Abstract
Asymptomatic and persistent colonization of the upper respiratory tract by Neisseria meningitidis occurs despite elicitation of adaptive immune responses against surface antigens. A putative mechanism for facilitating host persistence of this bacterial commensal and pathogen is alterations in expression of surface antigens by simple sequence repeat (SSR)-mediated phase variation. We investigated how often phase variation occurs during persistent carriage by analyzing the SSRs of eight loci in multiple isolates from 21 carriers representative of 1 to 6 months carriage. Alterations in repeat number were detected by a GeneScan analysis and occurred at 0.06 mutations/gene/month of carriage. The expression states were determined by Western blotting and two genes, fetA and nadA, exhibited trends toward low expression states. A critical finding from our unique examination of combinatorial expression states, “phasotypes,” was for significant reductions in expression of multiple phase-variable surface proteins during persistent carriage of some strains. The immune responses in these carriers were examined by measuring variant-specific PorA IgG antibodies, capsular group Y IgG antibodies and serum bactericidal activity in concomitant serum samples. Persistent carriage was associated with high levels of specific IgG antibodies and serum bactericidal activity while recent strain acquisition correlated with a significant induction of antibodies. We conclude that phase-variable genes are driven into lower expression states during long-term persistent meningococcal carriage, in part due to continuous exposure to antibody-mediated selection, suggesting localized hypermutation has evolved to facilitate host persistence.
INTRODUCTION
Heightened mutation or recombination rates in specific regions of bacterial genomes are a feature of many bacterial commensals and pathogens (1–4). The mechanisms responsible for this localized hypermutation are diverse (e.g., site-specific recombination and slippage in repetitive DNA tracts) but generate high frequencies of variation in a stochastic manner usually prior to exposure to a selective pressure (5). These hypermutable loci are termed contingency loci and are speculated to have evolved as a mechanism for adaptation to rapid, unpredictable, and undetectable fluctuations in selective pressures. Localized hypermutation is therefore likely to contribute to both host adaptation and the disease phenotypes of bacteria, but few studies have examined the frequency and types of events occurring during natural infections by these organisms (6–9).
Infections by Neisseria meningitidis can result in septicemia and meningitis. Disease is rapid in onset and has high rates of mortality and morbidity with infants exhibiting the highest prevalence of disease. Vaccine-elicited serum bactericidal antibodies (SBA) provide effective protection against N. meningitidis infections (10, 11). Despite this, N. meningitidis normally exists as a commensal on the naso/oropharyngeal surfaces of humans with high frequencies of asymptomatic carriage in teenagers and young adults (12). Meningococci can persist in individual hosts for 6 to 12 months with carriage eliciting adaptive immune responses against surface determinants (13). The SBA of carriers correlates with protection against disease by homologous strains (14, 15) but is also thought to mediate prevention of carriage of homologous strains. Evidence for the latter is based on observations such as sequential carriage and replacement of one strain by another strain with antigenically mismatched outer membrane proteins (OMPs) (16) and herd protection associated with meningococcal serogroup C (MenC) and serogroup A (MenA) conjugate vaccination (17, 18). This raises a puzzling feature of meningococcal carriage: namely, how does this bacterium persist in the face of an adaptive immune response directed against surface antigens?
Microsatellites or simple sequence repeat (SSR) tracts are a major mechanism of localized hypermutation being subject to high rates of insertions and deletions of repeats during DNA replication (3). The reversible nature of SSR mutations has enabled evolution of phase variation (PV), i.e., high frequency, reversible alterations in phenotypic expression, for a diversity of surface molecules. In N. meningitidis, 40 to 60 genes per genome are subject to SSR-mediated PV with these simple sequence contingency loci (SSCL) encoding outer membrane proteins, enzymes involved in modulation of the surface-exposed glycans of lipopolysaccharide and pilin, and restriction-modification systems (9, 19). The SSRs are present either within the reading frame, resulting in “ON/OFF” switches in expression, or the promoter, driving modulations of gene expression. The majority of N. meningitidis SSRs are poly(C) or poly(G) tracts, but there are also tetra-, penta-, hepta-, and longer repeat units. The outer membrane proteins encoded by N. meningitidis SSCLs include a porin (PorA), iron-acquisition proteins (HmbR, HpuAB, and FetA), adhesins and invasins (Opc, Opa, and NadA), and autotransporters (MspA/AusI, NalP/AspA, and NadA). These proteins have important primary functions—such as iron acquisition (e.g., HpuA, HmbR) and adhesion (e.g., Opc, NadA, and MspA) to host tissues—associated with their expression and hence phase-variants in an ON or high expression state are likely to contribute to survival on nasopharyngeal surfaces (20, 21). Host persistence may be further facilitated by the secondary functions of some of these proteins; for example, Opc contributes to complement resistance (22, 23). Selection for expression of the phenotypes associated with these SSCL is, therefore, potentially strong during host persistence. Conversely, an opposing “counter” selection for phase variants in an OFF or reduced expression state is presumed to occur due to adaptive immune responses. The PorA and Opc proteins elicit bactericidal antibodies during carriage (13, 24) and porA phase variants, exhibiting reduced expression of the PorA protein, can mediate escape of bactericidal antibodies in vitro (25). PV of meningococcal SSCL could therefore confer a major advantage as N. meningitidis persists on mucosal surfaces during carriage; however, it is currently unclear whether phase-variable switches actually occur during the carriage of N. meningitidis in native hosts or whether there are particular patterns of switching.
Characterization of genetic variation during natural infections provides key indications of how bacterial commensals and pathogens colonize and persist in their hosts. Localized hypermutation is a phenomenon that is presumed to facilitate bacterial adaptation to the fluctuating and opposing selective pressures (e.g., adherence versus immune avoidance) encountered in host environments. In the present study, we set out to determine how often SSR-mediated PV occurs in meningococci during natural infections and to examine whether changes were driven by adaptive immune responses. Our studies provide the first definitive information on the frequencies of localized hypermutation occurring during long-term persistence of meningococci on the pharyngeal tissues of their native hosts. Critically, our unique combinatorial investigation of multiple phase-variable loci indicates that host persistence is associated with a heightened prevalence of “phasotypes” with lower expression states for multiple surface proteins. A simultaneous evaluation of the immune responses in these carriers suggests that selection for low-expression phasotypes is driven by continuous exposure to immune selective pressures.
MATERIALS AND METHODS
Bacterial isolate growth and characterization.
All meningococcal isolates were obtained from a carriage study performed in Nottingham University between November 2008 and May 2009 as described previously (16). The study was approved by the Nottingham University Medical School Ethics Committee, and written informed consent was obtained from all volunteers. Up to 20 single colonies were restreaked from initial selective plates onto Columbia chocolate agar plates (Oxoid). After overnight growth at 37°C in 5% CO2, sweeps of growth were used for preparation of glycerol stocks in brain heart infusion (BHI) broth plus 20% glycerol and genomic DNA by extraction with a DNeasy blood and tissue kit (Qiagen). For expression analyses, strains were initially grown overnight in BHI broth at 37°C and then diluted 10-fold in BHI broth, followed by growth for 4 to 6 h. Iron-repressed genes were induced by the addition of desferal at a working concentration of 30 μM to a mid-log-phase culture, followed by incubation for an additional 1 to 2 h.
Typing of two isolates per time point was described previously (16). Typing of additional isolates was performed by PCR amplification with relevant capsule specific PCR primers and primers specific for the relevant variable regions of the porA and fetA genes as described previously (see reference 16 and Table SA1 in the supplemental material).
Enumeration of SSR repeat numbers.
The SSRs of each gene (i.e., fetA, porA, opc, hpuA, hmbR, nadA, mspA, and nalP) for each strain were amplified and sequenced using published or newly designed primers spanning the repeat tract by previously described methods (16, 25, 26, 27) (see also Table SA1 in the supplemental material). The numbers of repeats in SSRs of additional isolates for each strain were determined by a GeneScan protocol as described elsewhere (28, 29). Briefly, SSRs were amplified by using two oligonucleotides, one of which was labeled on the 5′ end with a fluorescent dye (6-carboxyfluorescein [FAM]) and subjected to electrophoresis on an ABI3730 autosequencer in comparison to a GeneScan500LIZ size standard (Applied Biosystems). Product sizes were calculated using PeakScanner v1.0 (Applied Biosystems), and repeat numbers were determined by comparison to controls of known size and repeat number from the same strain and hence presumed to have identical flanking sequences. A subset of tracts were analyzed by sequencing to confirm repeat numbers (see Tables SA2 and SA3 in the supplemental material). Mononucleotide repeat tracts of ≥9 U produced two or more labeled products due to slippage during PCR amplification. The ratio of the areas of the primary and secondary peaks was determined, and if the ratio was >1.2 the primary peak was utilized for determining repeat number; otherwise, the peak with the largest size was selected.
Comparative measurements of gene/protein expression levels.
A semiquantitative measure of protein expression levels for different phase variants of each strain was performed by probing Western blots of meningococcal whole-cell lysates with specific antibodies (Table 1). Meningococcal isolates were grown to mid-log phase either in the presence or in the absence of an iron chelator in order to induce genes repressed by high iron levels (i.e., fetA and hpuA). Cells were washed twice before being resuspended in phosphate-buffered saline (PBS). Cell numbers were adjusted to a constant optical density at 550 nm and then mixed with 2× sodium dodecyl sulfate loading buffer at a 1:1 ratio. Cell lysates were electrophoresed on 8% polyacrylamide gels and transferred to polyvinylidene difluoride membranes by application of fixed current for 1 h. Membranes were blocked overnight at 4°C in PBST-milk (PBS–0.5% Tween 20 plus 5% skimmed milk) and subsequently probed with an appropriate primary antibody diluted in PBST-milk for 2 h. Membranes were then washed three times with PBST and probed with either a 1:2,000 dilution of an anti-mouse or anti-rabbit IgG-horseradish peroxidase conjugate for 1 h. Bound antibodies were detected with an enhanced chemiluminescence detection kit and X-ray film. Quantification of bands was performed by scanning of blots and quantification using ImageJ.
