Abstract
The invasion of polarized epithelial cells by Salmonella enterica requires the cooperative activity of the Salmonella pathogenicity island 1 (SPI1)-encoded type III secretion system (T3SS) and the SPI4-encoded adhesin SiiE. The invasion of polarized cells is more efficient than that of nonpolarized cells, and we observed the formation of clusters of bacteria on infected cells. Here we demonstrate that the invasion of polarized cells is a highly cooperative activity. Using a novel live-cell imaging approach, we visualized the cooperative entry of multiple bacteria into ruffles induced on the apical surfaces of polarized cells. The induction of membrane ruffles by activity of Salmonella enables otherwise noninvasive mutant strains to enter polarized host cells. Bacterial motility and chemotaxis were of lower importance for cooperativity in polarized-cell invasion. We propose that cooperative invasion is a key factor for the very efficient entry into polarized cells and a factor contributing to epithelial damage and intestinal inflammation.
INTRODUCTION
Polarized epithelial cells in the intestinal layer form an efficient barrier to protect the sterile host tissue against the entry of microbes from the rich intestinal flora. Sophisticated virulence mechanisms are required for food-borne pathogens to overcome this intestinal barrier (1). Salmonella enterica is an important Gram-negative pathogen with the ability to cause self-limiting gastroenteritis as well as the systemic infection typhoid fever (reviewed in reference 2). Entry of S. enterica into epithelial cells is an important virulence trait and may initiate the intracellular lifestyle, the spread to other organs (3, 4), and intestinal inflammation (5, 6). Salmonella deploys a trigger mechanism to induce a macropinocytosis-related process in nonphagocytic cells such as enterocytes. Effector proteins translocated by the Salmonella pathogenicity island 1 (SPI1)-encoded type III secretion system (T3SS) control the invasion process, and the contribution of the various effector proteins to the manipulation of the host cell actin cytoskeleton is well characterized (7). A subset of effector proteins of the SPI1 T3SS takes control of the host actin cytoskeleton, with SipA and SipC acting as direct nucleators of actin (8, 9) and SopE and SopE2 functioning as guanine nucleotide exchange factors (GEFs) for CDC42 and Rac (10). A further effector, SopB, has phosphoinositide phosphatase activity affecting the surface charge of the Salmonella-containing vacuole during the intracellular phase (11) and indirectly activates Rho GTPases (12). SPI1 T3SS-mediated invasion allows entry into various types of nonphagocytic cells but also triggers rapid cell death or pyroptosis in phagocytes (13). Additional and alternative SPI1 T3SS-independent entry mechanisms have recently been described (reviewed in reference 14); however, the SPI1 T3SS-mediated process appears to be the dominant mode of entry into nonphagocytic cells.
The dynamics of Salmonella invasion of nonpolarized cells have been studied in some detail (15–17). Recent time-resolved analyses of invasion by Salmonella using nonpolarized-epithelial-cell models suggested that near-surface swimming and collision with mitotic cells are important for target cell selection (18). Other authors proposed plasma membrane cholesterol as a critical parameter for target cell selection (19).
While most of the analyses of SPI1 T3SS-mediated invasion have been performed using nonpolarized-cell-culture models, oral infection of host organisms by S. enterica clearly results in more complex interactions, for example, with polarized enterocytes of the intestinal mucosa. Some features of the tissue architecture of the intestinal epithelium can be mimicked by polarized-epithelial-cell-culture models (20), and these models are valuable tools to study Salmonella virulence functions. Our recent investigations of the interaction of Salmonella with polarized epithelial cells revealed the requirement for additional virulence factors and distinct dynamics of the invasion process. One example is the role of the giant adhesin SiiE, the substrate of the SPI4-encoded type I secretion system (SPI4 T1SS) (21). Without the function of the SPI4 T1SS or SiiE, Salmonella is highly reduced in adhesion to and subsequent invasion of polarized epithelial cells, while SPI4 T1SS and SiiE functions are entirely redundant for the invasion of nonpolarized epithelial cells (22). We also determined that the efficiency of invasion of polarized cells is much higher than for nonpolarized epithelial cells (23), while intracellular proliferation of Salmonella in these cells appeared to be low.
These differences in the interaction of Salmonella with epithelial cells in different cell culture models prompted us to analyze the dynamics of invasion of polarized cells by Salmonella in detail. Here we report that invasion of polarized cells is amplified after initiation of membrane ruffling. The massive alteration of the apical membrane allows efficient entry of additional Salmonella, including strains that are otherwise unable to invade polarized cells. We also used a new model that allowed us to follow actin dynamics during infection of polarized cells in a time-resolved fashion.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
For the experiments described here, Salmonella enterica serovar Typhimurium strain SL1344 was the wild-type strain and mutant strains were isogenic to SL1344. Characteristics of strains used in this study are listed in Table 1. Mutant strains deficient in fliI, cheY, or cheZ were generated in the strain background of S. enterica serovar Typhimurium NCTC12023 using Red recombinase-mediated allelic exchange basically as described before (26), using pKD13 as the template for amplification with the oligonucleotides listed in Table 2. Proper deletions in kanamycin-resistant mutant clones were confirmed using the check primers listed in Table 2, and motility or chemotaxis defects were analyzed using swim plate assays. Confirmed mutant alleles were subsequently moved into SL1344 using P22 transduction (27). If required for live-cell imaging, strains harboring pFPV25.1 or pWRG435 for constitutive expression of enhanced green fluorescent protein (eGFP) or red fluorescent protein (Tag-RFP) were used.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| S. enterica serovar Typhimurium strains | ||
| SL1344 | Wild-type strain | Lab collection |
| SB161 | ΔinvG | 24 |
| M712 (Δ5) | ΔsipA sopABEE2 | 25 |
| MvP602 | ΔSPI4::aph | 21 |
| MvP1622 | ΔfliI::aph | This study |
| MvP1623 | ΔcheY::aph | This study |
| MvP1624 | ΔcheZ::aph | This study |
| MvP1627 | ΔinvG ΔSPI4::aph | This study |
| Plasmids | ||
| pFPV25.1 | PrpsM::eGFP mut 3, constitutive GFP expression | Lab stock |
| pWRG435 | PrpsM::Tag-RFP in pFPV25.1 backbone | This study |
| pETcoco-1 | Single-copy vector, Cmr | Novagen |
| p3589 | PrpsM::mCherry in pETcoco-1, Cmr | This study |
| p3590 | PrpsM::eGFPmut3 in pETcoco-1, Cmr | This study |
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| fliI-Del13-For | CTGGCTTACCGCGCTCGACAACTTTGAAGCCAAAATGGCGATTCCGGGGATCCGTCGACC |
| fliI-Del13-Rev | CGAGCGCCTGCAGAGAGTCCTCCCAGTCGGCCCGTTCAAATGTAGGCTGGAGCTGCTTCG |
| cheY-Del13-For | GTAGTATTTTATGGCGGATAAAGAGCTTAAATTTTTGGTTATTCCGGGGATCCGTCGACC |
| cheY-Del13-Rev | cgcatccTCACATGCCCAGTTTCTCAAAGATTTTGTTGAGTGTAGGCTGGAGCTGCTTCG |
| cheZ-Del13-For | CTGGGCATGTGAGGATGCGATGATGATGCAACCATCTATCATTCCGGGGATCCGTCGACC |
| cheZ-Del13-Rev | TTCGAACCCGATAAGCGCAGCGCCATCAGGTCAAAAAAGCTGTAGGCTGGAGCTGCTTCG |
| fliI-DelCheck-For | CGATCCAACGTTGCATCACG |
| cheY-DelCheck-For | ACGAAGCAAGTTGTGTGGTG |
| cheZ-DelCheck-For | AAAACCATTCGCGCCGATAG |
| K1-Red-Del | CAGTCATAGCCGAATAGCCT |
Bacteria were routinely cultured in LB broth or on LB agar containing kanamycin or carbenicillin at 50 μg ml−1 if required for selection of markers.
