance. S. aureus acquired vancomycin resistance genes from E. faecalis and became resistant to vancomycin (16, 91, 95). S. aureus, during coinfection with C. albicans, showed increased vancomycin resistance (13, 101). This bacterium forms electron transport-deficient small-colony variants during coinfection with P. aeruginosa (57, 58). These SCVs persist better than their normal counterparts and are resistant to aminoglycosides and trimethoprim-sulfamethoxazols (102).
A 23-valent polysaccharide vaccine against S. pneumoniae which was recently introduced into the market indeed prevented S. pneumoniae nasopahryangeal colonization, but the vaccinated individuals were subject to an increased risk of S. aureus nasal colonization (72). Therefore, prevention of one pathogenic infection provides opportunities to the competing pathogens to cause disease. These findings highlight the potential complications that could arise from conventional treatment and disease prevention strategies that target a single organism, thereby necessitating the need to introduce modified therapeutic approaches that take into account the coinfecting organisms. Several strategies could be used to address the difficulties in treatment of polymicrobial infections of S. aureus. One could be the use of combined vaccines against two or more coinfecting microbes; however, such vaccines are still in the experimental stages. The next approach could be the judicious use of antimicrobial drugs. A coinfection of S. aureus and influenza virus should be treated with antiviral and appropriate antibacterial drugs. A third approach is the use of LAB strains to prevent not all but some of the S. aureus infections. Probiotic LAB can prevent intestinal and urogenital tract coinfections. Studies have shown that regular intake of probiotic LAB and fermented milk can even reduce S. aureus colonization in the upper respiratory tract. Similarly, probiotic LAB species also confer protection against influenza virus by modulating innate immunity. Thus, probiotic bacteria can be used to prevent coinfections of S. aureus and influenza virus.
In summary, S. aureus in polymicrobial infections represents a clinical challenge greater than that of S. aureus in monomicrobial infections. The coexisting microbes significantly influence the outcome of the infection by altering invasion ability, growth, gene expression, and drug sensitivity patterns. Further investigations are required to design appropriate treatment strategies to tackle polymicrobial infections mediated by S. aureus.
ACKNOWLEDGMENTS
N.N. is supported by a doctoral fellowship from Indian Council of Medical Research (ICMR; 45/16/2011/IMM-BMS), and R.B. is supported by a Ramalingaswami fellowship, Department of Biotechnology (DBT), Government of India. This study was supported by a DBT grant (BT/PR13125/GBD/27/193/2009) to R.B. and an ICMR grant (AMR/14/2011-ECD1) to L.B.
We thank Shantikumar V. Nair for valuable comments and for critically reviewing the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 18 March 2014
REFERENCES
- 1. Williams RE. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 27:56–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751–762. 10.1016/S1473-3099(05)70295-4 [DOI] [PubMed] [Google Scholar]
- 3. Götz F, Bannerman T, Schleifer KH. 2006. The genera Staphylococcus and Macrococcus. Prokaryotes 4:5–75. 10.1007/0-387-30744-3_1 [DOI] [Google Scholar]
- 4. McCaig LF, McDonald LC, Mandal S, Jernigan DB. 2006. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 12:1715–1723. 10.3201/eid1211.060190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garnier F, Tristan A, Francois B, Etienne J, Delage-Corre M, Martin C, Liassine N, Wannet W, Denis F, Ploy MC. 2006. Pneumonia and new methicillin-resistant Staphylococcus aureus clone. Emerg. Infect. Dis. 12:498–500. 10.3201/eid1203.051040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joo HS, Cheung GY, Otto M. 2011. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J. Biol. Chem. 286:8933–8940. 10.1074/jbc.M111.221382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prévost G, Mourey L, Colin DA, Menestrina G. 2001. Staphylococcal pore-forming toxins. Curr. Top. Microbiol. Immunol. 257:53–83 [DOI] [PubMed] [Google Scholar]