TABLE 1.
Longitudinal changes in expression states of phase variable meningococcal genes
| Clonal complex(es) and phasotype scorea | No. of isolates/observed period of carriage (%) |
|||
|---|---|---|---|---|
| Initial | 1 mo | 2–3 mos | 5–6 mos | |
| cc174 (FetA-Opc-NadA-HpuA-PorA-NalP-MspA) | ||||
| 10 | 5 (10) | 2 (5) | 0 | 0 |
| 9 | 4 (8) | 0 | 0 | 0 |
| 8 | 24 (50) | 22 (55) | 14 (38) | 0 |
| 7 | 8 (17) | 1 (3) | 7 (19) | 0 |
| 6 | 7 (15) | 15 (38) | 7 (19) | 15 (63) |
| 5 | 0 | 0 | 1 (3) | 2 (8) |
| 0–4 | 0 | 0 | 8 (21) | 7 (29) |
| Total | 48 | 40 | 37 | 24 |
| cc60 (FetA-Opc-HmbR-HpuA-PorA-NalP-MspA) | ||||
| 8 | 6 (20) | 17 (57) | 7 (58) | 6 (100) |
| 7 | 5 (17) | 5 (17) | 1 (8) | 0 |
| 6 | 11 (37) | 8 (27) | 4 (33) | 0 |
| 5 | 8 (27) | 0 | 0 | 0 |
| Total | 30 | 30 | 12 | 6 |
| cc167/cc23 (FetA-Opc-HpuA-PorA-NalP-MspA) | ||||
| 10–12 | 8 (21) | 5 (21) | 5 (14) | 0 |
| 9 | 6 (16) | 8 (33) | 11 (31) | 2 (8) |
| 8 | 2 (5) | 5 (21) | 8 (22) | 4 (17) |
| 7 | 5 (13) | 0 | 0 | 3 (13) |
| 6 | 5 (13) | 0 | 1 (3) | 2 (8) |
| 5 | 12 (32) | 6 (25) | 8 (22) | 8 (33) |
| 0–4 | 0 | 0 | 3 (8) | 5 (21) |
| Total | 38 | 24 | 36 | 24 |
| cc32 (FetA-Opc-NadA-HpuA-PorA-NalP-MspA) | ||||
| 10–12 | 6 (100) | 5 (83) | 4(67) | 3 (50) |
| 9 | 0 | 0 | 2 (33) | 3 (50) |
| 6 | 0 | 1 (17) | 0 | 0 |
| Total | 6 | 6 | 6 | 6 |
The phasotype score was determined from a combination of the expression states for six to seven genes (indicated in parentheses for each clonal complex) and in Table SA8 in the supplemental material.
Surface expression was analyzed by flow cytometry as described previously (25). Briefly, meningococcal cells were harvested from mid-log-phase cultures, washed and resuspended in assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM CaCl2, 0.05% Tween 20) containing primary antibody. Samples were incubated for 1 h at room temperature, washed thrice, and incubated for 1 h with a 1:100 dilution of an anti-mouse IgG-fluorescein isocyanate conjugate [Alexa Fluor 488 goat anti-mouse IgG (H+L); Life Technologies]. After three washes, the cells were resuspended in PBS containing 0.05% formalin. Samples were then analyzed on a fluorescence-activated cell sorter, and the mean fluorescence intensities were determined for each sample.
Expression of the porA gene was assessed by quantitative reverse transcription-PCR (RT-PCR). Meningococcal strains were grown to mid-log phase in Mueller-Hinton broth before being fixed in Bacteria Protect reagent (Qiagen). The total RNA was then extracted by using an RNeasy minikit with an additional RNase-free DNase step (Qiagen) to remove DNA contamination. The custom primers and probes used for gene expression assays are listed in Table SA1 in the supplemental material. RT-PCR assays were completed in MicroAmp Fast Optical 96-well reaction plates using a TaqMan RNA-to-CT 1-Step kit (Applied Biosystems). Reactions were set up in accordance with the Applied Biosystems protocol and analyzed on Applied Biosystems 7500. The housekeeping gene, gdh (glucose-6-phosphate 1-dehydrogenase), was used as an endogenous control gene, and H44/76 total RNA was used as a standard positive control. The fluorescence was recorded at the end of each extension step according to the probes present in the reaction, at wavelengths determined by the Applied Biosystems 7500 Fast System sequence detection software. Relative quantity values were calculated by the using the 2−ΔΔCT method. The gdh reaction was used as an endogenous control, and all samples were calibrated to the positive-control sample.
Quantification of anti-PorA and anti-CapY antibodies in serum samples.
An immunofluorescence bead-based immunoassay was utilized to quantify variant-specific PorA antibodies in serum samples. Eight PorA variants (P1.5-1,2-2, P1.19,15, P1.19-1,15-11, P1.5,2, P1.5-2,10, P1.7,16, P1.7-2,4, and P1.21,16) were cloned into an expression vector enabling production of His-tagged recombinant PorA proteins. A ninth protein (loopless, P1.−/−) was also produced from which the VR1 and VR2 regions had been deleted. Proteins were produced as inclusion bodies, purified on a His tag column in the presence of urea, refolded by droplet dilution, and finally dialyzed into an appropriate buffer. Polystyrene microspheres labeled with fluorophores (LiquiChip Carboxy beads; Bio-Rad) were coated with nickel-nitrilotriacetic acid. Each recombinant His-tagged PorA variant was bound to a spectrally distinct microsphere.
PorA-coupled fluorescent beads were mixed with four dilutions of each serum sample in LiquiChip assay buffer (PBS, 0.1% bovine serum albumin, 0.05% Triton X-100) in a 96-well filter plate and incubated for 30 min at room temperature on a rocking platform. Samples were washed three times and then incubated with a 1:25 dilution of a goat anti-human IgG R-phycoerythrin conjugate (ABD Serotec, Kidlington, United Kingdom) in LiquiChip assay buffer. Finally, samples were washed three times and analyzed in a LiquiChip200 workstation. Raw fluorescence data were converted into arbitrary units (AU) by comparison to a standard curve generated using serial dilutions of pooled sera from vaccinees who had been inoculated with either a MenBvac or MenNZB outer membrane vesicle vaccine (derived from two clinical studies MNB1 and MNB2, respectively). Separate standard curves were generated for each PorA variant; hence, comparisons between AU for each variant are not possible. The quality of each data set was assessed by statistical evaluation of the parallelism of the lines obtained for the standard curves using CombiStats version 5 (European Directorate for the Quality of Medicines and Healthcare, Council of Europe). The AU values for each variant were adjusted for nonspecific binding by subtraction of the AU value obtained for the loopless PorA variant; a value of 0.05 was arbitrarily assigned when binding to this control protein exceeded that to a particular PorA variant.
The amount of anti-CapY IgG antibodies in each serum samples were measured by enzyme-linked immunosorbent assay (ELISA) using purified serogroup Y capsular polysaccharide as a ligand as described in Gheesling et al. (30).
SBA assays.
Serum bactericidal antibody (SBA) assays were performed by standard techniques (31) in the Vaccine Evaluation Unit using a ST11 meningococcal strain expressing the serogroup Y antigen (M03-241125, CDC S1975, Y:P1.5,2:ST11, serotype 2a, fHBP-Oxford 2.2, NadA 2.49, and NHBA 29).
Statistical analyses.
Each carrier was examined for significant changes in repeat tract length between a pair of time points for every gene as follows. Repeat numbers were determined for six or more colonies for each time point (apart from four time points where fewer than six colonies were obtained). Repeat numbers were binned into two nonoverlapping categories for the two time points. The repeat number of each isolate was assigned to one of the two categories and placed into a contingency table. A difference was then deemed significant if a comparison yielded P < 0.05 in a Fisher exact test. Two-tailed Fisher exact tests were performed using GraphPad or Prism. Chi-squared tests were performed using an online program (www.physics.csbsju.edu/stats/). Geometric mean concentrations were calculated using Prism. Wilcoxon signed-rank tests were performed in R using the COINS package.
RESULTS
Frequent changes occur in the SSRs of eight genes encoding outer membrane proteins during persistent meningococcal carriage in the upper respiratory tracts of humans.
In order to examine the extent of localized hypermutation occurring during persistent meningococcal carriage, bacterial samples were obtained from the nasopharyngeal tissues of individuals colonized with the same meningococcal strain for up to 6 months. These samples were from a longitudinal study of meningococcal carriage performed with a cohort of 190 first-year students attending six catered halls at the University of Nottingham (16). This study was initiated in November 2008, 5 weeks after the start of term, and involved four time points (November [1st], December [2nd], February [3rd], and May [4th]). We have previously described the identification of persistent carriers and characterization of the colonizing strains (16). A subset of 21 persistent carriers were selected for further analysis. These carriers were colonized with one of six strains: Y:P1.21,16:F3-7:ST1466 (cc174, seven carriers), Y:P1.21,16:F3-7:ST8510 (cc174, one carrier), E:P1.5,2:F1-7:ST1383 (cc60, five carriers), Y:P1.5-1,10-1:F1-3:ST767 (cc167, three carriers), Y:P1.5-1,10-1:F4-1:ST1655 (cc23, four carriers), and B:P1.19,15:F5-1:ST5682 (cc32, one carrier). Ten of these carriers were persistently colonized for 5 to 6 months with the same strain, while six exhibited either clearance or replacement of the initial strain by the May (4th) time point, and the remainder were only examined at early time points. As described previously, oropharyngeal swabs were spread onto selective agar plates and then streaked to single colonies prior to overnight growth. Up to 20 single isolates were obtained by restreaking single colonies onto nonselective plates for preparation of glycerol stocks and DNA. Thus, all isolates were subject to minimal in vitro passage to reduce the potential for alterations in PV genotype. Where possible, six or more isolates were analyzed as a statistically representative sample. The similarity of each set of isolates was confirmed by PCR with primers specific for the relevant capsule, PorA and FetA type (data not shown).