Cell lines and culture conditions.
The canine polarized kidney epithelial cell line MDCK was cultured as described before (22). Under our culture conditions, MDCK cells formed a polarized monolayer, as revealed by the formation of an apical brush border and continuous cell contacts by tight junctions. For control experiments with nonpolarized cells, the human epithelioid cell line HeLa was used as described before. For quantification of invasion by Salmonella strains, cell lines were routinely cultured in 24-well cell culture-treated multiwell plates. For imaging of infection, cells were cultured on glass coverslips for subsequent fixation and staining, or in glass-bottom chamber slides (Nunc) for live-cell imaging.
In order to follow Salmonella-induced actin remodeling in living host cells, we generated an MDCK cell line constitutively expressing LifeAct as fluorescent marker for F-actin. Briefly, 5.0 × 105 MDCK cells were seeded in a petri dish 24 h before transfection. Ten micrograms of plasmid DNA of LifeAct-EGFP (28) was dissolved in 63 μl of 2 M CaCl2. To this solution, 500 μl of HGBS (280 mM NaCl, 10 mM KCl, 1.5 mM Na2HPO4, 12 mM dextrose, 50 mM HEPES; pH 7.05) was added slowly during strong stirring. DNA was precipitated during incubation on ice for 20 min. Fifty microliters of this solution was added dropwise into the cell culture. Thereafter, transfected cells were incubated for 6 h at 37°C at 5.0% CO2. Finally, medium containing 100 μg ml−1 Geneticin (Gibco, Life Technologies, Germany) was added. Medium was changed daily until confluence of the cell layer was reached. At this stage, clones were seeded at densities of 5.0 × 104 to 1 × 106 cells per 90-mm petri dish, and the Geneticin concentration was increased to 150 μg ml−1. Clone K4 showed 100% LifeAct-GFP expressing cells and retained the typical morphology of MDCK cells, and the infection rates were similar to the nontransfected cells (see Fig. S1 in the supplemental material). Thus, clone K4 was used for subsequent analyses.
Infection experiments.
Bacterial strains were cultured in borosilicate glass test tubes with aeration by agitation on a roller drum. Overnight cultures were diluted 1:31 and subcultured in LB broth for 3.5 h for maximal invasiveness. The cultures were diluted to an optical density at 600 nm (OD600) of 0.2, and aliquots of this suspension were used to infect host cells with the indicated multiplicity of infection (MOI) for 30 min. Infection was performed without centrifugation if not otherwise indicated. After incubation for various periods of time, noninternalized bacteria were removed by washing, and the remaining extracellular bacteria were killed by the addition of 100 μg ml−1 gentamicin and incubation for 1 h. The conditions for sequential infections are described in figure legends below (see Fig. 4 to 7).
For the quantification of internalization, infected cells were washed three times with phosphate-buffered saline (PBS), and lysed by addition of 0.5% deoxycholate in PBS. Internalized bacteria were quantified by plating lysates and inoculum onto agar plates. For sequential-infection experiments, equal amounts of the lysates were plated onto agar plates containing antibiotics selective for strain A or strain B. Statistical analyses were performed using SigmaPlot 11.2.
Microscopy and image analyses.
Imaging of fixed samples was performed on a wide-field microscope system, either Axiovert 200M using Neofluar 100× with a 1.30 numerical aperture (NA) or Plan-Apochromat 63× with 1.40 NA objectives (Zeiss). Images were acquired and further processed using AxioVision software (Zeiss).
Live-cell imaging was performed using a CellObserver microscopy system (Zeiss, Germany) equipped with Yokogawa spinning disc (SD) unit. Z stacks were acquired at maximal speed with intervals of 100 to 200 ms at distances between Z planes of 0.30 to 0.35 μm. Acquisitions were performed with a 63× water immersion objective with a numerical aperture of 1.333 and recorded with either a cooled charge-coupled device (CCD) camera (CoolSnap HQ2; Photometrics) with a chip of 1,040 by 1,392 pixels for high spatial resolution. Acquisition and processing of the time-lapse images were performed with ZEN 2011 (Zeiss). Images were deconvolved by Huygens V.4.2 (SVI; Hilversum, The Netherlands) using a theoretical point spread function (PSF). Bleaching and Z drift were also corrected with Huygens.
Atomic force microscopy (AFM).