- 8. Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE. 2013. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J. Immunol. 190:3417–3426. 10.4049/jimmunol.1202563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varella Coelho ML, Santos Nascimento JD, Fagundes PC, Madureira DJ, Oliveira SS, Vasconcelos de Paiva Brito MA, do Carmo de Freire Bastosa M. 2007. Activity of staphylococcal bacteriocins against Staphylococcus aureus and Streptococcus agalactiae involved in bovine mastitis. Res. Microbiol. 158:625–630. 10.1016/j.resmic.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 10. Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, Fry A, Hageman J, Gorwitz R, Bresee J, Uyeki T. 2008. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 122:805–811. 10.1542/peds.2008-1336 [DOI] [PubMed] [Google Scholar]
- 11. Adam B, Baillie GS, Douglas LJ. 2002. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 51:344–349 [DOI] [PubMed] [Google Scholar]
- 12. El-Azizi MA, Starks SE, Khardori N. 2004. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J. Appl. Microbiol. 96:1067–1073. 10.1111/j.1365-2672.2004.02213.x [DOI] [PubMed] [Google Scholar]
- 13. Harriott MM, Noverr MC. 2009. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob. Agents Chemother. 53:3914–3922. 10.1128/AAC.00657-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, Shirtliff ME. 2010. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 59:493–503. 10.1111/j.1574-695X.2010.00710.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ray AJ, Pultz NJ, Bhalla A, Aron DC, Donskey CJ. 2003. Coexistence of vancomycin-resistant enterococci and Staphylococcus aureus in the intestinal tracts of hospitalized patients. Clin. Infect. Dis. 37:875–881. 10.1086/377451 [DOI] [PubMed] [Google Scholar]
- 16. Flannagan SE, Clewell DB. 2002. Identification and characterization of genes encoding sex pheromone cAM373 activity in Enterococcus faecalis and Staphylococcus aureus. Mol. Microbiol. 44:803–817. 10.1046/j.1365-2958.2002.02922.x [DOI] [PubMed] [Google Scholar]
- 17. Crupper SS, Iandolo JJ. 1996. Purification and partial characterization of a novel antibacterial agent (Bac1829) produced by Staphylococcus aureus KSI1829. Appl. Environ. Microbiol. 62:3171–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madhi SA, Adrian P, Kuwanda L, Cutland C, Albrich WC, Klugman KP. 2007. Long-term effect of pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae—and associated interactions with Staphylococcus aureus and Haemophilus influenzae colonization—in HIV-infected and HIV-uninfected children. J. Infect. Dis. 196:1662–1666. 10.1086/522164 [DOI] [PubMed] [Google Scholar]
- 19. Margolis E, Yates A, Levin BR. 2010. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiol. 10:59. 10.1186/1471-2180-10-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niemann S, Ehrhardt C, Medina E, Warnking K, Tuchscherr L, Heitmann V, Ludwig S, Peters G, Loffler B. 2012. Combined action of influenza virus and Staphylococcus aureus Panton-Valentine leukocidin provokes severe lung epithelium damage. J. Infect. Dis. 206:1138–1148. 10.1093/infdis/jis468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tashiro M, Ciborowski P, Reinacher M, Pulverer G, Klenk HD, Rott R. 1987. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology 157:421–430. 10.1016/0042-6822(87)90284-4 [DOI] [PubMed] [Google Scholar]
- 22. Gan BS, Kim J, Reid G, Cadieux P, Howard JC. 2002. Lactobacillus fermentum RC-14 inhibits Staphylococcus aureus infection of surgical implants in rats. J. Infect. Dis. 185:1369–1372. 10.1086/340126 [DOI] [PubMed] [Google Scholar]
- 23. Li J, Wang W, Xu SX, Magarvey NA, McCormick JK. 2011. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. U. S. A. 108:3360–3365. 10.1073/pnas.1017431108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otero MC, Nader-Macias ME. 2006. Inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus gasseri isolated from the vaginal tract of cattle. Anim. Reprod. Sci. 96:35–46. 10.1016/j.anireprosci.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 25. Varma P, Nisha N, Dinesh KR, Kumar AV, Biswas R. 2011. Anti-infective properties of Lactobacillus fermentum against Staphylococcus aureus and Pseudomonas aeruginosa. J. Mol. Microbiol. Biotechnol. 20:137–143. 10.1159/000328512 [DOI] [PubMed] [Google Scholar]