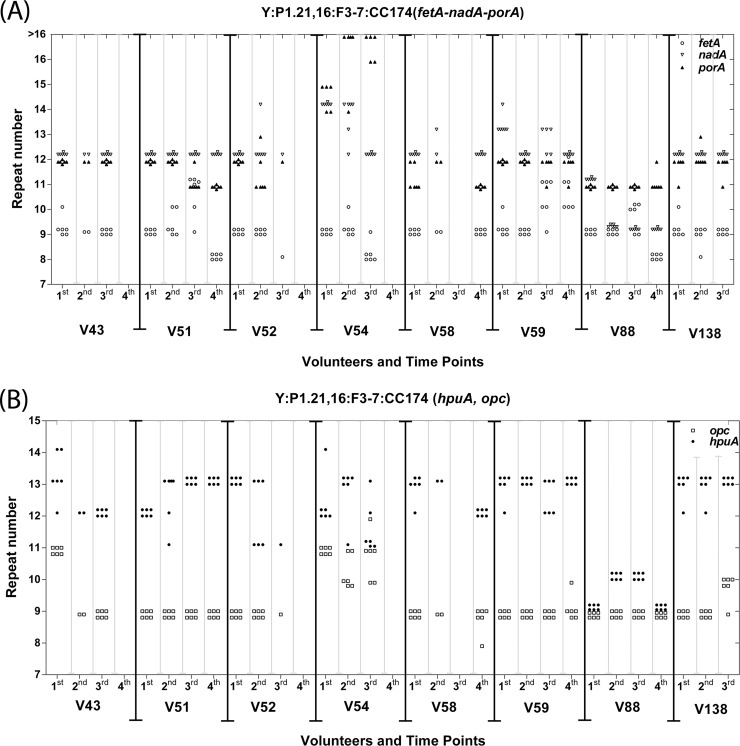
A coherent group of eight genes (porA, fetA, opc, hpuA, hmbR, nadA, mspA, and nalP) encoding phase-variable outer membrane proteins with known functional attributes were chosen for analysis. Most of the other phase-variable meningococcal genes encode glycosyltransferases, restriction-modification systems, or proteins of unknown function (9, 19). The repeat tract of each gene for each strain was amplified and sequenced. Two genes were not universally present, with hmbR only in the cc60 and cc32 strains and nadA only in the cc174 and cc32 strains (data not shown). The SSR was located in the reading frame for four genes and exhibited a consistent association between the number of repeats and whether the reading frame was “in-frame” or “out-of-frame” and hence predicted to have an ON or OFF expression state, respectively. The predicted ON expression states for each gene were as follows: 10G/13G, hpuA; 9G, hmbR; 10C/13C, nalP; and 6C/9C, mspA. For the other genes, the SSRs were either located upstream of (i.e., the 5′TAAA tract of nadA) or within the core promoter [poly(C) tracts in fetA and opc and a poly(G) tract in porA; see Fig. 1A]. For these genes, the expression state could not be directly predicted from the sequence data. Flanking sequences and repeat tracts were conserved between strains (data not shown) with the exception of the cc60 strains, which contained an interrupted SSR in fetA and a 1-nucleotide deletion in the putative −10 of porA (see Fig. SA1 in the supplemental material). A GeneScan assay was utilized to determine the repeat numbers for each gene in multiple isolates per time point (see Tables SA2 and SA3 in the supplemental material). Low levels of variation in tract lengths were observed within time points (see Fig. 1 and Fig. SA2 in the supplemental material for examples). Shifts in tract length between time points were observed for at least one gene in every carrier except V185, with specific trends being evident for some genes. For example, the fetA SSR in cc174 strains shifted from 9C to 8C or a mix of 10C and 11C variants in 5/8 carriers, while the nadA SSR remained at or shifted to 9 or 12 5′TAAA in all 8 of the cc174 carriers.
FIG 1.
Changes in repeat tract length of five genes during persistent carriage of cc174 strains. Multiple meningococcal isolates of the same strain were collected from eight volunteers (labeled as “V” in the figure) persistently colonized with a cc174 serogroup Y strain either ST1466 (V51, V52, V58, V59, V88, and V138) or ST8510 (V54; V43 exhibited replacement of ST8510 with ST1466 between the first and second time points). Up to six isolates per time point were analyzed for up to four time points (1st to 4th which were separated by 1, 2, or 3 months, respectively) for the number of simple sequence repeats in five phase-variable genes as follows: panel A, fetA [poly(C) tract, open circles], nadA (tetranucleotide 5′TAAA tract, open triangles), porA [poly(G) tract, filled triangles]; and panel B, hpuA [poly(G) tract, filled diamonds] and opc [poly(C) tract, open squares].
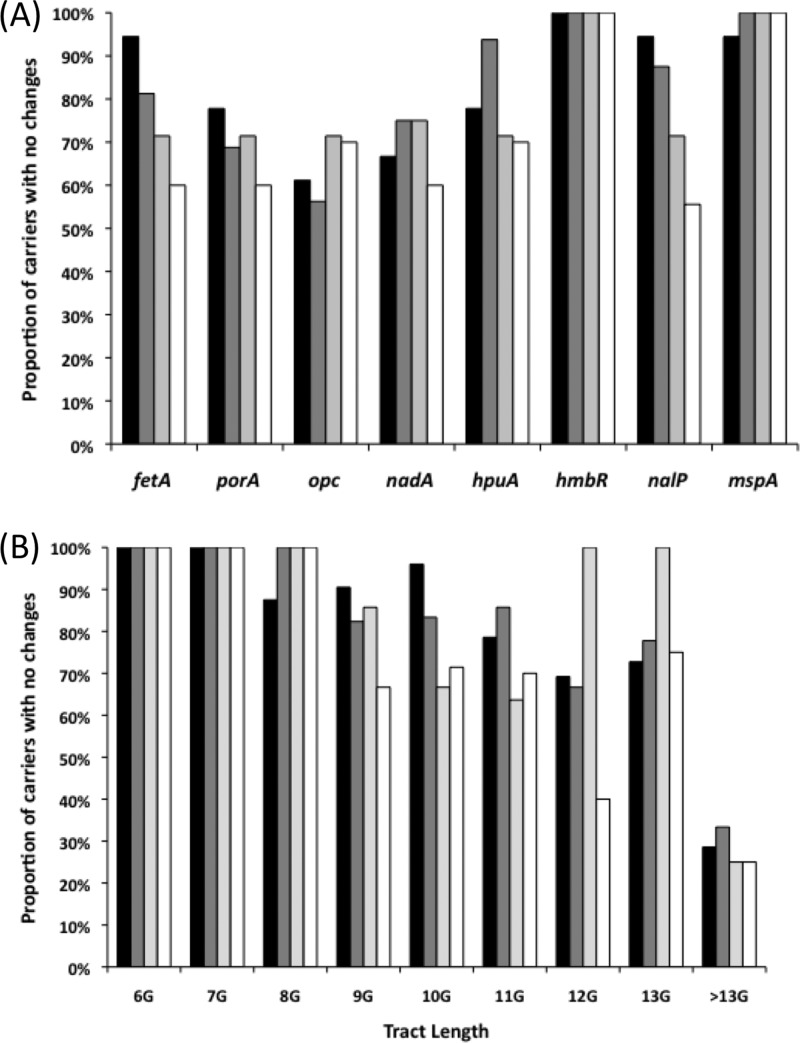
Comparisons between genes indicated very low levels of alterations in the repeat tracts of hmbR and mspA, whereas all of the other genes experienced alterations in 30 to 40% of the carriers (Fig. 2A). Both fetA and nalP exhibited significantly different frequencies of switching between the later, as opposed to the early time points (P < 0.05 for comparison of the 1st-to-2nd versus 1st-to-4th switching events using a two-tailed Fisher exact test), a finding indicative of a correlation between the length of carriage and the propensity for PV. A correlation between the mononucleotide repeat tract length and the propensity to PV was also observed, with no PV in short tracts (6 or 7 G/C repeats), very low levels in 8G/8C tracts, similar levels in tracts of 9 to 11 repeats, and a trend toward increasingly higher levels in longer tracts (Fig. 2B).
FIG 2.
Effect of persistent carriage on changes in the repeat tracts of phase-variable meningococcal genes. Each gene was examined for significant changes in repeat tract length between a pair of time points for carriers persistently colonized with the same meningococcal strain and plotted as the percentage of carriers with no significant changes. The total number of carrier samples examined for each of the four pairs of time points were as follows: fetA (18, 16, 7, and 10), porA (18, 16, 7, and 10), opc (18, 16, 7, and 10), nadA (9, 8, 4, and 5), hpuA (18, 16, 7, and 10), hmbR (5, 3, 1, and 2), nalP (18, 16, 7, and 10), and mspA (18, 16, 7, and 10). Time points were as follows: 1st to 2nd (1 month), black bars; 2nd to 3rd (2 months), dark gray bars; 3rd to 4th (3 months), light gray bars; and 1st-4th or 2nd-4th (5 or 6 months, respectively), white bars. (A) Changes per gene; (B) changes as a function of the repeat tract length relative to the tract length in the initial time point of each pair.