AFM measurements were conducted using the NanoWizard II AFM system (JPK Instruments AG, Berlin, Germany). High-resolution surface images were acquired by operating the AFM under ambient conditions in soft-contact mode using silicon nitride AFM probes with a nominal force constant of 0.06 N/m (SiNi; Budget Sensors, Wetzlar, Germany).
For AFM analyses, MDCK cells were infected with strain Δ5 (mutant strain lacking 5 effector genes, sipA, sopA, sopB, sopE, and sopE2) at an MOI of 50, fixed with 3% paraformaldehyde (PFA) 25 min postinfection (p.i.), and immediately used.
False-color height images were polynomial fitted and unsharpened mask filtered using JPK data processing software (JPK Instruments AG). Salmonella cells were pseudocolored using Corel Photo-Paint X5 software.
RESULTS
Salmonella forms clusters during invasion of polarized cells.
We previously investigated the interaction of Salmonella with polarized epithelial cells. In contrast to nonpolarized epithelial cells, highly increased levels of invasion were observed for the polarized epithelial cell lines MDCK, Caco-2, and T84 (22, 23). The microscopic investigation of infected polarized cells indicated that the high rates of invasion are also correlated to the formation of clusters of bacteria on individual host cells as well as intracellular microcolonies after internalization. We set out to investigate the mechanisms underlying the high rate of internalization and formation of microcolonies.
We followed the infection of polarized MDCK cells from the apical side by WT Salmonella (Fig. 1). Cells were fixed at various time points after the addition of the bacterial inoculum, and F-actin was visualized (Fig. 1A). F-actin-rich membrane ruffles were observed as early as 2 min postinfection (p.i.). These ruffles were mostly associated with a single bacterial cell. At 10 min p.i., the diameters of many membrane ruffles were increased, and many of the ruffles were associated with two or more bacteria. At 25 min p.i., membrane ruffles were highly reduced. F-actin-rich condensations were lost; however, the distribution of F-actin was different from that observed on the apical side of cells prior to invasion. The bacteria were mostly found in clusters. At later time points, such as 60 min p.i., F-actin-rich condensations were no longer visible, and F-actin staining was observed only at the cell-to-cell contacts. Most of the infected cells were associated with clusters of 5 or more bacteria.
FIG 1.
Dynamics of polarized-epithelial-cell infection by Salmonella. Polarized MDCK monolayers were infected with invasive WT S. enterica serovar Typhimurium expressing mCherry (red) at an MOI of 50. The infection was synchronized by centrifugation for 5 min. At various time points after infection, the cells were washed with PBS in order to remove nonadherent bacteria, fixed, and processed for epifluorescence microscopy. The actin cytoskeleton was labeled with phalloidin-Alexa 488 (white). (A) Representative images of patterns of bacterial association with MDCK cells at various time points after infection. Bar, 10 μm. (B) Quantification of host cell-associated bacteria. Magnification, ×63. Events of interaction of Salmonella with host cells, including adherent, invading, and already internalized bacteria, were scored per field of view. Next, for each event of interaction observed, the number of bacteria was quantified and categorized as single bacteria or groups of 2 to 5, 6 to 10, 11 to 15, or more than 15 bacteria per event. Bars indicate cumulative values from three independent experiments, each with three fields of view per time point.
We next quantified the frequency and size of events of bacterium-host cell interactions at various time points after infection (Fig. 1B). At 2 min p.i., most infected cells were associated with single bacteria. Clusters of 2 to 10 bacteria appeared from 10 min p.i. At 15 min p.i., we observed the appearance of larger clusters of bacteria with 11 or more bacteria associated with a single host cell. The number of these large clusters remained relatively constant at 60 min p.i., but a strong increase in smaller clusters of 2 to 10 bacteria was also evident at this time point.
We tested whether clustering of the bacteria is the result of a specific distribution pattern of ligands for adhesins of Salmonella or a result of the invasion process. The distributions of the WT, SPI1 T3SS- and SPI4 T1SS-deficient strains, and a SPI1 T3SS- and SPI1 T3SS-proficient noninvasive mutant strain on the apical side of MDCK were compared. For the latter, strain Δ5 with a deletion of the SPI1 T3SS effector genes sipA, sopA, sopB, sopE, and sopE2 was used. To directly visualize the distribution of surface-attached bacteria, surface topographies were scanned by atomic force microscopy (AFM) (Fig. 2). Frequent events of invasion this prominent membrane ruffles were observed for WT-infected cells, as well as formation of clusters of bacteria. No adherent bacteria were observed for the SPI4 strain, and few cell-associated bacteria were found for the SPI1 strain. Membrane ruffles were completely absent if cells were infected with strain Δ5. The adherent bacteria showed a random distribution, and only a few cells had a larger number of bacteria associated with the apical side (Fig. 2). We thus conclude that the biased distribution of Salmonella on polarized epithelial cells is a consequence of SPI1-mediated invasion process.
FIG 2.

Cluster formation during Salmonella invasion of polarized cells requires invasiveness. MDCK cells were infected with wild-type Salmonella (WT), strain M712 lacking the effector genes sipA and sopABEE2 and unable to induce trigger invasion (Δ5), siiE-deficient (SPI4) and invG-deficient (SPI1) strains at an MOI of 50 without centrifugation. Topographic height maps representative of the monolayer and adherent bacteria were recorded by AFM under ambient conditions. The false-color scale (green) indicates the height. For better visualization, adherent Salmonella cells were pseudocolored red. The inset for the WT-infected monolayer indicates clustering of bacteria. Bars, 5 μm (overview) and 2 μm (inset).
Salmonella bacteria preferentially infect polarized cells at sites of ongoing invasion.
