Abstract
Allogeneic hematopoietic stem cell transplant (HSCT) recipients are at high risk for invasive aspergillosis. Whereas adoptive immunotherapy transferring donor-derived anti-Aspergillus TH1 cells has been shown to be beneficial for HSCT recipients suffering from invasive aspergillosis, little is known about the impact of commonly used immunosuppressants on the functional properties of anti-Aspergillus TH1 cells. Anti-Aspergillus TH1 cells were coincubated with different concentrations of methylprednisolone, cyclosporine (CsA), mycophenolic acid (MPA), the active component of mycophenolate mofetil, and rapamycin. Immunosuppressants were tested in concentrations reflecting common target levels in serum and in significantly lower and higher concentrations. Apoptosis of anti-Aspergillus TH1 cells, as well as proliferation and production of gamma interferon (IFN-γ) and CD154 upon restimulation, was evaluated in the presence and absence of immunosuppressive compounds. All dosages of CsA, MPA, and methylprednisolone significantly decreased the number of viable anti-Aspergillus TH1 cells in the cell culture, which was due partly to an impaired proliferative capacity of the cells and partly to an increased rate of apoptosis. In addition, CsA significantly decreased the number of IFN-γ-producing cells and had the highest impact of all immunosuppressants on IFN-γ levels in the supernatant. CsA also significantly decreased the expression of CD154 by anti-Aspergillus TH1 cells. Variant dosages of immunosuppressants exhibit particular effects on essential functional properties of anti-Aspergillus TH1 cells. Our findings may have an important impact on the design of clinical trials evaluating the therapeutic benefit of anti-Aspergillus TH1 cells in allogeneic HSCT recipients suffering from invasive aspergillosis.
INTRODUCTION
Patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) are at high risk for invasive fungal disease, in particular, for invasive aspergillosis (1–3). Despite the availability of new antifungal compounds, the mortality rate for invasive aspergillosis reaches up to 70% in this patient population (4, 5). While severe and prolonged neutropenia is the single most important risk factor for invasive aspergillosis, it has become clear that also adaptive immunity, in particular, the T helper (TH) cell population, plays a critical role in the host response to Aspergillus (6). In this regard, the majority of invasive aspergillosis occurs in HSCT recipients at a time when neutropenia has generally resolved but adaptive immune responses are still hampered (4, 7). Since the presence of TH1 lymphocytes correlates with a favorable outcome of invasive aspergillosis (8), it was speculated that adoptive transference of donor-derived anti-Aspergillus TH1 cells to HSCT recipients will improve the prognosis of the infection. In fact, a small study of 10 allogeneic transplant recipients suffering from invasive aspergillosis demonstrated that the adoptive transfer of specific in vitro-generated anti-Aspergillus TH1 cells resulted in a clinical benefit (9); 9 out of the 10 patients who had received anti-Aspergillus TH1 cells cleared the infection and survived, whereas 6 out of 13 patients not receiving immunotherapy died due to invasive aspergillosis. Unfortunately, allogeneic HSCT recipients often require immunosuppressive agents as prophylaxis or treatment of graft-versus-host disease (GvHD). These compounds further increase the risk for invasive fungal disease, since they may have multiple negative effects on different arms of the immune system. For example, glucocorticosteroids not only suppress the phagocytic function of monocytes and neutrophils but also may impair antigen presentation, T cell function, and the expression of proinflammatory cytokines (10). However, data on the effect of various dosages of immunosuppressive compounds on the functional properties of anti-Aspergillus TH1 cells are lacking, which, in turn, has an important impact on the adoptive immunotherapeutic approach of using anti-Aspergillus TH1 cells. We therefore assessed the effect of commonly used immunosuppressive agents on important functional properties of anti-Aspergillus TH1 cells, such as proliferation and cytokine secretion.
(Part of this work was presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Denver, CO, 10 to 13 September 2013.)
MATERIALS AND METHODS
Study subjects.
A total of 200 ml peripheral blood obtained from healthy volunteers (n = 8) was used for the generation of anti-Aspergillus TH1 cells. None of the individuals had a history of invasive fungal infection or had received any medication within 4 weeks of the blood draw. The protocol was approved by the local ethics committee.
Fungal antigens.
The water-soluble cellular extract of Aspergillus fumigatus (strain CBS 144-89) was prepared as described before and stored in aliquots at −80°C (11). The extract was tested for sterility and endotoxin concentration.
Immunosuppressive compounds.
The immunosuppressive compounds cyclosporine (CsA; Novartis Pharma, Nuremberg, Germany), methylprednisolone (Sanofi Aventis, Frankfurt, Germany), mycophenolic acid (MPA; Sigma-Aldrich, Steinheim, Germany), the active component of mycophenolate mofetil (MMF), and rapamycin (Pfizer, New York, NY, USA) were obtained commercially. All agents were stored and dissolved according to the manufacturers' instructions. At least three concentrations were tested for each compound. In addition to testing the concentrations reflecting common target levels in serum (e.g., 0.1 to 0.15 μg/ml for CsA, 1 to 3.5 μg/ml for MPA, and 0.001 to 0.02 μg/ml for rapamycin) or concentrations based on pharmacokinetic data and common dose recommendations (e.g., 2 mg of methylprednisolone/kg of body weight/day) (12–15), we evaluated each compound in significantly lower and higher concentrations. Specifically, MPA was used at concentrations of 0.5, 5.0, and 50 μg/ml, methylprednisolone at 0.025, 0.25, and 2.5 μg/ml, rapamycin at 0.001, 0.01, and 0.1 μg/ml, and CsA at 0.000015, 0.00015, 0.005, 0.0015, 0.03, 0.15, and 0.75 μg/ml.
Generation of anti-Aspergillus TH1 cells.
