Abstract
Staphylococcus lugdunensis is a coagulase-negative staphylococcus that is a commensal of humans and an opportunistic pathogen. It can cause a spectrum of infections, including those that are associated with the ability to form biofilm, such as occurs with endocarditis or indwelling medical devices. The genome sequences of two strains revealed the presence of orthologues of the ica genes that are responsible for synthesis of poly-N-acetylglucosamine (PNAG) that is commonly associated with biofilm in other staphylococci. However, we discovered that biofilm formed by a panel of S. lugdunensis isolates growing in iron-restricted medium was susceptible to degradation by proteases and not by metaperiodate, suggesting that the biofilm matrix comprised proteins and not PNAG. When the iron concentration was raised to 1 mM biofilm formation by all strains tested was greatly reduced. A mutant of strain N920143 lacking the entire locus that encodes iron-regulated surface determinant (Isd) proteins was defective in biofilm formation under iron-limited conditions. An IsdC-null mutant was defective, whereas IsdK, IsdJ, and IsdB mutants formed biofilm to the same level as the parental strain. Expression of IsdC was required both for the primary attachment to unconditioned polystyrene and for the accumulation phase of biofilm involving cell-cell interactions. Purified recombinant IsdC protein formed dimers in solution and Lactococcus lactis cells expressing only IsdC adhered to immobilized recombinant IsdC but not to IsdJ, IsdK, or IsdB. This is consistent with a specific homophilic interaction between IsdC molecules on neighboring cells contributing to accumulation of S. lugdunensis biofilm in vivo.
INTRODUCTION
Coagulase-negative staphylococci (CoNS) and Staphylococcus aureus are the predominant etiological agents of medical device-related infections, largely owing to their ability to form biofilm. Biofilms are defined as communities of bacteria encased in a self-synthesized extracellular polymeric matrix (1) growing attached to biological or abiotic surfaces. Staphylococci in biofilms are resistant to antibiotics (2) and host immune responses (3), reducing the efficacy of available antimicrobials. The formation of biofilm is a complex, multifactorial process. Initially, bacteria adhere directly to the surface of implanted device or to devices coated with the host matrix components. In S. aureus biofilm the major autolysin, Atl, mediates primary attachment to plastic surfaces by promoting release of DNA from bacterial cells (4, 5), while adherence to surfaces conditioned by host plasma proteins is promoted by surface protein adhesins such as the fibrinogen-binding clumping factor A or fibronectin-binding proteins (6). This process is followed by proliferation, accumulation, and intercellular interactions mediated by the icaADBC-encoded polysaccharide intercellular adhesins (PIA) (7) or surface proteins such as Bap (8), SasG (9), SasC (10), protein A (11), or fibronectin-binding proteins (FnBPs) (12, 13). Likewise, biofilm formation by S. epidermidis is dependent on PIA or proteinaceous components such as Aap (14, 15) or SesC (16).
Staphylococcus lugdunensis is a coagulase-negative species with enhanced virulence compared to the other CoNS (17). S. lugdunensis causes a severe form of native valve endocarditis (18, 19) and infections of prosthetic heart valves (20), intravascular catheters (21), prosthetic joints (22), and ventriculoperitoneal shunts (23). This pathogenic potential is largely attributed to the ability of this bacterium to form biofilm. A previous study by Frank and Patel (24) demonstrated that despite the presence of icaADBC orthologues in S. lugdunensis, PIA is not the major component of the extracellular matrix of biofilms formed in vitro by this species. Rather, S. lugdunensis biofilms appear to be composed of proteins. S. lugdunensis expresses a fibrinogen-binding protein (Fbl) (25), a member of the family of MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules) family that is closely related to ClfA, which does not appear to have any role in biofilm formation (26), and a yet-uncharacterized von Willebrand factor binding protein (27).