In order to follow the dynamics of interaction of Salmonella with polarized epithelial cells, we deployed a novel infection model. MDCK cells were permanently transfected with a construct for the expression of LifeAct-GFP, which allows the visualization of F-actin formation and actin cytoskeleton dynamics in living cells. A MDCK clone was selected that stably expressed LifeAct-GFP and showed Salmonella adhesion and invasion to the same extent as the parental cell line (see Fig. S1 in the supplemental material). The MDCK LifeAct-GFP cells were used for infection experiments with spinning-disk microscopy for imaging with high spatial and temporal resolution (Fig. 3; see also Movie S1 in the supplemental material). Live-cell analyses enabled us to trace individual events of bacterium-host cell interactions. The contact of single Salmonella cells with the apical side resulted in rapid induction of membrane ruffles (Fig. 3), coinciding with the massive remodeling of the apical surface and the loss of the brush border. This was followed by a rapid accumulation of further bacteria at the same position. As shown in Fig. 1, host cells were either associated with a high number of bacteria or entirely devoid of adherent or invading Salmonella organisms. After an infection period of 30 min, only in very few cases were host cells with single bacteria observed. Following the fate of such infected cells, we found that the formation of clusters of bacteria was always linked to the initiation of ruffles, and we did not observe bacterial clusters in areas that had no previous ruffle formation.
FIG 3.
Salmonella preferentially enters polarized cells at sites of ongoing invasion. MDCK cells expressing LifeAct-GFP (green) were infected by WT Salmonella expressing Tag-RFP (red) at an MOI of 50 without centrifugation. The infection was analyzed by a Cell Observer SD microscopy system equipped with live-cell periphery (Zeiss). Z stacks were recorded every 10 s over a period of 30 min. Z stacks were assembled in Imaris, and projections are shown as indicated at 80% or 2.5% opacity of the GFP channel. The 2.5% opacity setting was used to visualize (partially) internalized bacteria and to label invading bacteria. Orthogonal projections are shown in the xy, yz, and xz planes, as indicated. The time stamp is in minutes and seconds. The corresponding time-lapse sequence is shown in Movie S1 in the supplemental material.
Since the generation time of Salmonella is about 30 min in the cell culture media used, we anticipate that the formation of the bacterial clusters is a function of the adhesion and invasion process and only to a minor extent the result of replication. In the example shown in Fig. 3 and in Movie S1 in the supplemental material, only the first invading Salmonella organisms showed cell division, and the clusters were formed by individual bacteria entering the site of ruffle formation. Based on this investigation, it appears that ruffle formation from initial infection events can promote subsequent infections at the same site.
Salmonella invades polarized epithelial cells in a cooperative manner.
We recently showed that the adhesion to and invasion of polarized epithelial cells requires the cooperative function both of the SPI1 T3SS and the SPI4 T1SS (22). The observed clustering and infection dynamics indicated that invasion is amplified if membrane ruffling is triggered by an initial invasion event. We speculated that such membrane ruffles may also be used for entry of mutant strains that are otherwise unable to invade polarized epithelial cells. To test this hypothesis, we performed sequential infection experiments (Fig. 4). Epithelial cells were subjected to a first round of infection with WT Salmonella. After a washing to remove noninternalized bacteria, a second infection was performed with WT, SPI1-deficient, or SPI4-deficient strains. Noninternalized bacteria were removed by washing, and the remaining bacteria were killed by the addition of gentamicin (Fig. 4A). The number of internalized, gentamicin-protected bacteria in MDCK cells was determined by plating cell lysates onto agar plates with antibiotics selective for either WT or mutant strains. As a control, the same experimental setting was used for the infection of nonpolarized HeLa cells.
FIG 4.
Invasion of polarized epithelial cells by Salmonella is highly cooperative. (A) Experimental design of sequential-invasion experiments. (B and C) Infections were performed with polarized MDCK cells (B) or nonpolarized HeLa cells (C). Single infections by WT, SPI1, and SPI4 strains were quantified by gentamicin protection assays (open bars) at an MOI of 1. For sequential infection experiments (filled bars), host cells were infected with WT Salmonella for 25 min at an MOI of 1. Noninternalized cells were removed by washing. A second infection was performed at an MOI of 5 with the WT, SPI1, or SPI4 strain, as indicated by the arrows. Single and sequential infections were performed without centrifugation. After incubation for 25 min, noninternalized bacteria were removed by washing, and the remaining cells were killed by the addition of gentamicin. Subsequently, host cells were lysed by addition of PBS containing 0.5% deoxycholate, and numbers of internalized bacteria were determined by plating on agar plates containing antibiotics selective for either the strain of the first infection or the strain of the second infection. Means and standard deviations for triplicates are shown, and the data are representative of three independent experiments. Statistical analyses were performed by one-way analysis of variance (ANOVA): n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Single-infection experiments with WT, SPI1, and SPI4 strains showed high rates of internalization of the WT strain, while internalization of SPI1 and SPI4 strains was reduced by 4 and 2 orders of magnitude compared to the WT, respectively (Fig. 4B). These data were in accordance with our previous observations for the invasion of polarized epithelial cells by Salmonella (22, 23). If sequential invasion was performed with the WT strain following initial invasion by the WT, no significant difference in invasion was observed for polarized cells. The preinfection by WT followed by an infection with an SPI1 strain resulted in an about 113-fold increase of internalization of the SPI1 strain, although the numbers of intracellular bacteria were still very low. Prior infection of cells with the WT resulted in a >28-fold increase in uptake of the SPI4 strain, and the absolute amounts of internalized bacteria were about 10-fold below that of the WT strain (Fig. 4B). In infection experiments with nonpolarized HeLa cells, rates of uptake also increased after sequential infection but to a much lower extent than for polarized cells (Fig. 4C). Here, prior invasion with the WT strain increased the subsequent invasion of WT or SPI1 strains by a factor of 3. SPI4-mediated adhesion is not required for polarized-cell invasion, and no amplification of invasion of the SPI4-deficient strain was observed (Fig. 4C). The data suggest that the initial infection event triggered by WT Salmonella is able to partially restore the infection efficiently in SPI1- or SPI4-deficient strains, presumably by facilitating entry into the host cells.
To visualize this process, we conducted subsequent experiments in which each strain was tagged either by GFP or Tag-RFP (Fig. 5). In experiments in which WT Salmonella bacteria were applied in the first as well as the second infection, bacterial cell clusters were composed randomly of either one of the strains or mixtures of both. If a combination of the WT plus the SPI1 strain or the WT plus the SPI4 strain was applied, we frequently found mixed clusters but very rarely events of invasion of SPI1 or SPI4 strains without an association with invading WT bacteria. These observations are in line with a cooperative invasion mechanism, with initial ruffle induction being triggered by WT Salmonella, enabling noninvasive strains to enter polarized epithelial cells.