Anti-Aspergillus T cells were enriched and cultivated as previously described, with some modifications (16). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by means of Ficoll-Paque density gradient centrifugation, incubated for up to 16 h with antigens of A. fumigatus (7.5 μg/ml), and coincubated with monoclonal antibodies (MAbs) against CD40 (Miltenyi Biotech, Bergisch-Gladbach, Germany). Then, activated CD4+ T cells were isolated using the CD154 MicroBead kit (Miltenyi Biotech), followed by a cultivation period of up to 14 days using cytotoxic T lymphocyte (CTL) medium containing RPMI 1640, 100 IU/ml penicillin G, 100 mg/ml streptomycin (Life Technologies, Darmstadt, Germany), and 10% pooled heat-inactivated human serum. Cells were cultivated in the presence of recombinant human interleukin 2 (rhIL-2) (50 U/ml; Chiron, Ratingen, Germany) and restimulated twice during the culturing period using irradiated autologous Aspergillus antigen-loaded antigen-presenting cells (APCs), which were obtained from PBMCs by the adherence method as described previously in detail (16). Cultivated cells were restimulated again for 2 h using Aspergillus antigen-loaded APCs. Then, a second purification and expansion step was performed by means of the gamma interferon (IFN-γ) cytokine secretion assay (CSA; Miltenyi Biotech), followed by rapid expansion for up to 7 days as previously described (16).
Phenotypic and functional analyses of generated anti-Aspergillus TH1 cells.
Generated cells were phenotypically characterized by means of flow cytometry using MAbs against CD3, CD4, CD8, CD14, CD19, CD56, CD69, human leukocyte antigen DR (HLA-DR), and 7-amino-actinomycin D (7-AAD) and a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA).
The viability of anti-Aspergillus TH1 cells was estimated by 7-AAD staining and flow cytometry. Apoptosis and cell death were analyzed by means of flow cytometry using annexin V and propidium iodide (PI) staining (BD Biosciences) after 24 h of coincubation with and without the immunosuppressive agent. Cell numbers were assessed using trypan blue staining and conventional microscopy.
Functional analyses of anti-Aspergillus TH1 cells, such as proliferation and cytokine production upon restimulation, were performed in the absence and presence of the different immunosuppressive agents. The percentage of cytokine-producing cells was assessed by means of the intracellular cytokine cytometry (ICC) assay as described previously (16). In brief, anti-Aspergillus TH1 cells were stimulated with autologous antigen-loaded APCs (effector/stimulator cell ratio, 5:1) and coincubated with the MAbs CD28 (BD Biosciences) and CD49d (Coulter Immunotech) (2 μg/ml each) for 6 h. Brefeldin A (10 μg/ml; Sigma, Deisenhofen, Germany) was added for the last 5 h. Finally, cells were permeabilized and stained using MAbs against IFN-γ, tumor necrosis factor alpha (TNF-α), CD3, CD4, CD62L, CD45RO, and CD154 (BD Biosciences). Negative controls were performed using unloaded autologous APCs; cells stimulated with phorbol-12-myristate-13-acetate (PMA; 0.5 μg/ml; Sigma) and ionomycin (1 μg/ml; Sigma) served as positive controls.
The proliferation of anti-Aspergillus TH1 cells was assessed by means of the carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling assay (Molecular Probes, Eugene, OR, USA) as described before (16). Briefly, cells were CFSE labeled and cultivated in CTL medium for 6 days. After additional staining with MAbs against CD3, CD4, and HLA-DR, the percentages of proliferating cells were estimated on days 3 and 6 by flow cytometry.
Assessment of cytokine concentration.
Cytokine levels were measured in the supernatant obtained on day 3 of the CFSE assay. Concentrations were assessed by means of the cytometric bead array (CBA; BD Biosciences) according to the manufacturer's instructions. The lower level of detection was 1.4 pg/ml for IL-4 and 0.8 pg/ml for IFN-γ.
Statistical analyses.
Data were analyzed using GraphPad Prism (version 5.04 for Windows; GraphPad Software, San Diego, CA, USA) and compared with the paired t test. A two-sided P of <0.05 was considered to be statistically significant. Since the generation of Aspergillus-specific T cells did not always result in a sufficient number of cells, the numbers of experiments occasionally differed.
RESULTS
Number and phenotypic characterization of generated anti-Aspergillus TH1 cells.
For the generation of anti-Aspergillus TH1 cells, a total number of 1.1 × 108 PBMCs were stimulated with the antigen extract of A. fumigatus. In eight independent experiments, a final median number (range) of 3.0 × 107 (0.6 × 107 to 6.0 × 107) viable anti-Aspergillus T cells were received. Flow cytometry analysis revealed a highly purified homogenous CD3+ CD4+ (97.8% [range, 91.7 to 99.4%]), HLA-DR-expressing (>90%) T cell population. Whereas only small numbers of CD3+ CD8+ T cells (<3%), CD3− CD56+ NK cells (<1%), and CD3+ CD56+ NKT cells (<5%) were detected, no B cells or monocytes were seen in the cell products (data not shown). Upon restimulation with antigen-loaded APCs, a significant percentage of CD3+ CD4+ T cells expressed the antigen-specific T helper cell activation marker CD154 (median [range], 14.2% [3.3 to 31.2%]) and produced IFN-γ and TNF-α (medians [ranges], 9.45% [3.1 to 12.5%] and 16.8% [5.9 to 24.7%], respectively). Consistently, concentrations of IFN-γ in the supernatant were highly elevated (median [range], 2,001 pg/ml [475 to 2,663 pg/ml]), whereas IL-4 could not be detected, indicating that the generated cells were activated TH1 cells.
CsA, methylprednisolone, and MPA result in a significantly reduced number of anti-Aspergillus TH1 cells in the cell culture.