A switching rate per month of carriage was determined for a combination of the eight phase-variable genes with mononucleotide tracts of 9 or more repeats in the 1st/2nd time point. A significant switch in repeat tract between the 1st/2nd and 4th time points for a particular gene was calculated as described in Materials and Methods. The number of significant switches was divided by the total number of events analyzed (i.e., 21/54) and by the number of months of carriage (i.e., 5 or 6), resulting in an estimate of 0.06 mutations/gene/month of carriage. We conclude that there is a relatively high level of alterations in the mononucleotide repeat tracts of N. meningitidis SSCL during carriage and that gene type, repeat number, and length of host persistence are important determinants of mutability.
Persistent meningococcal carriage is associated with accumulation of low expression states for some phase-variable genes.
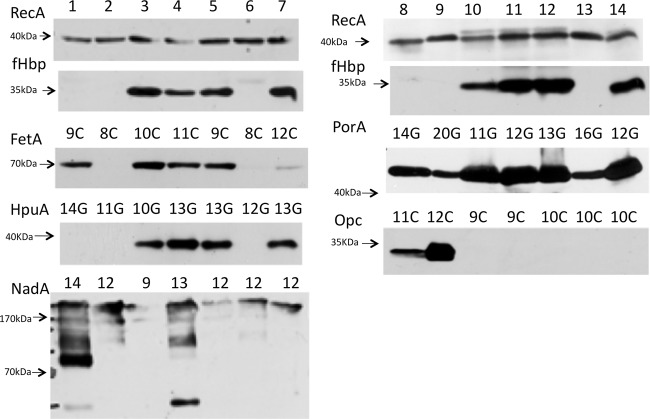
Alterations in the SSRs provide an indication of mutability but do not test whether there has been a change in phenotype. Detection of any phenotypic variation associated with these SSCL required analysis of whether alterations in SSRs mediated gene expression changes. Translational SSR-mediated switching has a predictable correlation between tract length and expression state. This was confirmed by analysis of SSRs predicted as “in-frame” (i.e., ON) or “out-of-frame” (i.e., OFF) for all four genes (Fig. 3; see also Fig. SA3 to SA7 in the supplemental material for hpuA; see Oldfield et al. [27] for mspA and nalP; and see Tauseef et al. [26] for hmbR). Transcriptional SSR-mediated switching causes changes in gene expression through modulation of promoter activity and hence is not readily predicted from repeat tract length. A series of Western blots were performed for each strain, generating associations between tract length and expression state for porA, fetA, nadA and opc (Fig. 3; see also Fig. SA3 to SA8 and Tables SA4 to SA6 in the supplemental material). All of the genes apart from porA exhibited at least three clear expression states ranging from low (>5-fold reduction) to intermediate to high expression (see Table SA5 in the supplemental material). For porA, high and/or intermediate levels of expression were detected for multiple tracts, while the lowest expression state was only ∼5-fold below the highest; larger differences but similar trends were detected in a quantitative RT-PCR analysis of a subset of strains (see Table SA7 in the supplemental material). To correlate surface expression with repeat number, six isolates were investigated by fluorescence-activated cell sorting (FACS). Variants of a cc32 strain with tracts of 10C or 9C exhibited 9- to 18-fold-higher surface expression of FetA than a variant with an 8C tract, while similar levels of high expression of porA were detected in variants of a cc174 with tracts of 11G, 12G, or 13G (see Fig. SA8 in the supplemental material).
FIG 3.
Comparison of protein expression levels for cc174 phase variants with different tract lengths. Whole-cell lysates were prepared from meningococcal cells grown to mid-log phase with (lanes 1 to 7) or without (lanes 8 to 14) induction of iron-repressed genes. Western blots were probed with 1:1,000 or 1:2,000 dilutions of primary antibodies/antisera (see Table S1A in the supplemental material), followed by an appropriate secondary antibody. Note that the an anti-F1-3 FetA variant mouse polyclonal and an antimeningococcal serotype P1.16 mouse MAb were used to detect FetA and PorA, respectively, whereas the other antisera recognize a wide range of antigenic types of the relevant protein. Repeat numbers are indicated as either the number of G's in a poly(G) tract or, for nadA, the number of 5′TAAA repeats. Lanes 1 and 8, N54.1; lanes 2 and 9, N343.5; lanes 3 and 10, N369.1; lanes 4 and 11, N352.3; lanes 5 and 12, N288.5; lanes 6 and 13, N343.2; lanes 7 and 14, N438.3. These isolates were from two different cc174 ST types: ST8510 (N54 and N343) and ST1466 (N288, N352, N369, and N438).
Expression state data were utilized to classify each SSR-gene combination into an expression code. Translational SSRs were coded into 0 and 2 for OFF and ON, respectively, and transcriptional SSRs were coded into 0, 1, and 2 representing undetectable/low, intermediate, and high expression states, respectively. Low and intermediate expression were defined as a >5-fold and 1.5- to 5-fold reductions, respectively, relative to high expression, as detected by Western blotting for fetA, opc, and nadA or a combination of Western blotting and RT-PCR for porA. A combined overview of all carriers for all time points detected genic differences in the proportions of carriage isolates with the highest expression states: PorA (91%), HpuA (66%), NalP (65%), FetA (42%), HmbR (24%), MspA (22%), Opc (18%), and NadA (7%) (see Table SA6 in the supplemental material). The largest temporal shift was observed for FetA, which started with 69% of isolates in the high state and ended with only 11% in this state by the 4th time point (i.e., after 5 to 6 months of carriage). A significant reduction in FetA expression (P = 0.008; Wilcoxon signed-rank test with continuity correction) was observed for a comparison of the mean initial and final expression states observed across all carriers (see Fig. SA9 in the supplemental material). Nonsignificant trends were noted for the other genes, with NadA and NalP also exhibiting reductions in expression state (see Fig. SA9 in the supplemental material). The expression of NadA was in the lowest state in all of the final time point samples, with the absence of a trend toward reductions in expression being due to this gene being in the lowest expression state in many of the initial samples.
Combinatorial reductions in phase-variable gene expression occur during persistent meningococcal carriage.
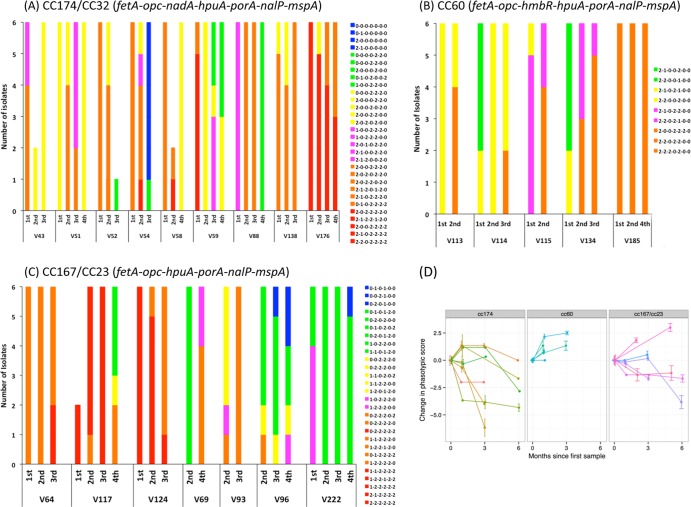
A major advantage of our analysis of multiple phase-variable genes is an ability to examine the combined expression states within an isolate. A combined code was generated for every isolate using the expression data for the six or seven phase-variable genes present and analyzed in each clonal group. These codes contain both genotypic and phenotypic information and hence are referred to as “phasotypes.” Seven genes—three with two states and four with three states—could generate 648 phasotypes. A total of 25 phasotypes were observed for the combined data for the 140 isolates of cc174, indicating a limited exploration of the potential expression states (see Table SA8 in the supplemental material).
Each phasotype contains information on the combined expression states of the genes, which is a simple sum of the individual gene states (i.e., 2-2-2-2-2-2-2 has score 14, 2-2-2-2-2-2-0 has score 12, etc.). The phasotypes were grouped by “expression score,” and the patterns of change between time points were examined for each carrier (Fig. 4A to C). The mean phasotype score was also calculated for each time point of every carrier (data not shown), and the change in this score was plotted against the months of carriage (Fig. 4D). The cc167 and cc23 strains were combined since they have identical PorA subtypes (P1.5-1,10-1) and gene combinations (i.e., the absence of nadA and hmbR). Only one carrier exhibited no change in expression score (V185; persistent carrier of a serogroup E, cc60 strain), while various patterns of changes were observed in other carriers. A reduction in phasotype score was observed in 11 of the 21 carriers between the initial and final time point of observed carriage but was variable between strains, with a shift toward lower expression phasotypes in 6/8, 4/7, 0/5, and 1/1 of the cc174, cc167/cc23, cc60, and cc32 strains, respectively. A significant change toward a lower mean phasotype score was detected for the cc174 carriers (P = 0.03 in a Wilcoxon rank test with continuity correction) but not for the cc167/cc23 or cc60 strains. The absence of a shift toward lower expression scores in the cc60 strains could be due to only one of these carriers exhibiting more than 3 months observed carriage and three of the genes having short repeat tracts (fetA, 6C; nalP, 7C; and mspA, 8C). Overall, persistent carriage is associated with a shift toward lower combined phasotype scores but exhibits a strain bias.