Uniquely for CoNS, S. lugdunensis contains a cluster of genes with similarity both in terms of organization and sequence to the iron-regulated surface determinant (isd) locus of S. aureus (28). Both systems are expressed under iron-restricted conditions (28, 29). Four of the S. lugdunensis Isd proteins are anchored to the cell wall peptidoglycan by sortases. In S. aureus and S. lugdunensis, the Isd proteins cooperate to capture heme and transfer it across the wall to a membrane-bound transporter, which delivers it to the cytoplasm, where heme is degraded to recover iron (30). There is evidence that surface-exposed Isd proteins may have additional roles in colonization and pathogenesis of both species. For example, IsdJ from S. lugdunensis (29) and IsdA from S. aureus (31) are multifunctional proteins that recognize and bind several host proteins and can confer resistance to skin fatty acids. In the present study, we investigated biofilm formation in vitro by a collection of S. lugdunensis isolates grown under low-iron conditions and assessed the role of isd locus in biofilm formation by this important pathogen.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The microorganisms used in the present study are reported in Table 1. S. aureus V329 (8) and SA113 (32) were kindly donated by J. R. Penades (Universidad Cardenal Herrera-CEU, Moncada, Valencia, Spain). The clinical isolate of S. epidermidis 5179R (14) was provided by H. Rohde (University Medical Centre Hamburg-Eppendorf, Hamburg, Germany). S. epidermidis RP62A was originally isolated from a patient with intravascular catheter-associated sepsis by Muller et al. (33). Staphylococci were grown in Trypticase soy broth (TSB; Difco, Detroit, MI) or in RPMI 1640 (RPMI; Biowest, Nuaillé, France) supplemented with 2 mM glutamine (Lonza Srl, Bergamo, Italy) at 37°C for 16 to 18 h with intense shaking (200 rpm). L. lactis transformants were grown in M17 medium (Difco) containing 0.5% glucose and 10% lactose at 30°C.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Staphylococcus lugdunensis | ||
| N920143 | Human breast abscess isolate | 28 |
| N930432 | Human endocarditis isolate | F. Vandenesch (unpublished data) |
| N940025 | Human endocarditis isolate | F. Vandenesch (unpublished data) |
| N940084 | Finger pulp infection isolate | This study |
| N940113 | Vertebral infection isolate | This study |
| N940135 | Perineal infection isolate | This study |
| N940164 | Perineal infection isolate | This study |
| 1871 | Orthopedic infection with internal fixation system | This study |
| 2050 | Orthopedic infection with knee implant | This study |
| N920143ΔisdB | isdB-null mutation in N920143 | 29 |
| N920143ΔisdC | isdC-null mutation in N920143 | 29 |
| N920143ΔisdCr | Reversion strain expressing isdCr in the native context | This study |
| N920143ΔisdJ | isdJ-null mutation in N920143 | 29 |
| N920143ΔisdK | isdK-null mutation in N920143 | 29 |
| N920143Δisd | isd-null mutation in N920143 | 29 |
| N920143ΔatlI | atlI-null mutation in N920143 | This study |
| N920143ΔsrtB | srtB-null mutation in N920143 | This study |
| Staphylococcus aureus | ||
| SA113 | ATCC 35556, restriction deficient | 31 |
| V329 | Isolate obtained from a bovine subclinical phenotype | 8 |
| Staphylococcus epidermidis | ||
| RP62A | ATCC 35984, isolated from a patient with sepsis | 32 |
| 5179R | Clinical isolate obtained from cerebrospinal fluid infection | 14 |
| Lactococcus lactis NZ9000 | L. lactis subsp. cremoris MG1363 carrying nisin resistance cassette | 33 |
| Escherichia coli | ||
| TOPP3 | E. coli cloning and protein purification host | Stratagene |
| Plasmids | ||
| pQE30 | E. coli cloning and expression vector; Apr | Stratagene |
| pQE30isdB | pQE30 encoding residues 45 to 655 of IsdB | 29 |
| pQE30isdC | pQE30 encoding residues 30 to 190 of IsdC | 29 |
| pQE30isdJ(45-610) | pQE30 encoding residues 45 to 610 of IsdJ | 29 |
| pQE30isdK | pQE30 encoding residues 35 to 426 of IsdK | 29 |
| pNZ8048 | L. lactis shuttle vector containing the PnisA promoter and start codon in NcoI site; Cmr allowing nisin-inducible expression of insert | 33 |
| pNZ8048isdC-LPQTG | pNZ8048 encoding isdC, for controlled expression of sortase A-anchored IsdC in L. lactis | This study |
| pIMAY | Themosensitive vector for allelic exchange; Cmr | 39 |
| pIMAYisdCr | Construct for restoration of the ΔisdC mutation | This study |
| pIMAYΔatl and pIMAYΔsrtB | Constructs for deleting atl and srtB, respectively | This study |
| pRMC2 | Inducible expression vector for staphylococci; Apr Cmr | 35 |
| pRMC2isdC-LPQTG | pRMC2 encoding isdC, for controlled expression of sortase A-anchored IsdC in S. lugdunensis Δisd | This study |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance. isdCr, recombined gene isdC.