The efficacy of adoptively transferred anti-Aspergillus TH1 cells depends on the number of viable and functionally active effector cells. After 6 days of culture, the number of viable anti-Aspergillus TH1 cells was significantly lower when cells were coincubated with methylprednisolone and MPA than when they were left untreated (controls) (P < 0.05 in each case) (Fig. 1 and see Fig. S1 in the supplemental material). Similarly, CsA at dosages of 0.03, 0.15, and 0.75 μg/ml resulted in significantly lower numbers of viable anti-Aspergillus TH1 cells than in the control (P < 0.05 each) (Fig. 1 and S1). Whereas CsA at a dosage of 0.0015 μg/ml decreased the number of viable anti-Aspergillus TH1 cells in the culture by approximately 50%, CsA at concentrations of 0.0005 μg/ml and lower revealed no effect (Fig. S2). In contrast, none of the dosages of rapamycin tested had a significant impact on the cell number (Fig. 1).
FIG 1.
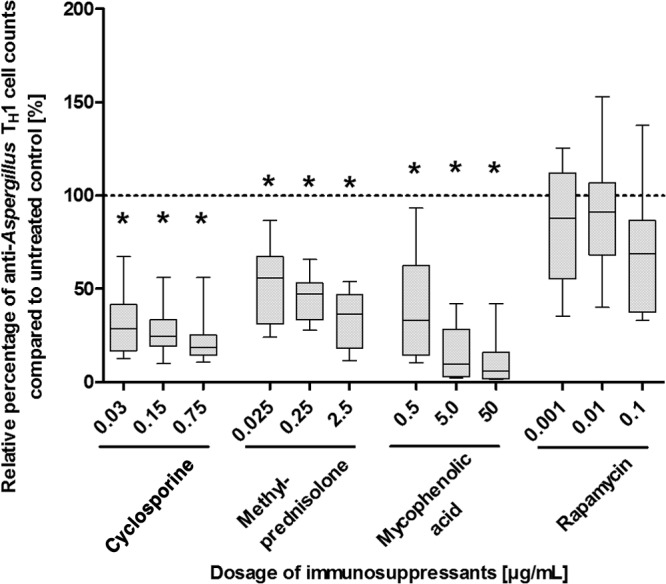
Effects of various dosages of immunosuppressants on cell counts of cultivated anti-Aspergillus TH1 cells. Counts of viable anti-Aspergillus TH1 cells were assessed after 6 days of cultivation with or without (control) different dosages of immunosuppressants. Presented are percentages of viable anti-Aspergillus TH1 cells relative to cell counts for the untreated controls (100% [dashed line]). Depicted are medians (horizontal bars), interquartile ranges (boxes), and ranges (whiskers). Results are derived from eight independent experiments. *, P < 0.05.
Since reduced cell numbers in the culture may be the result of a decreased proliferation and/or an increased rate of apoptosis, we evaluated both the proliferation and the apoptosis of anti-Aspergillus TH1 cells. Whereas approximately 90% of the anti-Aspergillus TH1 cells of the control cultivated in the absence of immunosuppressants underwent at least one cell cycle (median [range], 88% [46% to 98%]), methylprednisolone and MPA significantly reduced the proliferation of anti-Aspergillus TH1 cells (P < 0.05 in each case) (Fig. 2 and see Fig. S3 in the supplemental material). Notably, the strongest inhibition of proliferation was seen for MPA at dosages of 5 and 50 μg/ml. MPA did not significantly alter the rate of apoptosis of anti-Aspergillus TH1 cells, whereas apoptosis of the cells was increased by methylprednisolone; a statistically significant increase was seen for doses of 0.025 and 2.5 μg/ml (P < 0.05 in each case) (Fig. 3 and S4). Similarly, CSA dosages of 0.03 μg/ml and higher significantly decreased the proliferative response of anti-Aspergillus TH1 cells (P < 0.05 in each case) (Fig. 2), whereas CsA at concentrations of 0.0005 μg/ml and lower did not affect the proliferation (Fig. S5). CsA did not have a major impact on apoptosis (Fig. 3). In contrast to the other immunosuppressants evaluated, rapamycin did not significantly affect the proliferation and apoptosis of anti-Aspergillus TH1 cells (Fig. 2 and 3; see also Fig. S3 and S4). For all immunosuppressants tested, the effects on proliferation were similar when assessed at day 3 and at day 6 (data not shown).
FIG 2.
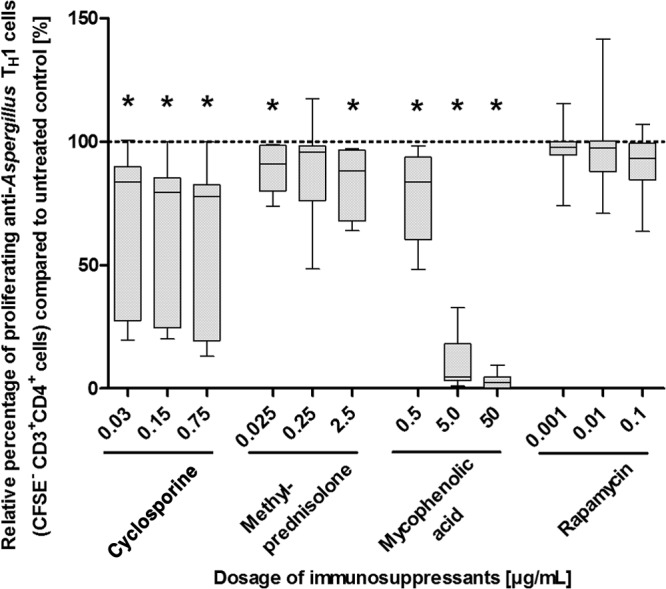
Effects of various dosages of immunosuppressants on the proliferation of anti-Aspergillus TH1 cells. Presented are percentages of proliferating anti-Aspergillus TH1 cells relative to cell counts for untreated controls (100%). Depicted are medians (horizontal bars), interquartile ranges (boxes), and ranges (whiskers). Results are derived from eight independent experiments. *, P < 0.05.