FIG 4.
Longitudinal alterations in the multiplex phase-variation expression states during persistent carriage of meningococcal strains. The expression states of phase-variable genes were determined from a combination of repeat number and direct assessments of expression state by Western blotting. The expression states of phase-variable loci were coded as 0 (OFF/low), 1 (intermediate), and 2 (ON/high) as described in the text. The combined pattern of expression states (i.e., phasotypes) for six or seven genes (as indicated in each panel) were determined for up to six isolates per time point. A total score was assigned to each phasotype by combining the expression scores of individual genes (i.e., seven genes in their maximum expression state scores 14), and then phasotypes with similar scores were color coded and plotted in panels A to C as follows: red (10 to 14), orange (8 and 9), magenta (7), yellow (6), green (4 and 5), and blue (0 to 3). A mean score for each time point of each volunteer was calculated from these total phasotype scores. (A) Eight cc174 carriers and one cc32 carrier (V176); (B) five cc60 carriers; (C) three cc167 and four cc23 carriers. (D) Graphs for three groups of strains (cc174, cc60, and cc167/cc23) showing the change in mean score relative to the initial time point when carriage was first detected.
The effect of persistence length on the reduction in phasotype score was examined for all 21 carriers. A trend was observed for reductions in phasotype score as a function of persistent carriage such that 44% (8/18), 50% (8/16), and 70% (7/10) of carriers exhibited reduced scores relative to the initial time point after 1, 2 to 3, or 5 to 6 months of carriage. Analysis of the phasotype scores for isolates provided further evidence of the effect of prolonged carriage (Table 1). Thus, for the cc174 isolates, there was a significant shift from 85% with a score of ≥7 in the initial time point to 100% with ≤6 after 5 to 6 months of carriage (P > 0.001 [chi-squared test]). Similarly, there was a reduction in expression score from 65% with ≥7 to 62% with ≤6 for the cc167/cc23 isolates (P = 0.002). Conversely, the cc60 strains exhibited a significant trend toward rising phasotype scores (P = 0.002); however, as discussed above, there was a lack of data for more than 3 months of carriage with this clonal complex. Thus, prolonged carriage is associated with reductions in phasotype score and the accumulation of phasotypes with lower expression states.
Variant-specific PorA antibodies can be detected using a multiplex microserology assay in sera from persistent meningococcal carriers.
In order to understand why switches in the phase-variable genes are occurring, it is important to characterize the selective pressures acting on the products of these genes. Adaptive immune responses exert a strong selective pressure on the surface antigens of meningococci with a significant potential impact on PV expression status. The phase-variable PorA protein is a major component of the outer membrane and contains two variable regions, VR1 and VR2, which are the main targets for bactericidal meningococcal antibodies and are also utilized for strain typing with VR specific monoclonal antibodies (MAbs) (32). We tested for the presence of a VR region specific PorA antibodies in serum samples collected concomitantly with nasopharyngeal swabs from the 21 persistent carriers. These sera were complemented with additional sera from the same carriage study (16); this included a set of sera from persistent noncarriers as controls and sera from volunteers exhibiting acquisition of carriage as a test of whether gain-of-carriage was associated with induction of meningococcal specific antibodies.
The levels of PorA-specific IgG antibodies were analyzed using a multiplex fluorescent-microsphere protocol and a LiquiChip workstation (Qiagen). A single combination of seven PorA variants was utilized for the majority of the assays, which included PorA variants with VRs similar or identical to those present in the carriage isolates and a modified PorA, lacking VR1 and VR2, used as a control for background reactivity (H. Patel et al., unpublished data). Antibody levels were measured in AU relative to pooled meningococcal vaccinee sera, and thus quantitative comparisons of reactivity between variants were not possible.
The specificity and reproducibility of the multiplex PorA assay was previously validated utilizing variant-specific PorA MAbs but has not been extensively tested with sera from carriers of known PorA variant type (Patel et al., unpublished data). We therefore analyzed the results for evidence of specificity and utility in detection of variant specific antibodies (see Tables SA9 and SA10 in the supplemental material and data not shown). Importantly, general reactivity to all PorA variants was noted as being very low, probably due to a nonspecific background, in persistent noncarriers (Table 2 and Fig. 5). High levels of monospecific activity were detected in at least one of the time points for five carriers (V51, V54, V64, V69, and V185) against the PorA variant protein containing one or both VRs matching the carriage isolate. Sera from other carriers exhibited a high level of reactivity mainly to the homologous PorA variant, while three carriers (V59, V88, and V124) had a pan-response against all variants, and three (V93, V138, and V176) had noncarrier levels of reactivity. Thus, some carriers exhibit monospecific responses, while others elicit various degrees of cross-reactivity, which could be due to cross-reactive epitopes, recent carriage of other strains, or a “bystander” induction of responses to all previously encountered PorA variants. The specificity of these responses was confirmed for a selection of samples by using VR specific MAbs to block binding of antibodies to one of the VRs (see Table SA10 in the supplemental material). Both VR1 and VR2 specific MAb antibodies reduced the binding of serum antibodies, but VR2 antibodies were generally more effective at blocking reactivity. Overall, the multiplex assay exhibited utility for detection of variant-specific IgG PorA antibodies in sera from meningococcal carriers.
TABLE 2.
Levels of anti-PorA, anti-CapY, and bactericidal antibodies in the sera of meningococcal carriers
| Clonal complex and antigen/antibody type or activity | Geometric mean antibody concn or titer at each time point (range; no. of samples)a |
|||
|---|---|---|---|---|
| 1st (November) | 2nd (December) | 3rd (March) | 4th (May) | |
| cc174 (Y:P1.21,16) | ||||
| P1.21,16/IgG | 1.4 (0.05–40.5; 8) | 1.5 (0.05–40.7; 8) | 3.4 (0.05–76.1; 8) | 1.7 (0.05–25.07; 7) |
| CapY/IgG | 1.8 (0.3–20.5; 5) | ND | 3.8 (0.4–17.1; 4) | 4.6 (0.6–27.5; 7) |
| CapY/SBA | 1,077 (3–8,192; 8) | 6,889 (4,096–16,384; 4) | 2,656 (128–8,192; 8) | 939 (32–4,096; 8) |
| cc60 (E:P1.5,2) | ||||
| P1.5,2/IgG | 1.5 (0.2–4.1; 3) | 2.7 (0.5–7.8; 3) | 1.5 (0.4–8.0; 3) | ND |
| cc167/cc23 (Y:P1.5-1,10-1) | ||||
| P1.5–2,10/IgG | 1.4 (0.5–6.8; 6) | 1.2 (0.3–2.7; 7) | 3.2 (0.3–17.2; 6) | 3.9 (2.8–8.0; 6) |
| CapY/IgG | 15.2 (8.6–38.5; 4) | 9.2 (7.5–11.2; 2) | 20.5 (6.4–29.8; 4) | 14.9 (4.4–67.3; 6) |
| CapY/SBA | 1,465 (3–16,384; 5) | 2,353 (128–16,384; 5) | 4,598 (2,048–16,384; 6) | 1,783 (1,024–8,192; 5) |
| cc32 (B:P1.19,15) | ||||
| P1.19,15 | 0.1 (NR; 1) | 0.1 (NR; 1) | 0.2 (NR; 1) | 0.3 (NR; 1) |
| Noncarriers | ||||
| PorA/IgG | 0.2 (0.1–0.5; 8) | 0.1 (0.1–0.6; 6) | 0.2 (0.1–0.6; 5) | 0.4 (0.2–0.9; 2) |
| CapY/IgG | 0.2 (0.1–0.3; 5) | ND | 0.2 (0.1–0.2; 4) | 0.2 (0.1–0.2; 4) |
| CapY/SBA | 5 (3–128; 8) | 7 (3–256; 5) | 3 (3–8; 3) | 9 (3–512; 8) |
Units for antibody concentrations are as follows: PorA (P1) variants, arbitrary units; serogroup Y capsular antigen (CapY), μg/ml; serum bactericidal activity (SBA) for an ST-11/Y strain, highest dilution resulting in ≥50% killing. ND, no data; NR, not relevant.
FIG 5.