- 26. Vesterlund S, Karp M, Salminen S, Ouwehand AC. 2006. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology 152:1819–1826. 10.1099/mic.0.28522-0 [DOI] [PubMed] [Google Scholar]
- 27. Zárate G, Nader-Macias ME. 2006. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 43:174–180. 10.1111/j.1472-765X.2006.01934.x [DOI] [PubMed] [Google Scholar]
- 28. Saeed S, Ahmad S, Rasool SA. 2004. Antimicrobial spectrum, production and mode of action of staphylococcin 188 produced by Staphylococcus aureus 188. Pak. J. Pharm. Sci. 17:1–8 [PubMed] [Google Scholar]
- 29. dos Santos Nascimento J, dos Santos KR, Gentilini E, Sordelli D, de Freire Bastos Mdo C. 2002. Phenotypic and genetic characterisation of bacteriocin-producing strains of Staphylococcus aureus involved in bovine mastitis. Vet. Microbiol. 85:133–144. 10.1016/S0378-1135(01)00476-X [DOI] [PubMed] [Google Scholar]
- 30. Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. 2003. Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl. Environ. Microbiol. 69:18–23. 10.1128/AEM.69.1.18-23.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valenza G, Tappe D, Turnwald D, Frosch M, Konig C, Hebestreit H, Abele-Horn M. 2008. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 7:123–127. 10.1016/j.jcf.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 32. Shirtliff ME, Peters BM, Jabra-Rizk MA. 2009. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 299:1–8. 10.1111/j.1574-6968.2009.01668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peters BM, Noverr MC. 2013. Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect. Immun. 81:2178–2189. 10.1128/IAI.00265-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peters BM, Ward RM, Rane HS, Lee SA, Noverr MC. 2013. Efficacy of ethanol against Candida albicans and Staphylococcus aureus polymicrobial biofilms. Antimicrob. Agents Chemother. 57:74–82. 10.1128/AAC.01599-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255–267. 10.1128/CMR.17.2.255-267.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. 2007. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 59:401–406. 10.1016/j.diagmicrobio.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 37. Peters BM, Ovchinnikova ES, Krom BP, Schlecht LM, Zhou H, Hoyer LL, Busscher HJ, van der Mei HC, Jabra-Rizk MA, Shirtliff ME. 2012. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 158:2975–2986. 10.1099/mic.0.062109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morales DK, Hogan DA. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 6:e1000886. 10.1371/journal.ppat.1000886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaminishi H, Miyaguchi H, Tamaki T, Suenaga N, Hisamatsu M, Mihashi I, Matsumoto H, Maeda H, Hagihara Y. 1995. Degradation of humoral host defense by Candida albicans proteinase. Infect. Immun. 63:984–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fehrmann C, Jurk K, Bertling A, Seidel G, Fegeler W, Kehrel BE, Peters G, Becker K, Heilmann C. 2013. Role for the fibrinogen-binding proteins coagulase and Efb in the Staphylococcus aureus-Candida interaction. Int. J. Med. Microbiol. 303:230–238. 10.1016/j.ijmm.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 41. Lee LY, Hook M, Haviland D, Wetsel RA, Yonter EO, Syribeys P, Vernachio J, Brown EL. 2004. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 190:571–579. 10.1086/422259 [DOI] [PubMed] [Google Scholar]
- 42. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 43. Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. 2006. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 50:1463–1469. 10.1128/AAC.50.4.1463-1469.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chellan G, Shivaprakash S, Karimassery Ramaiyar S, Varma AK, Varma N, Thekkeparambil Sukumaran M, Rohinivilasam Vasukutty J, Bal A, Kumar H. 2010. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J. Clin. Microbiol. 48:2097–2102. 10.1128/JCM.02035-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Popescu GA, Prazuck T, Poisson D, Picu C. 2005. A “true” polymicrobial endocarditis: Candida tropicalis and Staphylococcus aureus—to a drug user. Case presentation and literature review. Rom. J. Intern. Med. 43:157–161 [PubMed] [Google Scholar]