FIG 3.
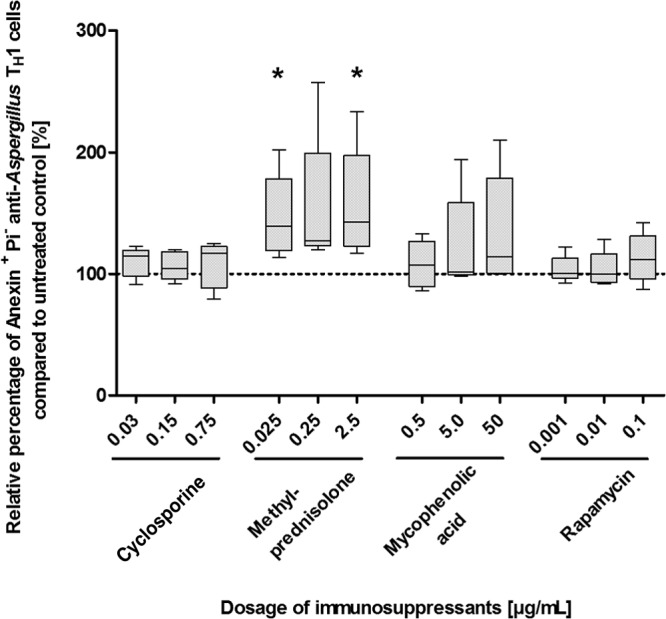
Effects of various dosages of immunosuppressants on apoptosis of anti-Aspergillus TH1 cells. Presented are percentages of apoptotic anti-Aspergillus TH1 cells relative to cell counts for untreated controls (100%). Depicted are medians (horizontal bars), interquartile ranges (boxes), and ranges (whiskers). Results are derived from five independent experiments. *, P < 0.05.
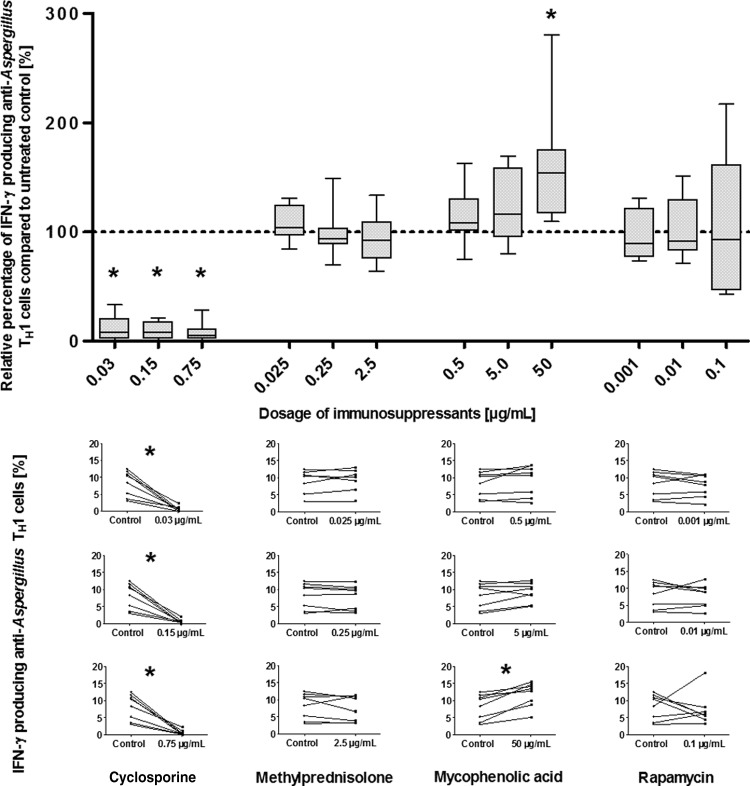
CsA significantly decreases the percentage of IFN-γ-producing anti-Aspergillus TH1 cells and concentrations of IFN-γ in the supernatant.
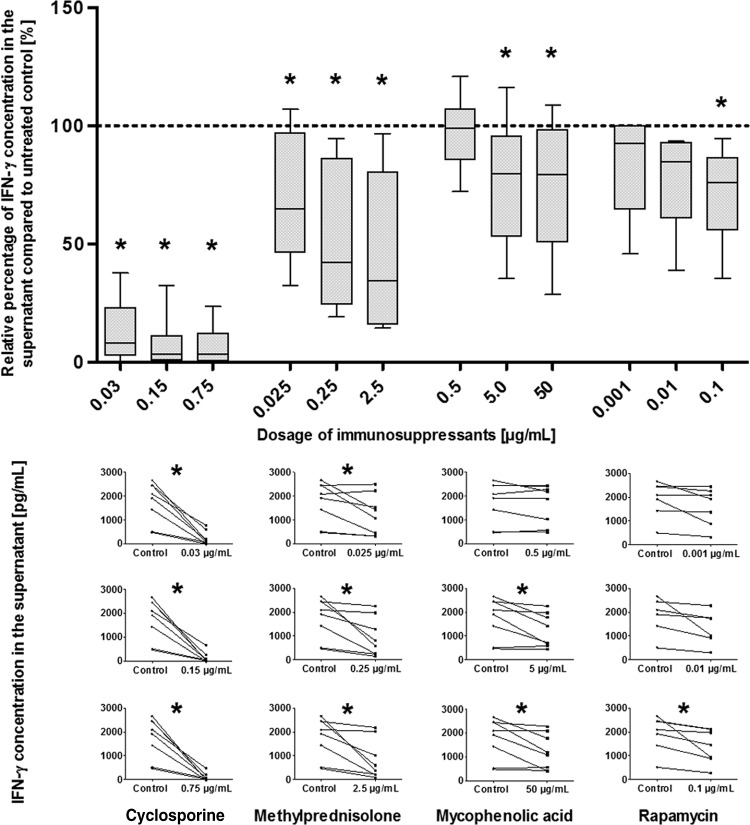
Since IFN-γ is an important molecule for the antifungal activity of anti-Aspergillus TH1 cells, we evaluated the impact of immunosuppressants on the percentage of IFN-γ-producing cells as well as on IFN-γ levels in the supernatant. Compared to no treatment, CsA at dosages of 0.03 μg/ml and higher significantly reduced both the percentage of IFN-γ-producing anti-Aspergillus TH1 cells and the IFN-γ levels (P < 0.05 in each case) (Fig. 4 and 5). Dilution experiments revealed that CsA at a dosage of 0.0005 μg/ml resulted in an inhibition of approximately 50% of IFN-γ production, whereas CsA at concentrations of 0.00015 μg/ml and lower did not affect IFN-γ production (see Fig. S6 in the supplemental material). Whereas none of the other immunosuppressants tested significantly impacted the number of IFN-γ-producing cells (with the exception of the highest dosage of MPA) (Fig. 4), a significant decrease in the concentration of IFN-γ in the supernatant was detected for all dosages of methylprednisolone as well as for higher dosages of MPA and rapamycin (P < 0.05 in each case) (Fig. 5). However, this impact was considerably less pronounced than the effect by CsA. For all immunosuppressants tested, the effects on IFN-γ production were similar when assessed at day 3 and at day 6 (data not shown).