FIG 5.

Live-cell imaging of cooperative invasion. MDCK cells were infected from the apical side as described for Fig. 4. WT Salmonella expressing Tag-RFP (red) was used for the first infection at an MOI of 10 for 25 min, followed by a second infection at an MOI of 50 for 25 min with WT (A), SPI1-deficient (B), or SPI4-deficient (C) strains all expressing GFP (green). Host cell membranes were stained with FM 4-64 FX (white in merge). Arrowheads indicate sites of cooperative invasion by two strains. Detail views show representative events of cooperative invasion and cluster formation by single strains. Representative still images of a time lapse series of images acquired over a period of 30 min are shown. Bars, 5 μm.
The formation of membrane ruffles is a highly dynamic process, and Salmonella effectors trigger actin rearrangements underlying membrane ruffles and also control their termination, restoring the normal cytoskeleton architecture (29). If polarized-cell invasion is amplified by Salmonella-induced membrane ruffles, we anticipate that this effect is time dependent and restricted to the phase of most pronounced membrane ruffling. We performed sequential infection experiments without and with an interval of 60 min between the first and second infection (Fig. 6A). Data shown in Fig. 1 and live-cell imaging (data not shown) indicated that 60 min was sufficient to terminate ruffle formation and to restore the normal architecture of the apical side of polarized cells. Comparison of invasion rates in sequential infections showed that amplification of invasion was ablated if a second infection was performed with an interval of 60 min after the first invasion. To further analyze the kinetics of the amplification, sequential invasion of WT and SPI4 strains was performed with different intervals between the first and second infections (Fig. 6B). Amplification of invasion was observed with intervals of 0 and 15 min but was reduced if the interval between first and second invasion was 30 min or longer. Next, we varied the number of WT Salmonella cells for the first infection from an MOI of 1 to an MOI of 50, followed by a second infection with the SPI4 strain at an MOI of 5 (Fig. 6C). Only a small increase in internalized SPI4-deficient Salmonella bacteria was observed with increasing MOI of the first infection, indicating that cooperative invasion can occur at a low number of invasion events.
FIG 6.
Cooperative invasion is maximal after onset of ruffle formation. (A) Single and sequential infections of MDCK cells were performed as described for Fig. 4. The second infection with SPI1- or SPI4-deficient Salmonella was either performed directly after the washing step to remove WT cells from the first infection (black bars) or with a delay of 60 min after the washing step (hatched bars). White bars indicate the rate of internalization of SPI1- or SPI4-deficient Salmonella in single infections. (B) Sequential infection by WT (MOI, 1) and SPI4-deficient (MOI, 5) strains was performed as described for panel A, and the time interval between the first and second infection was varied as indicated. The amounts of internalized SPI4-deficient Salmonella are shown. Means and standard deviations of triplicate assays are shown, and the data are representative of three independent experiments. (C) Sequential infections were performed by first infection with WT Salmonella at various MOIs as indicated, and second infection with the SPI4 strain at an MOI of 5. Statistical significances between single and sequential infections (A), direct versus delayed infection (B), or an MOI of 1 versus a higher MOI (C) were analyzed by one-way ANOVA: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Bacterial motility and chemotaxis are not required for cooperative invasion.
Previous work on invasion dynamics in a nonpolarized-cell model suggested a role of near-surface swimming for target selection and triggering of entry (18). We generated mutant strains deficient in flagellum assembly (fliI) or in chemotaxis (cheY, cheZ). Invasion of polarized cells by WT, SPI1-deficient, and SPI4-deficient strains was compared to that by fliI-, cheY-, and cheZ-deficient strains (Fig. 7). We first tested the contribution of motility in our model of sequential invasion. The fliI and cheZ strains were slightly reduced in invasion of MDCK cells, while invasion of the cheY strain was similar to WT levels (Fig. 7A). In contrast to SPI1 and SPI4 mutant strains, internalization of fliI, cheY, and cheZ strains was not increased by prior infection of MDCK cells with WT Salmonella. Invasion of nonpolarized cells by SPI1, fliI, and cheZ strains was highly reduced, while invasion by SPI4 and cheY strains was similar to that of the WT (Fig. 7B). Prior infection by WT Salmonella leads to slightly increased internalization of SPI1, fliI, and cheZ mutant strains. The flagellum-mediated motility is likely to increase the frequency of bacterium-to-host cell contacts, and we anticipated that this feature, in part, could be mimicked by cell contacts triggered by centrifugation of the bacterial inoculum. Centrifugation did not restore the invasion defects of SPI1- or SPI4-deficient strains in MDCK cells and did not lead to detectable increase of the invasion rates of the WT strain (Fig. 7C). Centrifugation restored invasion of the fliI strain to WT levels, while a minor increase of invasion rates was observed for cheZ and cheY strains.
FIG 7.
Bacterial chemotaxis and motility are not required for cooperative invasion of polarized epithelial cells by Salmonella. Salmonella WT SL1344 or isogenic strains deficient in SPI1, SPI4, flagellum assembly (fliI), or chemotaxis (cheY, cheZ) were used for infections of MDCK (A and C) or HeLa cells (B). (A and B) Single and sequential experiments were performed without centrifugation as described for Fig. 4. Single and first infections and second infections were performed at an MOI of 1 and MOI 5, respectively. (C) Effect of centrifugation of invasion of MDCK cells by various strains. Infection was performed without a centrifugation step (open bars) or with centrifugation of 5 min at 500 × g after addition of inoculum (gray bars). Means and standard deviations are shown for triplicate samples, and the data sets are representative of three independent replicates. Statistical significance between single and sequential infections or assays without and with centrifugation was determined by Student's t test: n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Sequential-invasion experiments showed that initial invasion with WT Salmonella did not result in increased invasion of polarized cells by flagellum- or chemotaxis-deficient mutant strains. Interestingly, invasion of nonpolarized cells by the fliI strain was highly reduced, and prior infection with WT Salmonella resulted in increased internalization of the fliI strain. These results indicate that bacterial motility is of minor importance for interaction of Salmonella with host cells in a polarized-epithelial-cell model.