- 46. Davison VE, Sanford BA. 1982. Factors influencing adherence of Staphylococcus aureus to influenza A virus-infected cell cultures. Infect. Immun. 37:946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yarovinsky TO, Mohning MP, Bradford MA, Monick MM, Hunninghake GW. 2005. Increased sensitivity to staphylococcal enterotoxin B following adenoviral infection. Infect. Immun. 73:3375–3384. 10.1128/IAI.73.6.3375-3384.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. MacDonald KL, Osterholm MT, Hedberg CW, Schrock CG, Peterson GF, Jentzen JM, Leonard SA, Schlievert PM. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenza like illness. JAMA 257:1053–1058 [DOI] [PubMed] [Google Scholar]
- 49. Zhang WJ, Sarawar S, Nguyen P, Daly K, Rehg JE, Doherty PC, Woodland DL, Blackman MA. 1996. Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice. J. Immunol. 157:5049–5060 [PubMed] [Google Scholar]
- 50. Nilsson IM, Hartford O, Foster T, Tarkowski A. 1999. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect. Immun. 67:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orlova OE, Elkina SI, Iastrebova NE, Vaneeva NP, Sergeev VV, Kalina NG, Tokarskaia MM. 2005. Influence of nicotinamide adenine dinucleotide and hemin concentrations on the growth of Haemophilus influanzae type b and the synthesis of capsular polysaccharide. Zh. Mikrobiol. Epidemiol. Immunobiol. 2005:12–15 (In Russian.) [PubMed] [Google Scholar]
- 52. Kemmer G, Reilly TJ, Schmidt-Brauns J, Zlotnik GW, Green BA, Fiske MJ, Herbert M, Kraiss A, Schlor S, Smith A, Reidl J. 2001. NadN and e (P4) are essential for utilization of NAD and nicotinamide mononucleotide but not nicotinamide riboside in Haemophilus influenzae. J. Bacteriol. 183:3974–3981. 10.1128/JB.183.13.3974-3981.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Artman M, Domenech E, Weiner M. 1983. Growth of Haemophilus influenzae in simulated blood cultures supplemented with hemin and NAD. J. Clin. Microbiol. 18:376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg. Infect. Dis. 14:1584–1591. 10.3201/eid1410.080119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, Wang X, Boonacker CW, Veenhoven RH, Bruin JP, Bogaert D, Sanders EA. 2012. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One 7:e47711. 10.1371/journal.pone.0047711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holley JL, Bernardini J, Piraino B. 1992. Polymicrobial peritonitis in patients on continuous peritoneal dialysis. Am. J. Kidney Dis. 19:162–166 [DOI] [PubMed] [Google Scholar]
- 57. Biswas L, Biswas R, Schlag M, Bertram R, Gotz F. 2009. Small-colony variant selection as a survival strategy for Staphylococcus aureus in the presence of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 75:6910–6912. 10.1128/AEM.01211-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Voggu L, Schlag S, Biswas R, Rosenstein R, Rausch C, Götz F. 2006. Microevolution of cytochrome bd oxidase in Staphylococci and its implication in resistance to respiratory toxins released by Pseudomonas. J. Bacteriol. 188:8079–8086. 10.1128/JB.00858-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoffman LR, Deziel E, D'Argenio DA, Lepine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 103:19890–19895. 10.1073/pnas.0606756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vaudaux P, Kelley WL, Lew DP. 2006. Staphylococcus aureus small colony variants: difficult to diagnose and difficult to treat. Clin. Infect. Dis. 43:968–970. 10.1086/507643 [DOI] [PubMed] [Google Scholar]
- 61. Kessler E, Safrin M, Olson JC, Ohman DE. 1993. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 268:7503–7508 [PubMed] [Google Scholar]
- 62. Lache M, Hearn WR, Zyskind JW, Tipper DJ, Strominger JL. 1969. Specificity of a bacteriolytic enzyme from Pseudomonas aeruginosa. J. Bacteriol. 100:254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mashburn LM, Jett AM, Akins DR, Whiteley M. 2005. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 187:554–566. 10.1128/JB.187.2.554-566.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Palmer KL, Mashburn LM, Singh PK, Whiteley M. 2005. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 187:5267–5277. 10.1128/JB.187.15.5267-5277.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rumke HC, Verbrugh HA, Hermans PW. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871–1872. 10.1016/S0140-6736(04)16357-5 [DOI] [PubMed] [Google Scholar]