FIG 4.
Effects of various dosages of immunosuppressants on IFN-γ-producing anti-Aspergillus TH1 cells. (Top) Percentages of IFN-γ-producing anti-Aspergillus TH1 cell cells relative to cell counts for untreated controls (100%). Depicted are medians (horizontal bars), interquartile ranges (boxes), and ranges (whiskers). Results are derived from eight independent experiments. *, P < 0.05. (Bottom) Individual percentages of IFN-γ-producing anti-Aspergillus TH1 cells in cells treated with different dosages of the immunosuppressants (right) and in untreated (control) cells without immunosuppressant (left). *, P < 0.05.
FIG 5.
Effects of various dosages of immunosuppressants on the levels of IFN-γ in the supernatant of anti-Aspergillus TH1 cells. (Top) Percentage of the IFN-γ concentration in the supernatant relative to the IFN-γ level in the untreated control (100%). Depicted are medians (horizontal bars), interquartile ranges (boxes), and ranges (whiskers). Results are derived from eight independent experiments. *, P < 0.05. (Bottom) Individual levels of IFN-γ in the supernatant of anti-Aspergillus TH1 cells treated with different dosages of the immunosuppressants (right) and in the supernatant of untreated (control) cells without immunosuppressant (left).*, P < 0.05.
CsA negatively influences the expression of the antigen-specific activation marker CD154.
Since CD154 is an important regulatory molecule in host immunity, we evaluated the impact of immunosuppressive compounds on this specific marker for antigen-activated T helper cells. At dosages of 0.03 μg/ml and higher, CsA significantly decreased the percentage of CD154-expressing T cells compared to the percentages in controls (P < 0.05 each) (Fig. 6 and see Fig. S7 in the supplemental material), whereas a decrease of approximately 50% of the CD154-expressing cells was seen at a dosage of 0.0005 μg/ml. CsA at dosages of 0.00015 μg/ml and lower did not have a relevant impact on CD154 expression (Fig. S8). A slight but significant increase in the percentage of CD154-expressing cells was seen for the highest dosages of MPA, whereas none of the other dosages of the immunosuppressants methylprednisolone and rapamycin tested affected the CD154 expression of anti-Aspergillus TH1 cells (Fig. 6 and S7).
FIG 6.
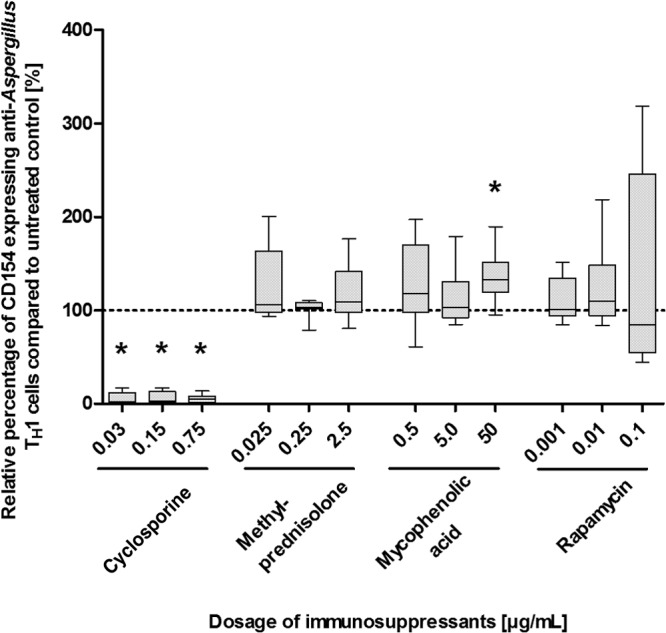
Effects of various dosages of immunosuppressants on CD154 expression of anti-Aspergillus TH1 cells. Presented are percentages of CD154-expressing anti-Aspergillus TH1 cells relative to cell counts for the untreated controls (100%). Depicted are medians (horizontal bars), interquartile ranges (boxes), and ranges (whiskers). Results are derived from eight independent experiments. *, P < 0.05.