Construction of S. lugdunensis mutants.
S. lugdunensis N920143 mutants deficient in individual genes isdB, isdC, isdJ, and isdK and the mutant deficient in the entire isd locus were as reported earlier (29) (Table 1).
In order to reverse the deletion mutation in N920143, the program SILENT (emboss.bioinformatics.nl/cgi-bin/emboss/silent) was used to identify single nucleotides within isdC that can be mutated to create a novel restriction site without causing translational changes in the protein sequence. The novel restriction sites allow the discrimination of the wild type and the restored allele. Primers isdC-E and isdC-F exchanged nucleotide 459 of isdC (A to C), creating a novel SmaI site. Primers isdC-A and isdC-E were used to amplify a 500-bp upstream sequence and the 5′-fragment of isdC, and primers isdC-F and isdC-D were used to amplify a 500-bp downstream region, together with the 3′ fragment of isdC. PCR products were gel-purified and used for the spliced overlap extension PCR using primers A and D. The cassette was gel purified, cleaved at endonuclease cleavage sites introduced in primers A and D, and cloned into pIMAY by using SLIC (sequence and ligase independent cloning), forming pIMAYisdCr. The isdCr gene was recombined into the chromosome of S. lugdunensis N920143ΔisdC by allelic exchange.
Additional mutants deficient in srtB and the autolysin atlI were created using the thermosensitive vector pIMAY and allelic exchange. A detailed protocol of the procedure is described elsewhere (34). Primers used for the construction of deletion cassettes are summarized in Table S1 in the supplemental material.
Construction and expression of isdC in S. lugdunensis Δisd mutant.
In order to create a S. lugdunensis strain expressing IsdC in the absence of any other Isd proteins, the isdC gene was cloned into the anhydrotetracycline-inducible vector pRMC2 (35). The gene was PCR amplified with the primers isdC-pRF and isdC-pRR using pNZ8048isdC-LPQTG as the template. The S. lugdunensis Δisd mutant is deficient in sortase B, which will prevent cell wall sorting of the wild-type IsdC protein harboring an NPQTS motif. The IsdC-LPQTG protein will be sorted by sortase A.
The isdC LPQTG gene was cloned into pRMC2 using SLIC (36, 37). This method involves PCR amplification of the vector backbone and of the insert. Amplification of the vector backbone generates a linear product with blunt ends at the site required for the cloning. Primers incorporate short identical DNA sequences in vector and insert. Treatment of both PCR products with T4 polymerase (3′-5′ exonuclease activity) creates single-stranded, 5′ overhangs in both vector and insert. The DNA fragments are assembled in vitro (without ligation) and used to transform Escherichia coli.
Primers pRA and pRB were used to amplify the pRMC2 backbone (see Table S1 in the supplemental material). Identical sequences (20 to 25 nucleotides) were integrated in the primers for the amplification of the insert (isdC-pRF/isdC-pRR; see Table S1 in the supplemental material). Next, 10 ng of plasmid DNA was used as the template for the amplification of the plasmid backbone with Phusion polymerase (Keilaranta 16A; Finnzymes, Espoo, Finland). The PCR products were purified, and the vector product was digested with DpnI to remove methylated template DNA. Then, 1 μg of vector and insert DNA was treated with T4 polymerase in a final volume of 40 μl of NEB buffer 2 (New England BioLabs, Ipswich, MA) with highly purified bovine serum albumin at 100 μg/ml (New England BioLabs), 5 mM dithiothreitol, 200 mM urea, and 3 U of T4 DNA polymerase (New England BioLabs), followed by incubation for 20 min at 23°C, and the reaction was stopped by the addition of 25 mM EDTA and subsequent incubation for 20 min at 73°C.