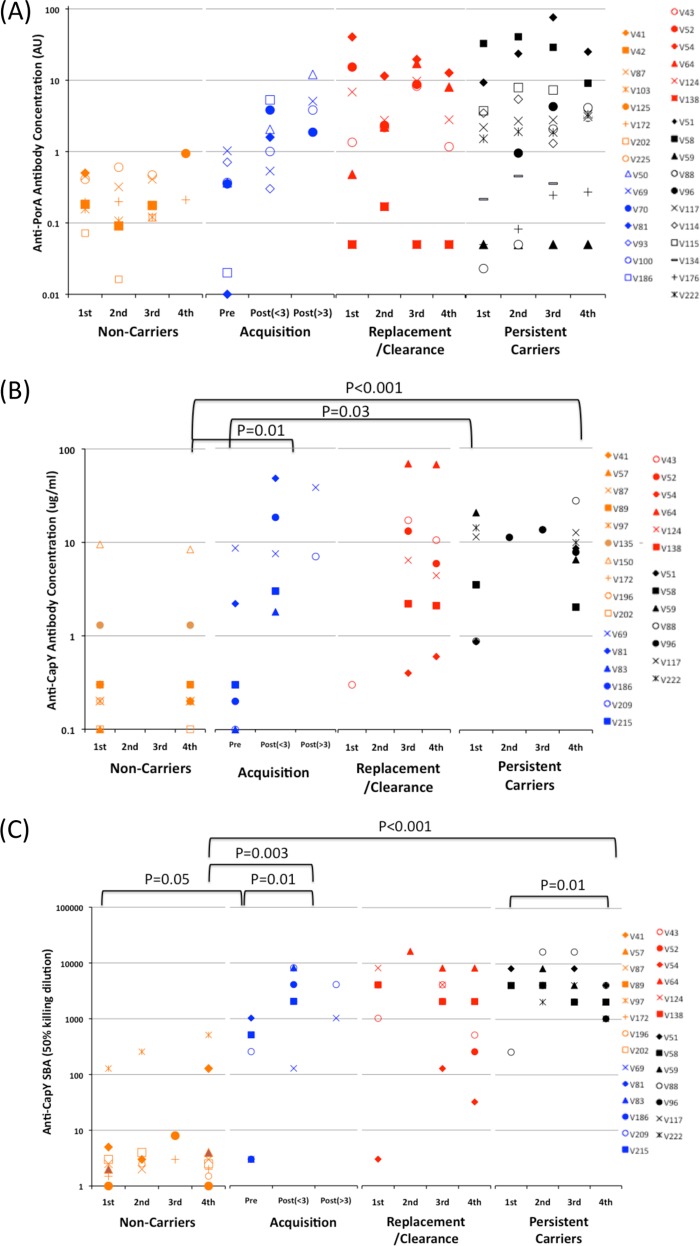
Antigen-specific immune responses in serum samples from meningococcal carriers. Volunteers were grouped into four categories according to the type of meningococcal carriage detected by nasopharyngeal swabbing as follows: persistent carriers, same strains detected at all time points; acquisition, progression from absence to presence of carriage with time points separated into precolonization (Pre) and less than or more than 3 months after colonization [i.e., Post(<3) and Post(>3), respectively]; replacement/clearance, initial strain either replaced by antigenically different strain or not detected in the 4th time point; and noncarriers, no meningococcal carriage detected at any time point. One to four sera were analyzed for each volunteer. (A) Anti-PorA IgG antibodies detected by a multiplex fluorescence-bead assay (expressed in arbitrary units [AU]). (B) Anti-CapY IgG antibodies detected by ELISA against purified capsular antigen. (C) Serum bactericidal activity against an ST-11 meningococcal strain expressing a serogroup Y capsular antigen. Values represent the dilution providing 50% killing.
Persistent meningococcal carriage is associated high levels of adaptive immune responses against surface determinants.
The studies described above indicated that variant-specific PorA antibodies could be readily detected in the sera of these carriers. However, since variability in the antigenic regions of PorA prevented direct comparisons between strains, antibody responses against the serogroup Y (MenY) capsular antigen were also investigated to facilitate comparisons between volunteers and due to the high prevalence of MenY strains among our persistent carrier samples. Immune responses to the serogroup Y capsular antigen were assessed by a CapY-IgG specific ELISA (i.e., with purified capsular polysaccharide). The sera were also tested for bactericidal antibodies using an ST-11 strain expressing a serogroup Y capsule and a P1.5,2 PorA variant. Since the PorA protein was mismatched in either both VRs (cc174) or VR2 (cc167/cc23), this assay will mainly detect bactericidal IgM and IgG anti-CapY antibodies. The expression states and/or sequence variation, compared to test strains, in other phase-variable genes were, however, unknown and may make a minor contribution to the bactericidal titer.
Sera from carriers subject to different types of carriage (acquisition, clearance and replacement, and persistent) were analyzed to determine the levels and temporal behavior of antibody responses (see Table SA9 in the supplemental material; Fig. 5). As observed for PorA, most of the noncarriers had low levels of CapY IgG antibodies and low SBA titers apart from three who had with either high CapY IgG (V135 and V150) or high SBA (V97) titers, which could be due to carriage of homologous meningococcal strains prior to enrollment in the study or vaccination with a CapY-conjugate vaccine. Acquisition of carriage was associated with a significant rise in variant-specific PorA antibodies and SBA titer and a nonsignificant increase in anti-CapY IgG. Antibody levels and SBA titers increased as a function of time postcolonization. Replacement or clearance of the initial strain was associated with high PorA and CapY IgG antibody concentrations and high SBA titers, similar to persistent carriers with no significant overall decrease following the loss of carriage, suggesting persistence of antibodies in the absence of ongoing stimulation. Significantly higher levels of reactivity were detected in sera from persistent carriers compared to noncarriers to the homologous PorA type and CapY at all time points (Fig. 5A and B). Similarly, SBA titers were higher in persistent carriers than in noncarriers, although a small but significant drop in SBA titer was found for the fourth time point, possibly due to the waning of a specific IgM response (Fig. 5C). A major increase between the November (i.e., the 1st time point) and later time points in both anti-PorA and anti-CapY antibodies was observed in two carriers, V88 and V43, while two others exhibited increases in PorA IgG (V64) or CapY IgG (V51). These rises were indicative of recent colonization and commensurate with the timing of sample collection, which was performed near the start of the term when high levels of spread of meningococcal strains are anticipated among this typical group of university students.
Relative amounts of antibodies were assessed for the 21 carriers subject to PV analysis in groups comparable to those utilized for the phasotype scores (Table 2). The cc174 and cc167/cc23 strains exhibited higher concentrations of CapY IgG and SBA titers than noncarriers at all time points (P < 0.05 with an unpaired t test) apart from a nonsignificant difference in CapY IgG at the 1st time point in cc174 carriers, probably due to recent colonization in three of these carriers (V43, V51, and V88) and hence insufficient time for elicitation of antibodies. The levels of variant-specific PorA antibodies were significantly higher in the cc167/23 and cc60 carriers than noncarriers (P < 0.05). Six of the cc174 carriers had 10-fold-higher levels of P1.21,16 specific PorA antibodies levels than noncarriers at two or more time points but specific antibodies were not detectable in two (V59 and V138) carriers.
In 9 of the 11 carriers exhibiting a drop in average phasotype score, high variant-specific PorA antibodies, CapY responses, and SBA titers were detected at multiple time points during persistence of the meningococcal clone. Of the others, one (V88) exhibited a late rise in PorA levels at the third time point, correlating with a major reduction in phasotype score, while in V54 the CapY IgG and SBA response were weak but the PorA specific responses were very high. The exception was for carrier V176, wherein no PorA antibodies could be detected. Collectively, we detected high levels of surface-antigen-specific and bactericidal antibodies in the majority of persistent carriers in which phase-variable reductions in OMP expression were observed.
DISCUSSION
Contingency loci are present in many pathogenic and commensal bacteria and are thought to generate high levels of genetic variation, enabling adaptation to fluctuations in the stringent selective pressures exerted by the host milieu. A significant gap in our understanding of contingency loci is the extent of their contributions to natural infections, whether asymptomatic or disease associated. We determined here the frequencies and patterns of SSR-mediated PV occurring during asymptomatic, persistent carriage of N. meningitidis, a pathogen/commensal with multiple contingency loci, in the upper respiratory tract of humans—their only hosts—and detected trends toward lower expression states for specific and combinations of phase-variable surface proteins (herein termed “phasotypes”). Our report highlights the importance of examining bacterial isolates from natural environments and implies a role for PV in facilitating persistent carriage of a bacterial pathogen.
By measuring variation in eight contingency loci, we estimate that the SSR-mediated PV rate for poly(G/C) tracts of 9 or more repeats is 0.06 mutations/gene/month of carriage for N. meningitidis in the upper respiratory tract in humans. In contrast, genetic variation in the VRs of PorA was only detected in one of these 21 carriers, and no variation was detected in the FetA VRs (16; data not shown), indicating an antigenic variation rate of 0.006/gene/month of carriage (1 mutation in 172 months of carriage). Thus, changes in the structure of an antigen by point mutation or recombination after lateral gene transfer are infrequent compared to phase-variable alterations in expression mediated by mutations in SSR tracts. There are 30 to 40 phase-variable genes in each N. meningitidis strain, most of which modify OMPs or the structures of surface determinants. Thus, localized hypermutation is the major source of genetic variability occurring during asymptomatic carriage of a meningococcal clone within an individual person.
Temporal fluctuations in the selective pressures acting on the different expression states of phase-variable loci are likely to be the major force driving any temporal patterns in alterations to the SSR tracts. Thus, selection for the ON state may be balanced and exceeded by selection for the OFF state during long-term host persistence of a meningococcal strain resulting in an observation of ON-to-OFF switching. A key finding from our phenotypic analysis of SSCL was for switches to lower the expression states of specific OMPs—namely, NadA, FetA, and NalP—during persistent carriage. The other phase-variable OMPs showed either variability in their expression states or, in the case of PorA, a continuous high expression state. It should be noted that all our results pertain to events on the mucosal surface since sampling of carriers was done by swabbing epithelial surfaces. One implication of the observed accumulation of OFF variants as a function of persistent carriage is that once colonization has been established within an individual, selection for high/ON expression states of phase-variable OMPs is reduced, and selection for OFF expression states can drive phase-variable loci into minimal expression states. The nadA gene was found in a low expression state in all isolates except a few from early time points (Fig. 1A; see Table SA2 in the supplemental material). Since this gene encodes an adhesin (33, 34), our results suggest that NadA is only required for initial colonization and is rapidly subject to selection for loss of expression as N. meningitidis persists in a host. Similar requirements during colonization may be ascribed to Opc and MspA, which encode known and putative adhesins (35, 36) and were also found mainly in low expression states (52% for Opc and 78% for MspA; see Table SA6 in the supplemental material). The FetA OMP showed a significant trend toward lower expression states as a function of persistence (see Fig. SA10 in the supplemental material) and a high prevalence of ON variants during early time points (see Table SA6 in the supplemental material). The prevalence of highly expressing variants of FetA was partially due to clonal expansion of the cc174 strains in one hall of residence (16). However, the siderophore-binding iron uptake attributes of FetA (37) may mean that siderophores are a potent source of iron during initial colonization by N. meningitidis but are replaced by other sources (transferrin and hemoglobin) as bacterial numbers increase and perturb the normal mucosal surface. Finally, NalP showed a trend toward an OFF expression state possibly connected with a growing requirement for establishment of a biofilm, which NalP antagonizes by cleavage of other surface meningococcal proteins (38, 39).