DISCUSSION
CsA, methylprednisolone, MMF, and rapamycin are commonly used immunosuppressants in the allogeneic HSCT setting for the prevention of graft rejection and the prophylaxis and treatment of GvHD. In the present study, we evaluated for the first time the effects of these immunosuppressants on important functional properties of specific anti-Aspergillus TH1 cells. These analyses are important since the beneficial effects of adoptively transferred anti-Aspergillus TH1 cells, which has been demonstrated in a pivotal clinical trial in HSCT recipients suffering from invasive aspergillosis (9), may be compromised or even abolished by the concomitant use of immunosuppressants. Our results demonstrate that different dosages of the immunosuppressants tested have particular effects on cell proliferation and/or the upregulation of important effector molecules, such as IFN-γ and CD154 of anti-Aspergillus TH1 cells (10). Although several previous studies have evaluated the influence of immunosuppressive compounds on lymphocytes, our studies are novel and important, since older studies either investigated bulk lymphocytes/PBMCs, which may exhibit functional properties different from those of antigen-specific T cells, or investigated CD8+ T cells specific for cytomegalovirus (CMV) or Epstein-Barr virus (EBV) stimulated with nonphysiologic stimuli, such as ionomycin. It has also to be noted that the present, rather complex approach to generating Aspergillus-specific T cells was not designed for generating cells for a clinical trial but was needed to generate extremely large numbers of Aspergillus-specific cells required for the experiments. Importantly, the present approach results in the generation of a highly homogenous cell population which has phenotypic characteristics and functionality comparable to those of cells suitable for a clinical trial (16, 17). Although the anti-Aspergillus TH1 cells that result in a clinical benefit have been generated with a strategy different from that used for the anti-Aspergillus TH1 cells in the present study (9), similar functional properties suggest that the cells used in the present study also exhibit a therapeutic effect.
CsA inhibits calcineurin, which is activated through T cell receptor signaling and leads to dephosphorylation of the nuclear factor of activated T cells (NFATc). Dephosphorylated NFATc is required for the transcription of several key genes for T cell activation, differentiation, and proliferation, such as the IFN-γ, TNF-α, or IL-2 gene (18). Therefore, it is not surprising that CsA at dosages higher than 0.0015 μg/ml significantly reduced the number of cultivated anti-Aspergillus TH1 cells, which was caused mainly by the inhibition of proliferation. This observation is important, since the efficacy of adoptively transferred anti-Aspergillus TH1 cells depends on the number of viable and functionally active effector cells. Unfortunately, due to the low percentages of anti-Aspergillus TH1 cells in the peripheral blood of healthy individuals, clinical-scale isolation strategies result in a limited number of isolated anti-Aspergillus TH1 cells (19). On the other hand, in vitro data clearly demonstrate that the isolated anti-Aspergillus TH1 cells are not end differentiated and are able to proliferate when restimulated by a naturally processed antigen (19). This corroborates the in vivo observation for haploidentical transplant recipients in whom increasing numbers of anti-Aspergillus TH1 cells were found within 3 weeks of adoptive transfer of the donor-derived cells (9). Therefore, our data suggest that the concomitant use of CsA with adoptively transferred donor-derived anti-Aspergillus TH1 cells may result in an insufficient number of effector cells in vivo. In addition, our results show that CsA in subtherapeutic levels significantly inhibited the production of IFN-γ upon restimulation with naturally processed Aspergillus antigens, which is in line with recently reported data on purified memory CD4+ T cells stimulated with PMA and ionomycin (20), as well as with data on CMV-specific CD8+ T cells (21). This is an important observation, since IFN-γ is well known as one of the key molecules in the combat against Aspergillus (6). In clinical settings, it has been demonstrated that in patients with invasive aspergillosis, a predominant release of IFN-γ in culture supernatants upon stimulation with A. fumigatus antigens is associated with a favorable outcome (8). Our results also demonstrate that subtherapeutic levels of CsA significantly reduce the percentage of CD154-producing CD4+ T cells, which corroborates previous results on unspecific CD4+ T cells stimulated with PMA and ionomycin (22). Notably, CD154, also known as CD40L, plays an important role in T cell-dependent B cell proliferation, memory cell formation, and B cell antibody response (23, 24). Our results suggest that CsA should be avoided, whenever possible, in patients receiving adoptive immunotherapy with anti-Aspergillus TH1.
MPA, the active component of MMF, is an inhibitor of the type II isoform of IMP dehydrogenase, which is involved in the synthesis of guanine nucleotides mandatory for cell proliferation (25). This corroborates our results demonstrating that MPA significantly reduced the number of cultivated anti-Aspergillus TH1 cells. In particular, MPA decreased proliferation at high dosages, whereas apoptosis was only slightly increased, and this increase did not reach statistical significance. A recent study reported on the negative impact of MPA on the proliferation of highly purified nonspecific CD4+ T cells stimulated by anti-CD3/anti-CD28 MAbs (26), consistent with our results. In contrast, data on the effect of MPA on IFN-γ production are less clear. Corroborating our results with anti-Aspergillus TH1 cells, Abadja et al. observed a significant suppression of IFN-γ mRNA by MPA in CD4+ T cells stimulated by anti-CD3/anti-CD28 MAbs (27). In contrast, Egli et al. (28) demonstrated only minor inhibition of IFN-γ production by CMV-specific T cells, and Heidt et al. (24) found highly varied results in IFN-γ mRNA expression without a significant impact of MPA on IFN-γ cytokine levels in the supernatant. However, due to the different study designs with different cell populations tested and different stimuli tested, the results of these studies are difficult to compare.