We then mixed 5 μl of vector DNA and 5 μl of insert DNA, and the single-stranded overhangs were allowed to anneal. The tube was placed in a PCR machine for 10 min at 65°C, followed by a slow decrease in temperature from 65 to 25°C with a 1-min hold for each degree. A 2.5-μl portion of the reaction mixture was used to transform E. coli to isolate pRMC2isdC-LPQTG, which was confirmed by DNA sequencing. The plasmid was transformed into the S. lugdunensis Δisd strain (34). Induction of IsdC-LPXTG expression from pRMC2 in S. lugdunensis Δisd mutant was carried out by adding anhydrotetracycline (0.125 μg/ml; Sigma, St. Louis, MO) to exponentially growing cultures.
Construction of Lactococcus lactis expressing IsdC.
To express IsdC in the surrogate host L. lactis, the isdC gene was amplified (IsdC-F/IsdC-R) and cloned into the nisin-inducible expression vector pNZ8048 (38). L. lactis was transformed with the recombinant plasmid as described earlier (39).
IsdC possesses a NPQTS sorting signal at its C terminus and is therefore anchored to the cell wall by the transpeptidase sortase B, which is encoded within the isd operon. L. lactis does not encode sortase B, which will prevent sorting of IsdC to the cell wall. To allow sorting, pNZ8048isdC was isolated, and DNA encoding the NPQTS signal was exchanged to LPQTG using 5′-phosphorylated primers (LPXTG-A/LPXTG-B) and inverse PCR. The primers allowed the amplification of the entire plasmid and introduced the required nucleotide substitutions in the cell wall anchoring region of isdC. The PCR product was treated with T4 ligase to allow circularization of the plasmid and transformed into L. lactis. The resulting plasmid (pNZ8048isdC-LPQTG) was confirmed by DNA sequencing. Primers are summarized in Table S1 in the supplemental material. Induction of IsdC-LPXTG expression from pNZ8048 in L. lactis was carried out by adding nisin (0.4 ng/ml) to exponentially growing cultures.
Biofilm formation.
Overnight cultures of staphylococci were diluted 1:200 in TSB containing 0.3% glucose (TSB0.3%glucose) or RPMI supplemented with 0.3% glucose (RPMI0.3%glucose) and 2 mM glutamine. Aliquots (200 μl) of the diluted bacterial suspensions were added to 96-well flat-bottom sterile polystyrene microplates (Costar; Corning, New York, NY) and incubated statically for 24 h at 37°C. Biofilm formation was detected by the method of Christensen et al. (40). Briefly, biofilms formed on the plates were gently washed twice with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 [pH 7.4]) to remove planktonic and loosely adhering bacteria. Adherent cells were fixed with 96% ethanol for 10 min, stained with 0.1% crystal violet for 15 min, and—after several washings—the wells were air dried. For a quantitative estimation of biofilm density, bound crystal violet was solubilized with 10% glacial acetic acid, and the absorbance of the solubilized dye was read at 595 nm in a microplate reader (model 680; Bio-Rad Laboratories, Inc., Hercules, CA). To test the role of iron on biofilm formation, S. lugdunensis strains N920143, N940025, N940113, and N940135 were cultured in RPMI0.3%glucose–2 mM glutamine supplemented with 1,000 μM FeCl3 in 96-well flat-bottom sterile polystyrene microplates and treated as described above. Iron-depleted growth medium was obtained by treatment of RPMI with divalent metal chelator Chelex 100 according to the manufacturer's instructions (Bio-Rad).
Enzymatic and chemical treatment of biofilms.
Chemical and enzymatic treatments of biofilms were carried out as described previously (41, 42). Briefly, the biofilms grown in microtiter plates were rinsed with 200 μl of 0.9% NaCl and then treated for 2 h at 37°C with 100 μl of 10 mM sodium metaperiodate (Sigma) in 50 mM sodium acetate buffer (pH 4.5). Alternatively, biofilms were incubated with 100 μl of proteinase K (Sigma) at 1 mg/ml in 20 mM Tris buffer containing 100 mM NaCl (pH 7.5) or 100 μl of DNase I (Sigma) at 2 mg/ml in PBS. Enzymes or sodium metaperiodate were replaced with the appropriate amounts of buffer in the controls. To rule out the possibility that DNase I could be contaminated with proteases, the enzyme was incubated with albumin for 2 h, and the mixture was subjected to SDS-PAGE. Under these conditions, no difference in the electrophoretic mobility of DNase I-treated and untreated samples of albumin was observed.
Expression and purification of recombinant proteins.