- 66. Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E, Rahav G, Rubinstein E. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292:716–720. 10.1001/jama.292.6.716 [DOI] [PubMed] [Google Scholar]
- 67. Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. 2006. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 188:4996–5001. 10.1128/JB.00317-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Selva L, Viana D, Regev-Yochay G, Trzcinski K, Corpa JM, Lasa I, Novick RP, Penades JR. 2009. Killing niche competitors by remote-control bacteriophage induction. Proc. Natl. Acad. Sci. U. S. A. 106:1234–1238. 10.1073/pnas.0809600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pericone CD, Park S, Imlay JA, Weiser JN. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815–6825. 10.1128/JB.185.23.6815-6825.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clauditz A, Resch A, Wieland KP, Peschel A, Götz F. 2006. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 74:4950–4953. 10.1128/IAI.00204-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lijek RS, Weiser JN. 2012. Co-infection subverts mucosal immunity in the upper respiratory tract. Curr. Opin. Immunol. 24:417–423. 10.1016/j.coi.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lijek RS, Luque SL, Liu Q, Parker D, Bae T, Weiser JN. 2012. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. Proc. Natl. Acad. Sci. U. S. A. 109:13823–13828. 10.1073/pnas.1208075109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reid G, Burton J. 2002. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 4:319–324. 10.1016/S1286-4579(02)01544-7 [DOI] [PubMed] [Google Scholar]
- 74. Reid G. 2008. Probiotic Lactobacilli for urogenital health in women. J. Clin. Gastroenterol. 42(Suppl 3 Pt 2):S234–S236. 10.1097/MCG.0b013e31817f1298 [DOI] [PubMed] [Google Scholar]
- 75. Walter J, Ley R. 2011. The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 65:411–429. 10.1146/annurev-micro-090110-102830 [DOI] [PubMed] [Google Scholar]
- 76. Ocaña VS, de Ruiz Holgado AA, Nader-Macías ME. 1999. Growth inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus paracasei subsp. paracasei isolated from the human vagina. FEMS Immunol. Med. Microbiol. 23:87–92. 10.1111/j.1574-695X.1999.tb01227.x [DOI] [PubMed] [Google Scholar]
- 77. Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, Holmes KK. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 174:1058–1063. 10.1093/infdis/174.5.1058 [DOI] [PubMed] [Google Scholar]
- 78. Varma P, Dinesh KR, Menon KK, Biswas R. 2010. Lactobacillus fermentum isolated from human colonic mucosal biopsy inhibits the growth and adhesion of enteric and foodborne pathogens. J. Food Sci. 75:M546–M551. 10.1111/j.1750-3841.2010.01818.x [DOI] [PubMed] [Google Scholar]
- 79. Drider D, Fimland G, Hechard Y, McMullen LM, Prevost H. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564–582. 10.1128/MMBR.00016-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Charlier C, Cretenet M, Even S, Le Loir Y. 2009. Interactions between Staphylococcus aureus and lactic acid bacteria: an old story with new perspectives. Int. J. Food Microbiol. 131:30–39. 10.1016/j.ijfoodmicro.2008.06.032 [DOI] [PubMed] [Google Scholar]
- 81. Li J, Wang W, Xu SX, Magarvey NA, McCormick JK. 2011. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. U. S. A. 108:3360–3365. 10.1073/pnas.1017431108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Netz DJ, Pohl R, Beck-Sickinger AG, Selmer T, Pierik AJ, Bastos Mdo C, Sahl HG. 2002. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J. Mol. Biol. 319:745–756. 10.1016/S0022-2836(02)00368-6 [DOI] [PubMed] [Google Scholar]