The discussion above indicates how selection for the functions of phase-variable OMPs could result in an elevated prevalence of high/ON expression at specific times during persistent carriage but, apart from NalP, has not elaborated on how selection for low/OFF expression states is exerted. A novel approach pioneered for C. jejuni isolates (40) and utilized in the present study was to examine isolates for alterations in the combined expression states of multiple phase-variable loci. The term “phasotypes” has been adopted herein (see also reference 41) to convey the idea that these types are based on conversion of nonarbitrary genotypic information (i.e., SSR number) into a potential phenotypic state. Phasotype does not indicate an actual in vivo phenotypic state since the expression of some of these phase-variable genes may be controlled by external signals (i.e., fetA is iron regulated). Nevertheless, changes in the phasotype are indicative of how the bacterial cells are responding to selective forces. We note that the phasotype system is portable and could be utilized by other laboratories for comparisons of the expression states of phase-variable genes.
A novel finding from our analysis of phasotypes is for an overall decrease in the surface expression of multiple OMPs during persistent mucosal carriage. This observation implies that selection is acting on the combination of genes present on the N. meningitidis surface. One explanation for this finding is that meningococcal cells expressing smaller amounts of OMPs have a growth advantage and replace high OMP expressers. This selective pressure is, however, probably very weak and easily counteracted by any selection for expression of a gene. A second explanation is that adaptive immune responses against surface OMPs select for antigenic variation and reduced expression states.
Multiple studies have shown that N. meningitidis carriage elicits serum IgG and bactericidal antibodies against a range of OMPs, including PorA, Opc, PilE, and NadA, and other surface molecules such as the capsule and lipopolysaccharide (13, 42). Mucosal IgA responses to whole N. meningitidis cells and to PorA have also been detected (43, 44). We demonstrated the presence of high levels of IgG antibodies specific for the homologous PorA variant in the majority of persistent carriers and high levels of anti-CapY specific IgG antibodies (Table 2 and Fig. 5). Furthermore, we show that these high levels persist through 6 months of continuous carriage, that specific antibodies are rapidly elicited upon acquisition of carriage, and that there is also a strong serum bactericidal activity response. Although we have yet to confirm whether antibody responses are elicited against all nine OMPs, our results suggest that a robust anti-OMP response is generated, as observed in other N. meningitidis carriers. Since selection of low-expression PorA phase variants has been observed in vitro (25), the absence of an effect of the specific antibodies on PorA expression levels is a contraindication for a role of the adaptive immune response. However, adaptive immune responses may exert only a weak effect that could be counteracted by a stronger selection for expression of this OMP. Variability in the strength of selection for the high/ON states of other OMPs may permit weak immune selection to drive these proteins into lower expression states. Variability in the patterns of switching between OMPs may also emanate from another source. Reduction in the combination of surface expressed OMPs implies an effect on the complexity of the bacterial surface rather than the amount of each protein. This could be due to the observed synergistic effects of bactericidal antibodies against multiple minor OMPs (45) or cross-linking and neutralization of bacterial cells by a polyclonal secretory IgA response (46), resulting in variability in which OMP is downregulated between isolates and carriers. Overall, our findings are supportive of the hypothesis that prolonged exposure to antibody-mediated selection drives N. meningitidis cells toward reduced expression states for phase-variable OMPs during persistent carriage in their natural hosts. Thus, PV may facilitate host persistence by mediating escape of adaptive immune responses while simultaneously rendering the resident N. meningitidis strain more sensitive to clearance by innate immune effectors or to competition and replacement by an antigenically divergent N. meningitidis strain.
The reductions in expression of OMPs have potential implications for meningococcal protein vaccines. Two of these proteins, NadA and PorA, are present within the recently licensed Bexsero vaccine, while NalP modifies NHBA another component of this vaccine (47, 48). The FetA protein has also been proposed as a potential vaccine candidate (49). The accumulation of low-expression variants of NadA and FetA indicates that vaccines, including these antigens, would not prevent persistent carriage but would be most likely to act during the initial stages of host colonization. Testing for herd immunity by meningococcal vaccines will therefore require a focus on prevention of acquisition of carriage in naive individuals and a careful design of vaccine trials to monitor the relative times of strain acquisition and vaccine-induced antibody responses.
We have defined here the frequency and patterns of alterations in SSCL encoding eight OMPs during persistent N. meningitidis carriage. Our results indicate that persistent carriage of N. meningitidis populations is associated with reductions in the expression of single and combinations of SSCLs, with evidence for adaptive immune responses being one of the major selective pressures driving the population into this state. Comparisons of the phasotypes of these carriage isolates with disease isolates of similar strain types can now be performed to determine whether particular phasotypes are required for meningococci to cause disease and whether the lack of disease associated with long-term carriage of meningococci is due simply to the specific immune responses or is also prevented by the accumulation of noninvasive phasotypes.
Supplementary Material
ACKNOWLEDGMENTS
M.A. received a Ph.D. scholarship from the government of Saudi Arabia. Sample collection was supported by research grants from Sanofi Pasteur and the Healthcare and Bioscience iNET.
We thank Salil Redkar and Jessica Abulimhe for contributions to analysis of the SSRs, Hema Patel for advice with setting up of the LiquiChip assays, and Mohamed Youssif for help with the FACS assays. We are grateful to Mumtaz Virji for provision of the Opc serum and Christoph Tang for the fHBP serum.
Footnotes
Published ahead of print 31 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01521-14.
REFERENCES
- 1.Moxon ER, Bayliss CD, Hood DW. 2007. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Annu. Rev. Genet. 40:307–333. 10.1146/annurev.genet.40.110405.090442 [DOI] [PubMed] [Google Scholar]
- 2.van der Woude MW, Baumler AJ. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17:581–611. 10.1128/CMR.17.3.581-611.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayliss CD. 2009. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol. Rev. 33:504–520. 10.1111/j.1574-6976.2009.00162.x [DOI] [PubMed] [Google Scholar]
- 4.Deitsch KW, Lukehart SA, Stringer JR. 2009. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat. Rev. Microbiol. 7:493–503. 10.1038/nrmicro2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moxon ER, Rainey PB, Nowak MA, Lenski R. 1994. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr. Biol. 4:24–33. 10.1016/S0960-9822(00)00005-1 [DOI] [PubMed] [Google Scholar]
- 6.Cholon DM, Cutter D, Richardson SK, Sethi S, Murphy TF, Look DC, St Geme JW., III 2008. Serial isolates of persistent Haemophilus influenzae in patients with chronic obstructive pulmonary disease express diminishing quantities of the HMW1 and HMW2 adhesins. Infect. Immun. 76:4463–4468. 10.1128/IAI.00499-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobbs MM, Sparling PF, Cohen MS, Shafer WM, Deal CD, Jerse AE. 2011. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front. Microbiol. 2:123. 10.3389/fmicb.2011.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods JP, Cannon JG. 1990. Variation in expression of class 1 and class 5 outer membrane proteins during nasopharyngeal carriage of Neisseria meningitidis. Infect. Immun. 58:569–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayliss CD, Field D, Moxon ER. 2001. The simple sequence contingency loci of Haemophilus influenzae and Neisseria meningitidis. J. Clin. Invest. 107:657–662. 10.1172/JCI12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granoff DM. 2010. Review of meningococcal group B vaccines. Clin. Infect. Dis. 50(Suppl 2):S54–S65. 10.1086/648966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truck J, Pollard AJ. 2010. Challenges in immunization against bacterial infection in children. Early Hum. Dev. 86:695–701. 10.1016/j.earlhumdev.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 12.Caugant DA, Maiden MC. 2009. Meningococcal carriage and disease–population biology and evolution. Vaccine 27(Suppl 2):B64–B70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordens JZ, Williams JN, Jones GR, Christodoulides M, Heckels JE. 2004. Development of immunity to serogroup B meningococci during carriage of Neisseria meningitidis in a cohort of university students. Infect. Immun. 72:6503–6510. 10.1128/IAI.72.11.6503-6510.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala'Aldeen DA, Bayliss CD. 2011. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J. Clin. Microbiol. 49:506–512. 10.1128/JCM.01322-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiden MC, Stuart JM. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359:1829–1831. 10.1016/S0140-6736(02)08679-8 [DOI] [PubMed] [Google Scholar]