Recombinant His-tagged proteins were expressed and purified by Ni2+ chelate chromatography as described previously (29) (Table 1). E. coli strain TOPP3 (Stratagene, La Jolla, CA), used for the expression of recombinant His-tagged proteins, was grown in Luria-Bertani (LB) broth (Difco) supplemented with ampicillin (100 μg/ml; Sigma) at 37°C for 18 h with shaking (150 rpm). Overnight cultures were diluted 1:100 in LB medium and grown at 37°C, with shaking, until the optical density at 600 nm (OD600) reached 0.5 to 0.6. Expression was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside; Inalco, Milan, Italy) to a final concentration of 1 mM. Bacteria were harvested by centrifugation at 1,700 × g for 20 min and lysed by passage through a French press. The cell debris was removed by centrifugation (20,000 × g), and the filtered supernatant (0.45-μm-pore-size membrane) was applied to a 1-ml Ni2+-Sepharose His-Trap HP column (GE Healthcare, Buckinghamshire, United Kingdom). Fusion proteins were eluted with 20 column volumes of a 0.00 to 500 mM imidazole (Sigma) gradient in 20 mM sodium phosphate–0.5 M NaCl buffer (pH 7.4). Fractions corresponding to the recombinant protein were pooled and extensively dialyzed against PBS. Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Primary attachment assay.
The attachment assay was performed as reported by Geoghegan et al. (9). Briefly, bacteria were grown overnight in RPMI–2 mM glutamine and diluted in RPMI0.3%glucose–2 mM glutamine, and ∼300 CFU in 100 μl was then spread onto the bases of empty petri dishes. Dishes were incubated upright at 37°C for 30 min, washed three times with 5 ml of sterile PBS, and covered with TSB agar. Bacterial plate counts were run in parallel, and the percent attachment was calculated. Each experiment was repeated three times. The statistical significance was determined with Student t test using GraphPad software (GraphPad Software, Inc., San Diego, CA).
Aggregation assays.
The aggregation assay was based on the method described by Geoghegan et al. (9). Bacteria were grown overnight in RPMI–2 mM glutamine and diluted in RPMI0.3%glucose–2 mM glutamine to an OD600 of 1.0. Tubes were incubated statically at 37°C for up to 24 h. A 1-ml portion of broth was removed from the top of the tube at the indicated times, and the OD600 was measured. The remaining culture was vortexed to resuspend the cells, and the OD600 was measured again. The percent aggregation was calculated using the following formula: 100 × [(OD600 of vortexed sample − OD600 before vortexing)/(OD600 of vortexed sample)]. The statistical significance was determined with a Student t test using GraphPad software.
Gel filtration chromatography.
The size exclusion chromatography experiment was performed using a Superose 12 HR 10/30 column (GE Healthcare) connected to an AKTA design chromatography system (GE Healthcare). A 100-μl portion of 100 μM IsdC was loaded onto a gel filtration column equilibrated in PBS with or without 100 μM FeCl3 and eluted with one column volume (24 ml) at a flow rate of 0.5 ml/min. Recorded data were analyzed using UNICORN 5.10 control software (GE Healthcare). The Mr of IsdC was determined from the calibration curve (plot of the partition coefficient [Kav] versus log Mr) once its Kav value was calculated from the measured elution volume.
Preparation of bacterial lysates.
The lysates from S. lugdunensis and L. lactis strains were prepared as previously described (29) with minor modifications. Briefly, bacterial cultures were harvested by centrifugation, washed in PBS, and adjusted to an OD600 of 10. A 1-ml portion of the bacterial suspension was pelleted and resuspended in 250 μl of digestion buffer (50 mM Tris-HCl, 20 mM MgCl2, 30% [wt/vol] raffinose; pH 7.5) containing Complete Mini-Protease inhibitors (Roche). Cell wall proteins were solubilized by digestion with lysostaphin (500 μg/ml) (S. lugdunensis) or a combination of mutanolysin (1,000 U/ml) and lysozyme (900 μg/ml) (L. lactis) at 37°C for 30 or 15 min, respectively. Protoplasts were harvested by centrifugation (5,000 × g, 15 min), and the supernatants were subjected to SDS-PAGE and Western blotting.
Western immunoblotting.