DISCUSSION
We investigated the dynamic interaction of Salmonella enterica with monolayers of polarized epithelial cells. This infection model more closely resembles the tissue organization of the intestinal epithelium, and we anticipated that penetration of polarized-cell monolayers would require the ability to destroy the barrier functions. While analyzing the invasion of polarized epithelial cells by Salmonella, we observed striking differences relative to the commonly used infection model of nonpolarized epithelial cells. Polarized cells were preferentially invaded in a cooperative manner, i.e., the sequential entry of additional bacteria at sites where an initial invasion event took place. This mechanism resulted in formation of clusters of bacteria on individual cells. Furthermore, cooperation increased entry of Salmonella strains that are otherwise unable to invade if host cells have been subjected to an initial event of invasion.
The dynamics of the infection process suggests that invasion is strongly promoted if membrane ruffles are already initiated on individual host cells. An initial invasion event by an adhesive and invasive bacterium promotes the binding and subsequent uptake of a large number of further bacteria, regardless of their ability to adhere to and invade polarized cells. This model is depicted in Fig. 8. We propose that membrane ruffles induced by initially invading bacteria result in a massive disturbance of the apical surface, leading to increased frequency of contacts between luminal Salmonella and host cells. The dense array of microvilli on the apical side represents an efficient barrier against bacterial invasion, and only a few bacteria are able to breach this barrier. Invasion by a founding bacterial invader leads to destruction of this barrier, allowing the subsequent entry of additional bacteria. The enhanced uptake of SPI1- or SPI4-deficient bacteria indicates that the requirements for virulence functions of such passenger bacteria are much lower. We define this process as cooperative invasion, with initial invaders facilitating the entry of additional bacteria. Future work is needed to reveal if this mechanism also leads in internalization of nonrelated bacteria such as intestinal microbiota. Cooperativity in host cell invasion by Salmonella has been described for nonpolarized infection models (16, 18, 30), but we demonstrate here that cooperativity is a much more pronounced phenomenon during infection of polarized epithelia.
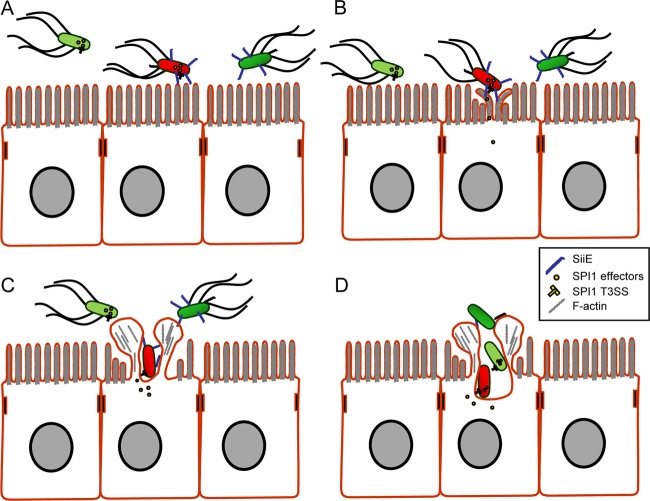
FIG 8.
Model for cooperative invasion of polarized epithelial cells by Salmonella. SPI1- and SPI4-proficient WT bacteria are depicted in red, and SPI1- or SPI4-deficient bacteria are depicted in dark green or light green, respectively. (A) Contact of luminal Salmonella with the enterocyte brush border. (B) Mediated by SPI4-dependent adhesion, Salmonella establishes close contact with the brush border membrane. The translocation by the SPI1 T3SS is subsequently initiated. (C) The activity of SPI1 T3SS effector proteins results in induction of membrane ruffles and effacement of the microvilli. The disturbance of the brush border surface results in increased contacts of Salmonella with enterocytes. This favors an increased frequency of contact between additional bacteria and enterocytes already infected by Salmonella. (D) The membrane ruffles induced by SPI1 T3SS-proficient Salmonella allow cooperative invasion by other WT Salmonella as well as by mutant strains otherwise unable to enter polarized epithelial cells. The loss of SiiE and the flagella during the invasion process is hypothetical.
Our data demonstrate significant differences between invasion dynamics of polarized and nonpolarized epithelial cells. One explanation of the divergent effects is that the host cell's surface is available for interaction with the bacteria. With nonpolarized host cells, cells are separated by areas of substrate or allowed to come into contact with one another. In contrast, a polarized epithelial cell layer consists of columnar cells in continuous tight contact with neighboring cells. The apical surface consists of a dense array of microvilli with a topology distinct from that of adherent nonpolarized cells. Once effacement of the brush border has been initiated, large amounts of plasma membrane and G-actin are available for the generation of membrane ruffles at the apical side. This situation is specific for polarized epithelial cells and may explain why Salmonella invasion of polarized cells is more efficient than that of nonpolarized cells. While polarized-cell models more closely resemble the intestinal mucosa, additional factors, such as the glycocalyx and the secretion of mucus, are missing.
Whether similar cooperative effects during cell invasion occur under in vivo conditions is open and probably difficult to analyze. The low numbers of internalized mutant strains in the experiments shown in Fig. 4 indicate that uptake of invasion-deficient bacteria by cooperative invasion are rare events. Most likely, only a low number of infecting Salmonella cells are in contact with enterocytes, in contrast to the high MOI applied in cell culture-based assays. Furthermore, in the intestine, a large number of commensal bacteria are present in addition to the exogenous pathogen. Interestingly, the early ultrastructural analysis by Takeuchi (31) of the invasion of guinea pig intestinal epithelium by Salmonella revealed formation of bacterial clusters concomitant with brush border effacement and formation of membrane ruffles. This observation indicates that cooperativity occurs during Salmonella infection of host tissue. Currently available techniques do not allow us to follow the dynamics of invasion of the intestinal epithelium in an in vivo setting. Only a few experimental approaches are able to follow the dynamics of Salmonella interaction with enterocytes in vivo, such as the study by Müller et al. (32). Intravital analyses of cooperativity and cluster formation demand in vivo imaging of the apical surface of the intestine, which remains a technical challenge.