- 83. Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. 2010. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One 5:e10598. 10.1371/journal.pone.0010598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malone CL, Boles BR, Horswill AR. 2007. Biosynthesis of Staphylococcus aureus autoinducing peptides by using the synechocystis DnaB mini-intein. Appl. Environ. Microbiol. 73:6036–6044. 10.1128/AEM.00912-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dufour P, Jarraud S, Vandenesch F, Greenland T, Novick RP, Bes M, Etienne J, Lina G. 2002. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 184:1180–1186. 10.1128/jb.184.4.1180-1186.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Otto M, Echner H, Voelter W, Gotz F. 2001. Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69:1957–1960. 10.1128/IAI.69.3.1957-1960.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349. 10.1038/nature09074 [DOI] [PubMed] [Google Scholar]
- 88. Chen C, Krishnan V, Macon K, Manne K, Narayana SV, Schneewind O. 2013. Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J. Biol. Chem. 288:29440–29452. 10.1074/jbc.M113.502039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, Gallo RL. 2010. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Investig. Dermatol. 130:192–200. 10.1038/jid.2009.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Clark NC, Weigel LM, Patel JB, Tenover FC. 2005. Comparison of Tn1546-like elements in vancomycin-resistant Staphylococcus aureus isolates from Michigan and Pennsylvania. Antimicrob. Agents Chemother. 49:470–472. 10.1128/AAC.49.1.470-472.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Périchon B, Courvalin P. 2009. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4580–4587. 10.1128/AAC.00346-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhu W, Murray PR, Huskins WC, Jernigan JA, McDonald LC, Clark NC, Anderson KF, McDougal LK, Hageman JC, Olsen-Rasmussen M, Frace M, Alangaden GJ, Chenoweth C, Zervos MJ, Robinson-Dunn B, Schreckenberger PC, Reller LB, Rudrik JT, Patel JB. 2010. Dissemination of an Enterococcus Inc18-Like vanA plasmid associated with vancomycin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:4314–4320. 10.1128/AAC.00185-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Flannagan SE, Chow JW, Donabedian SM, Brown WJ, Perri MB, Zervos MJ, Ozawa Y, Clewell DB. 2003. Plasmid content of a vancomycin-resistant Enterococcus faecalis isolate from a patient also colonized by Staphylococcus aureus with a VanA phenotype. Antimicrob. Agents Chemother. 47:3954–3959. 10.1128/AAC.47.12.3954-3959.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Muscholl-Silberhorn A, Samberger E, Wirth R. 1997. Why does Staphylococcus aureus secrete an Enterococcus faecalis-specific pheromone? FEMS Microbiol. Lett. 157:261–266. 10.1111/j.1574-6968.1997.tb12782.x [DOI] [PubMed] [Google Scholar]
- 95. Sletvold H, Johnsen PJ, Wikmark OG, Simonsen GS, Sundsfjord A, Nielsen KM. 2010. Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J. Antimicrob. Chemother. 65:1894–1906. 10.1093/jac/dkq219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Arthur M, Depardieu F, Cabanie L, Reynolds P, Courvalin P. 1998. Requirement of the VanY and VanX D,D-peptidases for glycopeptide resistance in enterococci. Mol. Microbiol. 30:819–830. 10.1046/j.1365-2958.1998.01114.x [DOI] [PubMed] [Google Scholar]
- 97. Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. 2011. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 186:1666–1674. 10.4049/jimmunol.1002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A. 2010. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect. Immun. 78:4234–4242. 10.1128/IAI.00447-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B. 2009. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5:e1000703. 10.1371/journal.ppat.1000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McHugh KJ, Mandalapu S, Kolls JK, Ross TM, Alcorn JF. 2013. A novel outbred mouse model of 2009 pandemic influenza and bacterial co-infection severity. PLoS One 8:e82865. 10.1371/journal.pone.0082865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Harriott MM, Noverr MC. 2010. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob. Agents Chemother. 54:3746–3755. 10.1128/AAC.00573-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, Tulkens PM, Van Bambeke F. 2013. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 68:1455–1464. 10.1093/jac/dkt072 [DOI] [PubMed] [Google Scholar]