Whole-cell lysates of transformants of L. lactis were subjected to 12.5% polyacrylamide gel electrophoresis and then electroblotted onto a nitrocellulose membrane (GE Healthcare). The membrane was treated with a solution containing 5% (wt/vol) dried milk in PBS, washed, and incubated with anti-IsdC rabbit IgG for 1 h at 22°C. After additional washings with 0.5% (vol/vol) Tween 20 in PBS, the membrane was incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG. The membrane was treated with enhanced chemiluminescence detection reagents 1 and 2, as recommended by the manufacturer (GE Healthcare), and exposed to an X-ray film for 30 to 60 s.
Attachment of L. lactis transformants to Isd proteins.
Isd proteins (1 μg/well) were coated in microtiter wells in bicarbonate buffer overnight. L. lactis(pNZ8048isdC) and L. lactis(pNZ8048) (5 × 108 cells/well) were added to the wells, followed by incubation for 1 h at 22°C. After extensive washing with PBS, adhering cells were fixed with 25% formaldehyde (Sigma) and stained with 2.5% crystal violet, and the A595 was measured.
Statistical methods.
Continuous data were expressed as means and standard deviations. Two-group comparisons were performed by Student t test. One-way analysis of variance, followed by Bonferroni's post hoc tests, was exploited for comparison of three or more groups. Analyses were performed using Prism 4.0 (GraphPad). Two-tailed P values of <0.05 were considered statistically significant.
RESULTS
S. lugdunensis clinical isolates form proteinaceous biofilm in iron-restricted conditions.
It was reported previously that S. lugdunensis strains growing in rich broth formed biofilm that was predominantly proteinaceous in that preformed biofilm could be disrupted by proteases but not by periodate (24). In order to investigate the nature of S. lugdunensis biofilm in more detail, we tested a panel of clinical isolates along with S. aureus and S. epidermidis controls where the composition of the biofilm matrix is known to be composed predominantly of protein (V329, 5179R) or polysaccharide (SA113, RP62A) growing in iron-replete TSB or in iron-deficient RPMI. The controls formed protein or polysaccharide dependent biofilms as previously reported.
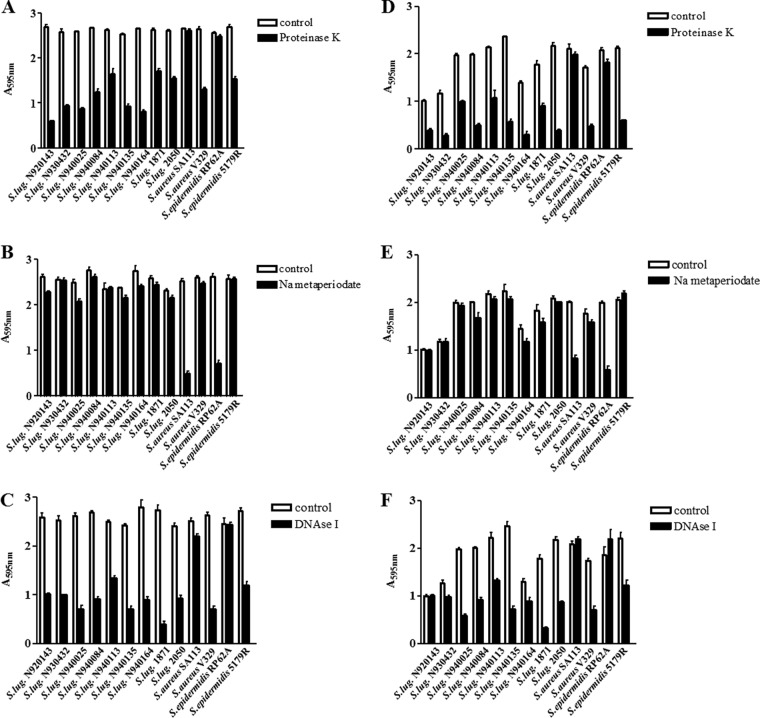
All S. lugdunensis strains tested formed biofilm when growing in both media. Glucose concentrations of >0.25% supported higher levels of biofilm formation compared to lower concentrations (P < 0.001, data not shown). We confirmed the previous report that biofilms formed by S. lugdunensis growing in TSB-glucose were susceptible to detachment by proteinase K but not by sodium metaperiodate (Fig. 1). We also found that biofilms formed by S. lugdunensis growing in RPMI were susceptible to protease and not to periodate. Furthermore, DNase caused significant detachment of S. lugdunensis biofilm formed under both growth conditions. DNase also detached the control protein-dependent biofilm but not that involving a polysaccharide matrix (Fig. 1).