Glucocorticosteroids are widely used immunosuppressants in the prevention and treatment of GvHD, and they exhibit their potent immunosuppressive effects by a number of mechanisms, such as the suppression of the transcription of various proinflammatory genes, the promotion of apoptosis of T cells, and the strong inhibition of the proliferation of specific TH1 and TH2 clones (10, 29, 30). In line with this, we observed that methylprednisolone at dosages of 0.025 μg/ml and 2.5 μg/ml significantly increased both the rate of apoptosis and the rate of proliferation of anti-Aspergillus TH1 cells. In addition, as with our results, a negative effect of glucocorticosteroids on IFN-γ production was reported, which seems to be more pronounced for dexamethasone than for methylprednisolone (30, 31). Since methylprednisolone is recommended for the treatment of acute GvHD and used by the majority of centers as a first-line treatment, we chose this compound for our analysis (32, 33).
Rapamycin, an immunosuppressive macrolide, specifically inhibits mammalian target of rapamycin complex 1 (mTORC1), which plays an important role in the differentiation and function of T cells and dendritic cells (DCs) (34). It was recently reported that rapamycin markedly reduced the proliferation of CD4+ T cells (35), in contrast to our results with anti-Aspergillus TH1 cells. However, this discrepancy may be explained by different dosages of rapamycin, since Battaglia et al. (35) observed this effect only when using rapamycin at a dosage of 1 μg/ml, a dosage 10- to 1,000-fold higher than the dosages that we used in our experiments. Similarly, others have reported only a weak inhibition of T cell activity with rapamycin concentrations lower than 0.1 μg/ml (36), which was the highest dose in our setting. Notably, we used in our experiments the recommended target range of rapamycin in allogeneic HSCT recipients, which is between 0.001 and 0.02 μg/ml (15). However, it is also important to note that rapamycin exhibits its immunosuppressive activity by a variety of mechanisms on different cell populations, such as the induction of proliferation of immunosuppressive CD4+ CD25+ FOXP3+ T regulatory cells or the limitation of the proinflammatory capacity of mature DCs by downregulating IL-12 and upregulating IL-10 (34, 35).
In conclusion, we studied for the first time the effects of various commonly used immunosuppressants on important functional properties of anti-Aspergillus TH1 cells, such as proliferation and IFN-γ production, upon their restimulation with naturally processed antigens. Our in vitro data demonstrate that CsA, MPA, and methylprednisolone exhibit significant and particular effects on these functional properties, whereas our data did not exhibit a major impact of rapamycin on the generated anti-Aspergillus TH1 cells. Although further studies are warranted to assess both newer immunosuppressants, such as anti-inflammatory antibodies, and the complex effects of immunosuppressants in vivo, our data may have an important impact on the design of clinical studies evaluating the effect of adoptively transferred anti-Aspergillus TH1 cells to allogeneic HSCT recipients suffering from invasive aspergillosis.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Uniscientia Stiftung.
Footnotes
Published ahead of print 7 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01700-14.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 50:1091–1100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 2.Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, Aloisi T, Irrera G, Bonini A, Picardi M, Caramatti C, Invernizzi R, Mattei D, Melillo L, de Waure C, Reddiconto G, Fianchi L, Valentini CG, Girmenia C, Leone G, Aversa F. 2007. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study—Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin. Infect. Dis. 45:1161–1170. 10.1086/522189 [DOI] [PubMed] [Google Scholar]
- 3.Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. 2005. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 36:621–629. 10.1038/sj.bmt.1705113 [DOI] [PubMed] [Google Scholar]
- 4.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48:265–273. 10.1086/595846 [DOI] [PubMed] [Google Scholar]
- 5.Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, Ibatici A, Del Bono V, Bacigalupo A, Viscoli C. 2009. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 44:361–370. 10.1038/bmt.2009.39 [DOI] [PubMed] [Google Scholar]
- 6.Romani L. 2011. Immunity to fungal infections. Nat. Rev. Immunol. 11:275–288. 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 7.Hamza NS, Lisgaris M, Yadavalli G, Nadeau L, Fox R, Fu P, Lazarus HM, Koc ON, Salata RA, Laughlin MJ. 2004. Kinetics of myeloid and lymphocyte recovery and infectious complications after unrelated umbilical cord blood versus HLA-matched unrelated donor allogeneic transplantation in adults. Br. J. Haematol. 124:488–498. 10.1046/j.1365-2141.2003.04792.x [DOI] [PubMed] [Google Scholar]
- 8.Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, Loeffler J, Monod M, Latge JP, Einsele H. 2002. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood 100:4521–4528. 10.1182/blood-2002-01-0265 [DOI] [PubMed] [Google Scholar]
- 9.Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, Martelli MF, Romani L, Velardi A. 2005. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood 106:4397–4406. 10.1182/blood-2005-05-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandevyver S, Dejager L, Tuckermann J, Libert C. 2013. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology 154:993–1007. 10.1210/en.2012-2045 [DOI] [PubMed] [Google Scholar]
- 11.Braedel S, Radsak M, Einsele H, Latge JP, Michan A, Loeffler J, Haddad Z, Grigoleit U, Schild H, Hebart H. 2004. Aspergillus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br. J. Haematol. 125:392–399. 10.1111/j.1365-2141.2004.04922.x [DOI] [PubMed] [Google Scholar]
- 12.Rohatagi S, Barth J, Mollmann H, Hochhaus G, Soldner A, Mollmann C, Derendorf H. 1997. Pharmacokinetics of methylprednisolone and prednisolone after single and multiple oral administration. J. Clin. Pharmacol. 37:916–925. 10.1002/j.1552-4604.1997.tb04266.x [DOI] [PubMed] [Google Scholar]