What determines selective invasion of individual host cells? One cause may be the differential surface expression of molecules acting as receptors for SiiE, the SPI1 T3SS translocon, or other bacterial adhesion factors. Despite the fact that the cells used in this study are clonal, differences in the expression of glycosylated surface molecules on MDCK cells have been previously reported (33). We found that an adhesive but invasion-deficient strain was randomly distributed on the apical surface. Our previous analysis of the binding specificity of SiiE also did not indicate preferential binding to subpopulations of polarized cells (34). Thus, we exclude heterogeneity in adhesion to polarized cells as a cause of the formation of clusters.
Recent analyses of Salmonella invasion in nonpolarized-cell models propose that invasion preferentially occurs in mitotic host cells. This observation was explained by Santos et al. (19) by the requirement of SPI1 T3SS translocon subunit SipB for cholesterol-rich target membranes (35). In mitotic cells, an asymmetric distribution of cholesterol with higher concentrations in the outer leaflet of the plasma membrane was detected and postulated as course for preferential binding of Salmonella (19). An alternative explanation was provided by the work of Misselwitz et al. (18). The tracking of Salmonella motility during infection indicated preferential near-surface swimming (NSS) and the cessation of swimming at cells with disturbances of the surface. Such disturbances can be the more elevated cell shape during mitosis or membrane ruffles due to already initiated invasion. The NSS-mediated collision of Salmonella with obstacles on host cells was given as an explanation for the association of multiple bacteria with certain nonpolarized cells (18). Our data for the polarized-epithelial-cell model would also support the idea that cells with Salmonella-induced membrane ruffles are sites of preferential entry by additional bacteria. The analyses of various live-cell series of polarized-cell invasion did not indicate a preference of invasion of dividing cells (data not shown) and other, yet-unknown cell-individual factors to determine target site selection of the initially invading Salmonella.
Our analyses indicate only a minor contribution of motility and chemotaxis to invasion of polarized epithelial cells. In contrast, the invasion of nonpolarized cells by a nonmotile strain and a cheZ-deficient mutant was highly reduced. Flagellum-mediated motility may increase the frequency of contacts between bacteria and host cells. While the fliI strain lacks flagellum-mediated motility, the cheZ strain is motile but arrested in the tumbling mode of motility. In contrast, the cheY strain is arrested in the swimming mode of motility. Both nonmotile and tumbling-only phenotypes result in decreased frequency of contact with host cells. This lack of contact has much stronger effects in nonpolarized-cell invasion. These contact events appear to be of minor importance in polarized-cell invasion.
We observed that cooperative invasion leads to high bacterial burden of a subset of cells in a polarized epithelial layer. What are the consequences of this clustering? We have recently reported that the net replication of Salmonella in polarized epithelial cells is low and independent of SPI2 T3SS function (23). The multiple uptake events may lead to increased loss of vesicular containment of the bacteria and release into the cytosol. High bacterial load due to cytosolic replication has reported by Knodler et al. (36) as stimulus for extrusion of cells from an epithelial layer. This phenomenon was considered a means of bacterial dissemination. Those authors also reported that extruded, Salmonella-containing cells undergo inflammatory cell death, thus contributing to intestinal inflammation (36). Under our experimental conditions, we also observed loss of highly infected cells starting 90 to 120 min after infection, at a stage that likely is prior to increased burden due to intracellular replication (data not shown).
Cells that are subjected to cooperative invasion receive a high dose of SPI1 T3SS effector molecules during invasion, and the translocation of the effector proteins continues during the early stages of the intracellular lifestyle. Some of the SPI1 T3SS effectors and translocon proteins have been reported as inducers of caspase 1-dependent inflammatory cell death (37). We propose that epithelial cells that are highly infected due to cooperative invasion are subject to inflammatory cell death. Cell invasion by Salmonella allows the penetration of the epithelial barrier. Recent research revealed that invasion-dependent induction of an inflammatory response is important for Salmonella to outcompete the intestinal microbiota (reviewed in references 6 and 38), by mounting a host immune defense mainly affecting the commensal microflora (39), or by providing novel nutritional supplies mainly used by Salmonella (40). Competitive invasion of polarized cells will lead to an acceleration and amplification of these reactions, resulting in a rapid-growth benefit for infecting Salmonella in the host intestine.
We propose that cooperativity and amplification of invasion of polarized epithelial cells by Salmonella is a further community effort of this highly successful pathogen to efficiently colonize the host organism.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the DFG though grant P4 within Sonderforschungsbereich 944 ‘Dynamics and physiology of cellular microcompartments' and the Niedersächsisches Ministerium für Wissenschaft und Kultur. A.F.-L. was supported by a fellowship of the DAAD.
Footnotes
Published ahead of print 7 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00023-14.