- 18.Daugla D, Gami J, Gamougam K, Naibei N, Mbainadji L, Narbe M, Toralta J, Kodbesse B, Ngadoua C, Coldiron M, Fermon F, Page AL, Djingarey M, Hugonnet S, Harrison O, Rebbetts L, Tekletsion Y, Watkins E, Hill D, Caugant D, Chandramohan D, Hassan-King M, Manigart O, Nascimento M, Woukeu A, Trotter C, Stuart J, Maiden M, Greenwood B. 2014. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet 383:40–47. 10.1016/S0140-6736(13)61612-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders NJ, Jeffries AC, Peden JF, Hood DW, Tettelin H, Rappouli R, Moxon ER. 2000. Repeat-associated phase-variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol. Microbiol. 37:207–215. 10.1046/j.1365-2958.2000.02000.x [DOI] [PubMed] [Google Scholar]
- 20.Virji M. 2009. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 7:274–286. 10.1038/nrmicro2097 [DOI] [PubMed] [Google Scholar]
- 21.Perkins-Balding D, Ratliff-Griffin M, Stojiljkovic I. 2004. Iron transport systems in Neisseria meningitidis. Microbiol. Mol. Biol. Rev. 68:154–171. 10.1128/MMBR.68.1.154-171.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths NJ, Hill DJ, Borodina E, Sessions RB, Devos NI, Feron CM, Poolman JT, Virji M. 2011. Meningococcal surface fibril (Msf) binds to activated vitronectin and inhibits the terminal complement pathway to increase serum resistance. Mol. Microbiol. 82:1129–1149. 10.1111/j.1365-2958.2011.07876.x [DOI] [PubMed] [Google Scholar]
- 23.Hubert K, Pawlik MC, Claus H, Jarva H, Meri S, Vogel U. 2012. Opc expression, LPS immunotype switch, and pilin conversion contribute to serum resistance of unencapsulated meningococci. PLoS One 7:e45132. 10.1371/journal.pone.0045132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenqvist E, Hoiby EA, Wedege E, Kusecek B, Achtman M. 1993. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J. Infect. Dis. 167:1065–1073. 10.1093/infdis/167.5.1065 [DOI] [PubMed] [Google Scholar]
- 25.Tauseef I, Ali YM, Bayliss CD. 2013. Phase variation of PorA, a major outer membrane protein, mediates escape of bactericidal antibodies by Neisseria meningitidis. Infect. Immun. 81:1374–1380. 10.1128/IAI.01358-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tauseef I, Harrison OB, Wooldridge KG, Feavers IM, Neal KR, Gray SJ, Kriz P, Turner DP, Ala'Aldeen DA, Maiden MC, Bayliss CD. 2011. Influence of the combination and phase variation status of the haemoglobin receptors HmbR and HpuAB on meningococcal virulence. Microbiology 157:1446–1456. 10.1099/mic.0.046946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldfield NJ, Matar S, Bidmos FA, Alamro M, Neal KR, Turner DP, Bayliss CD, Ala'aldeen DA. 2013. Prevalence and phase-variable expression status of two autotransporters, NalP and MspA, in carriage and disease isolates of Neisseria meningitidis. PLoS One 8:e69746. 10.1371/journal.pone.0069746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Bolle X, Bayliss CD, Field D, van de Ven T, Saunders NJ, Hood DW, Moxon ER. 2000. The length of a tetranucleotide repeat tract in Haemophilus influenzae determines the phase variation rate of a gene with homology to type III DNA methyltransferases. Mol. Microbiol. 35:211–222. 10.1046/j.1365-2958.2000.01701.x [DOI] [PubMed] [Google Scholar]
- 29.Lucidarme J, Findlow J, Chan H, Feavers IM, Gray SJ, Kaczmarski EB, Parkhill J, Bai X, Borrow R, Bayliss CD. 2013. The distribution and ‘in vivo' phase variation status of haemoglobin receptors in invasive meningococcal serogroup B disease: genotypic and phenotypic analysis. PLoS One 8:e76932. 10.1371/journal.pone.0076932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gheesling LL, Carlone GM, Pais LB, Holder PF, Maslanka SE, Plikaytis BD, Achtman M, Densen P, Frasch CE, Kayhty H, Mays JP, Nencioni L, Peeters C, Phipps DC, Poolman JT, Rosenqvist E, Siber GR, Thiesen B, Tai J, Thompson CM, Vella PP, Wenger JD. 1994. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 32:1475–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, Kriz-Kuzemenska P, Lemmon RD, Lorange M, Peeters CC, Quataert S, Tai JY, Carlone GM, The Multilaboratory Study Group 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poolman JT, Kriz-Kuzemenska P, Ashton F, Bibb W, Dankert J, Demina A, Froholm LO, Hassan-King M, Jones DM, Lind I, Prakash K, Xujing H. 1995. Serotypes and subtypes of Neisseria meningitidis: results of an international study comparing sensitivities and specificities of monoclonal antibodies. Clin. Diagn. Lab. Immunol. 2:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metruccio MM, Pigozzi E, Roncarati D, Berlanda Scorza F, Norais N, Hill SA, Scarlato V, Delany I. 2009. A novel phase variation mechanism in the meningococcus driven by a ligand-responsive repressor and differential spacing of distal promoter elements. PLoS Pathog. 5:e1000710. 10.1371/journal.ppat.1000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tavano R, Capecchi B, Montanari P, Franzoso S, Marin O, Sztukowska M, Cecchini P, Segat D, Scarselli M, Arico B, Papini E. 2011. Mapping of the Neisseria meningitidis NadA cell-binding site: relevance of predicted α-helices in the NH2-terminal and dimeric coiled-coil regions. J. Bacteriol. 193:107–115. 10.1128/JB.00430-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner DP, Marietou AG, Johnston L, Ho KK, Rogers AJ, Wooldridge KG, Ala'Aldeen DA. 2006. Characterization of MspA, an immunogenic autotransporter protein that mediates adhesion to epithelial and endothelial cells in Neisseria meningitidis. Infect. Immun. 74:2957–2964. 10.1128/IAI.74.5.2957-2964.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virji M, Makepeace K, Peak IR, Ferguson DJ, Jennings MP, Moxon ER. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells; molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741–754. 10.1111/j.1365-2958.1995.mmi_18040741.x [DOI] [PubMed] [Google Scholar]
- 37.Carson SD, Klebba PE, Newton SM, Sparling PF. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arenas J, Nijland R, Rodriguez FJ, Bosma TN, Tommassen J. 2013. Involvement of three meningococcal surface-exposed proteins, the heparin-binding protein NhbA, the alpha-peptide of IgA protease and the autotransporter protease NalP, in initiation of biofilm formation. Mol. Microbiol. 87:254–268. 10.1111/mmi.12097 [DOI] [PubMed] [Google Scholar]
- 39.Roussel-Jazede V, Jongerius I, Bos MP, Tommassen J, van Ulsen P. 2010. NalP-mediated proteolytic release of lactoferrin-binding protein B from the meningococcal cell surface. Infect. Immun. 78:3083–3089. 10.1128/IAI.01193-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayliss CD, Bidmos FA, Anjum A, Manchev VT, Richards RL, Grossier JP, Wooldridge KG, Ketley JM, Barrow PA, Jones MA, Tretyakov MV. 2012. Phase variable genes of Campylobacter jejuni exhibit high mutation rates and specific mutational patterns but mutability is not the major determinant of population structure during host colonization. Nucleic Acids Res. 40:5876–5889. 10.1093/nar/gks246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bidmos FA, Bayliss CD. 2014. Genomic and global approaches to unravelling how hypermutable sequences influence bacterial pathogenesis. Pathogens 3:164–184. 10.3390/pathogens3010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litt DJ, Savino S, Beddek A, Comanducci M, Sandiford C, Stevens J, Levin M, Ison C, Pizza M, Rappuoli R, Kroll JS. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 190:1488–1497. 10.1086/424464 [DOI] [PubMed] [Google Scholar]
- 43.Horton RE, Stuart J, Christensen H, Borrow R, Guthrie T, Davenport V, Finn A, Williams NA, Heyderman RS. 2005. Influence of age and carriage status on salivary IgA to Neisseria meningitidis. Epidemiol. Infect. 133:883–889. 10.1017/S0950268805004097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson K, Neal KR, Howard C, Stockton J, Atkinson K, Scarth E, Moran J, Robins A, Todd I, Kaczmarski E, Gray S, Muscat I, Slack R, Ala'Aldeen DA. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun. 70:1301–1309. 10.1128/IAI.70.3.1301-1309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weynants VE, Feron CM, Goraj KK, Bos MP, Denoel PA, Verlant VG, Tommassen J, Peak IR, Judd RC, Jennings MP, Poolman JT. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 75:5434–5442. 10.1128/IAI.00411-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corthesy B. 2013. Multifacted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4:1–11. 10.3389/fimmu.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serruto D, Spadafina T, Ciucchi L, Lewis LA, Ram S, Tontini M, Santini L, Biolchi A, Seib KL, Giuliani MM, Donnelly JJ, Berti F, Savino S, Scarselli M, Costantino P, Kroll JS, O'Dwyer C, Qiu J, Plaut AG, Moxon R, Rappuoli R, Pizza M, Arico B. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770–3775. 10.1073/pnas.0915162107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, Carannante A, Deghmane AE, Fazio C, Frosch M, Frosi G, Gilchrist S, Giuliani MM, Hong E, Ledroit M, Lovaglio PG, Lucidarme J, Musilek M, Muzzi A, Oksnes J, Rigat F, Orlandi L, Stella M, Thompson D, Pizza M, Rappuoli R, Serruto D, Comanducci M, Boccadifuoco G, Donnelly JJ, Medini D, Borrow R. 2013. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect. Dis. 13:416–425. 10.1016/S1473-3099(13)70006-9 [DOI] [PubMed] [Google Scholar]
- 49.Urwin R, Russell JE, Thompson EA, Holmes EC, Feavers IM, Maiden MC. 2004. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect. Immun. 72:5955–5962. 10.1128/IAI.72.10.5955-5962.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.