- 13.Punnett A, Sung L, Price V, Das P, Diezi M, Doyle J, Dupuis LL. 2007. Achievement of target cyclosporine concentrations as a predictor of severe acute graft versus host disease in children undergoing hematopoietic stem cell transplantation and receiving cyclosporine and methotrexate prophylaxis. Ther. Drug Monit. 29:750–757. 10.1097/FTD.0b013e31815c12ca [DOI] [PubMed] [Google Scholar]
- 14.Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G, Garvin J, George D, Bradley MB, Wolownik K, Wischhover C, Levy J, Skerrett D, Cairo MS. 2004. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol. Blood Marrow Transplant. 10:246–258. 10.1016/j.bbmt.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 15.Goyal RK, Han K, Wall DA, Pulsipher MA, Bunin N, Grupp SA, Mada SR, Venkataramanan R. 2013. Sirolimus pharmacokinetics in early postmyeloablative pediatric blood and marrow transplantation. Biol. Blood Marrow Transplant. 19:569–575. 10.1016/j.bbmt.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, Lehrnbecher T. 2006. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood 107:2562–2569. 10.1182/blood-2005-04-1660 [DOI] [PubMed] [Google Scholar]
- 17.Tramsen L, Beck O, Schuster FR, Hunfeld KP, Latge JP, Sarfati J, Roger F, Klingebiel T, Koehl U, Lehrnbecher T. 2007. Generation and characterization of anti-Candida T cells as potential immunotherapy in patients with Candida infection after allogeneic hematopoietic stem-cell transplant. J. Infect. Dis. 196:485–492. 10.1086/519389 [DOI] [PubMed] [Google Scholar]
- 18.Sommerer C, Meuer S, Zeier M, Giese T. 2012. Calcineurin inhibitors and NFAT-regulated gene expression. Clin. Chim. Acta 413:1379–1386. 10.1016/j.cca.2011.09.041 [DOI] [PubMed] [Google Scholar]
- 19.Tramsen L, Koehl U, Tonn T, Latge JP, Schuster FR, Borkhardt A, Uharek L, Quaritsch R, Beck O, Seifried E, Klingebiel T, Lehrnbecher T. 2009. Clinical-scale generation of human anti-Aspergillus T cells for adoptive immunotherapy. Bone Marrow Transplant. 43:13–19. 10.1038/bmt.2008.271 [DOI] [PubMed] [Google Scholar]
- 20.Tsuda K, Yamanaka K, Kitagawa H, Akeda T, Naka M, Niwa K, Nakanishi T, Kakeda M, Gabazza EC, Mizutani H. 2012. Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naive T cells into cytokine-producing mature T cells. PLoS One 7:e31465. 10.1371/journal.pone.0031465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrmann S, Lachmann R, Streitz M, Hetzer R, Volk HD, Lehmkuhl H, Kern F. 2012. Cyclosporin A and tacrolimus reduce T-cell polyfunctionality but not interferon-gamma responses directed at cytomegalovirus. Immunology 136:408–413. 10.1111/j.1365-2567.2012.03594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuleihan R, Ramesh N, Horner A, Ahern D, Belshaw PJ, Alberg DG, Stamenkovic I, Harmon W, Geha RS. 1994. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J. Clin. Invest. 93:1315–1320. 10.1172/JCI117089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. 2009. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229:152–172. 10.1111/j.1600-065X.2009.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidt S, Roelen DL, Eijsink C, Eikmans M, van Kooten C, Claas FH, Mulder A. 2010. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin. Exp. Immunol. 159:199–207. 10.1111/j.1365-2249.2009.04051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison AC, Eugui EM. 2000. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47:85–118. 10.1016/S0162-3109(00)00188-0 [DOI] [PubMed] [Google Scholar]
- 26.He X, Smeets RL, Koenen HJ, Vink PM, Wagenaars J, Boots AM, Joosten I. 2011. Mycophenolic acid-mediated suppression of human CD4+ T cells: more than mere guanine nucleotide deprivation. Am. J. Transplant. 11:439–449. 10.1111/j.1600-6143.2010.03413.x [DOI] [PubMed] [Google Scholar]
- 27.Abadja F, Atemkeng S, Alamartine E, Berthoux F, Mariat C. 2011. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation 92:396–403. 10.1097/TP.0b013e3182247b5f [DOI] [PubMed] [Google Scholar]
- 28.Egli A, Kumar D, Broscheit C, O'Shea D, Humar A. 2013. Comparison of the effect of standard and novel immunosuppressive drugs on CMV-specific T-cell cytokine profiling. Transplantation 95:448–455. 10.1097/TP.0b013e318276a19f [DOI] [PubMed] [Google Scholar]
- 29.Herold MJ, McPherson KG, Reichardt HM. 2006. Glucocorticoids in T cell apoptosis and function. Cell. Mol. Life Sci. 63:60–72. 10.1007/s00018-005-5390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun CM, Huang SK, Bashian GG, Kagey-Sobotka A, Lichtenstein LM, Essayan DM. 1997. Corticosteroid modulation of human, antigen-specific Th1 and Th2 responses. J. Allergy Clin. Immunol. 100:400–407. 10.1016/S0091-6749(97)70255-0 [DOI] [PubMed] [Google Scholar]
- 31.Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O'Shea JJ. 2000. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J. Immunol. 164:1768–1774 [DOI] [PubMed] [Google Scholar]
- 32.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, Shaughnessy PJ, Wall DA, Carpenter PA. 2012. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 18:1150–1163. 10.1016/j.bbmt.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruutu T, van Biezen A, Hertenstein B, Henseler A, Garderet L, Passweg J, Mohty M, Sureda A, Niederwieser D, Gratwohl A, de Witte T. 2012. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: a survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 47:1459–1464. 10.1038/bmt.2012.45 [DOI] [PubMed] [Google Scholar]
- 34.Salmond RJ, Zamoyska R. 2011. The influence of mTOR on T helper cell differentiation and dendritic cell function. Eur. J. Immunol. 41:2137–2141. 10.1002/eji.201141523 [DOI] [PubMed] [Google Scholar]
- 35.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. 2006. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177:8338–8347 [DOI] [PubMed] [Google Scholar]
- 36.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. 2006. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 107:1018–1023. 10.1182/blood-2005-07-3032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.