REFERENCES
- 1.Sansonetti PJ. 2004. War and peace at mucosal surfaces. Nat. Rev. Immunol. 4:953–964. 10.1038/nri1499 [DOI] [PubMed] [Google Scholar]
- 2.de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. 2012. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 8:e1002933. 10.1371/journal.ppat.1002933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraga A, Ohlson MB, Miller SI. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53–66. 10.1038/nrmicro1788 [DOI] [PubMed] [Google Scholar]
- 4.Jantsch J, Chikkaballi D, Hensel M. 2011. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol. Rev. 240:185–195. 10.1111/j.1600-065X.2010.00981.x [DOI] [PubMed] [Google Scholar]
- 5.Stecher B, Maier L, Hardt WD. 2013. ‘Blooming' in the gut: how dysbiosis might contribute to pathogen evolution. Nat. Rev. Microbiol. 11:277–284. 10.1038/nrmicro2989 [DOI] [PubMed] [Google Scholar]
- 6.Thiennimitr P, Winter SE, Baumler AJ. 2012. Salmonella, the host and its microbiota. Curr. Opin. Microbiol. 15:108–114. 10.1016/j.mib.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agbor TA, McCormick BA. 2011. Salmonella effectors: important players modulating host cell function during infection. Cell. Microbiol. 13:1858–1869. 10.1111/j.1462-5822.2011.01701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, Mooseker MS, Galan JE. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092–2095. 10.1126/science.283.5410.2092 [DOI] [PubMed] [Google Scholar]
- 9.Hayward RD, Koronakis V. 1999. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18:4926–4934. 10.1093/emboj/18.18.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friebel A, Ilchmann H, Aepfelbacher M, Ehrbar K, Machleidt W, Hardt WD. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035–34040. 10.1074/jbc.M100609200 [DOI] [PubMed] [Google Scholar]
- 11.Bakowski MA, Braun V, Lam GY, Yeung T, Do Heo W, Meyer T, Finlay BB, Grinstein S, Brumell JH. 2010. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe 7:453–462. 10.1016/j.chom.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Patel JC, Galan JE. 2006. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J. Cell Biol. 175:453–463. 10.1083/jcb.200605144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7:99–109. 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velge P, Wiedemann A, Rosselin M, Abed N, Boumart Z, Chausse AM, Grepinet O, Namdari F, Roche SM, Rossignol A, Virlogeux-Payant I. 2012. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. Microbiologyopen 1:243–258. 10.1002/mbo3.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlumberger MC, Muller AJ, Ehrbar K, Winnen B, Duss I, Stecher B, Hardt WD. 2005. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc. Natl. Acad. Sci. U. S. A. 102:12548–12553. 10.1073/pnas.0503407102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cain RJ, Hayward RD, Koronakis V. 2008. Deciphering interplay between Salmonella invasion effectors. PLoS Pathog. 4:e1000037. 10.1371/journal.ppat.1000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrett CA, Jepson MA. 2009. Regulation of Salmonella-induced membrane ruffling by SipA differs in strains lacking other effectors. Cell. Microbiol. 11:475–487. 10.1111/j.1462-5822.2008.01268.x [DOI] [PubMed] [Google Scholar]
- 18.Misselwitz B, Barrett N, Kreibich S, Vonaesch P, Andritschke D, Rout S, Weidner K, Sormaz M, Songhet P, Horvath P, Chabria M, Vogel V, Spori DM, Jenny P, Hardt WD. 2012. Near surface swimming of Salmonella Typhimurium explains target-site selection and cooperative invasion. PLoS Pathog. 8:e1002810. 10.1371/journal.ppat.1002810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos AJ, Meinecke M, Fessler MB, Holden DW, Boucrot E. 2013. Preferential invasion of mitotic cells by Salmonella reveals that cell surface cholesterol is maximal during metaphase. J. Cell Sci. 126:2990–2996. 10.1242/jcs.115253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazmierczak BI, Mostov K, Engel JN. 2001. Interaction of bacterial pathogens with polarized epithelium. Annu. Rev. Microbiol. 55:407–435. 10.1146/annurev.micro.55.1.407 [DOI] [PubMed] [Google Scholar]
- 21.Gerlach RG, Jäckel D, Stecher B, Wagner C, Lupas L, Hardt WD, Hensel M. 2007. Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell. Microbiol. 9:1834–1850. 10.1111/j.1462-5822.2007.00919.x [DOI] [PubMed] [Google Scholar]
- 22.Gerlach RG, Claudio N, Rohde M, Jäckel D, Wagner C, Hensel M. 2008. Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell. Microbiol. 10:2364–2376. 10.1111/j.1462-5822.2008.01218.x [DOI] [PubMed] [Google Scholar]
- 23.Hölzer SU, Hensel M. 2012. Divergent roles of Salmonella pathogenicity island 2 and metabolic traits during interaction of S. enterica serovar Typhimurium with host cells. PLoS One 7:e33220. 10.1371/journal.pone.0033220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaniga K, Bossio JC, Galan JE. 1994. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol. Microbiol. 13:555–568. 10.1111/j.1365-2958.1994.tb00450.x [DOI] [PubMed] [Google Scholar]
- 25.Ehrbar K, Hapfelmeier S, Stecher B, Hardt WD. 2004. InvB is required for type III-dependent secretion of SopA in Salmonella enterica serovar Typhimurium. J. Bacteriol. 186:1215–1219. 10.1128/JB.186.4.1215-1219.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maloy SR, Stewart VL, Taylor RK. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 28.Riedl J, Flynn KC, Raducanu A, Gartner F, Beck G, Bosl M, Bradke F, Massberg S, Aszodi A, Sixt M, Wedlich-Söldner R. 2010. Lifeact mice for studying F-actin dynamics. Nat. Methods 7:168–169. 10.1038/nmeth0310-168 [DOI] [PubMed] [Google Scholar]
- 29.Galan JE, Zhou D. 2000. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl. Acad. Sci. U. S. A. 97:8754–8761. 10.1073/pnas.97.16.8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginocchio C, Pace J, Galan JE. 1992. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 89:5976–5980. 10.1073/pnas.89.13.5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi A. 1967. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109–136 [PMC free article] [PubMed] [Google Scholar]
- 32.Müller AJ, Kaiser P, Dittmar KE, Weber TC, Haueter S, Endt K, Songhet P, Zellweger C, Kremer M, Fehling HJ, Hardt WD. 2012. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 11:19–32. 10.1016/j.chom.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 33.Kovbasnjuk ON, Spring KR. 2000. The apical membrane glycocalyx of MDCK cells. J. Membr. Biol. 176:19–29. 10.1007/s002320001072 [DOI] [PubMed] [Google Scholar]
- 34.Wagner C, Barlag B, Gerlach RG, Deiwick J, Hensel M. 17 December 2013. The Salmonella enterica giant adhesin SiiE binds to polarized epithelial cells in a lectin-like manner. Cell Microbiol. 10.1111/cmi.12253 [DOI] [PubMed] [Google Scholar]
- 35.Hayward RD, Cain RJ, McGhie EJ, Phillips N, Garner MJ, Koronakis V. 2005. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol. Microbiol. 56:590–603. 10.1111/j.1365-2958.2005.04568.x [DOI] [PubMed] [Google Scholar]
- 36.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. 2010. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. U. S. A. 107:17733–17738. 10.1073/pnas.1006098107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink SL, Cookson BT. 2007. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 9:2562–2570. 10.1111/j.1462-5822.2007.01036.x [DOI] [PubMed] [Google Scholar]
- 38.Stecher B, Hardt WD. 2011. Mechanisms controlling pathogen colonization of the gut. Curr. Opin. Microbiol. 14:82–91. 10.1016/j.mib.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 39.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD. 2007. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5:2177–2189. 10.1371/journal.pbio.0050244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Baumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. 10.1038/nature09415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.