FIG 1.
Characterization of S. lugdunensis biofilm matrix composition. Biofilms were grown in sterile 96-well polystyrene plates in TSB (A, B, and C) or RPMI (D, E, and F) supplemented with 0.3% glucose, for 24 h at 37°C, and the adherent cells were treated with proteinase K (A and D), sodium metaperiodate (B and E), or DNase I (C and F). After washing, cells were stained with 0.1% crystal violet, and the absorbance was measured at 595 nm. Bars represent the averages of measurements taken from four wells. Error bars represent the standard deviations. Controls for protein-dependent biofilm (S. aureus V329 and S. epidermidis 5179R) and PIA-dependent biofilm (S. aureus SA113 and S. epidermidis RP62a) were included in experiments to test the sensitivity to proteinase K, DNase, and sodium metaperiodate. This experiment was performed three times with similar results.
Involvement of the IsdC protein in S. lugdunensis biofilm.
To determine whether S. lugdunensis biofilm formed during growth in RPMI was influenced by the availability of iron, three strains were tested in RPMI supplemented with 1 mM FeCl3. Interestingly, the addition of iron reduced the biofilm density, suggesting that the involvement of proteins whose expression was regulated by iron (Fig. 2). S. lugdunensis is the only species of coagulase-negative staphylococcus that harbors an iron-regulated surface determinant (Isd) locus. It is only expressed under iron-limited conditions and is responsible for the acquisition of iron from hemoglobin and heme in vivo (28, 29). To determine whether the isd locus is involved in biofilm formation, S. lugdunensis N920143 wild type and a mutant in which the entire isd locus is deleted (Δisd) were compared. A schematic representation of the isd locus and mutations is shown in Fig. 3. The level of biofilm formed by the isd mutant was the same as that formed by the wild-type strain in the presence of FeCl3. This indicates that one or more proteins expressed by the isd locus are involved in biofilm formation in iron-limited conditions (Fig. 4). The S. lugdunensis isd locus expresses four cell envelope-associated proteins IsdC, IsdB, IsdJ, and IsdK (29). Mutants lacking each of the Isd proteins were tested for biofilm formation in RPMI. Notably, only the IsdC mutant was defective and showed the same low level of biofilm formation as the wild type supplemented with 1 mM FeCl3 or the Δisd mutant. To exclude the possibility that the reduction in biofilm formation was due to a difference in growth in RPMI, the growth curves for the wild type and each of the mutants were compared and found to be superimposable. A revertant strain where the isdC gene had been restored to wild type by allele exchange (ΔisdCr), expressed biofilm normally, indicating that the isdC mutation and not a secondary mutation is responsible.
FIG 2.
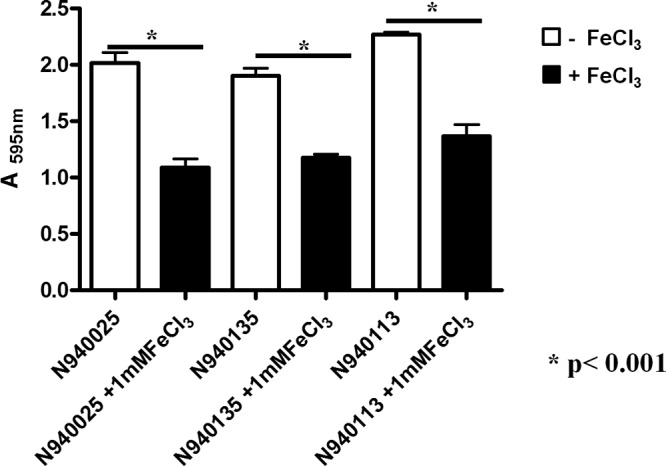
Biofilm formation by S. lugdunensis clinical isolates grown in RPMI with or without 100 μM FeCl3. Bacteria were grown for 24 h in RPMI and diluted 1:200 in RPMI-glucose (0.3% [wt/vol]) in the presence or absence of 1 mM FeCl3. Diluted bacteria (200 μl) were added to sterile 96-well polystyrene plates and statically incubated at 37°C for 24 h. Wells were washed three times with phosphate-buffered saline and fixed with 96% ethanol. Adherent cells were stained with 0.1% (wt/vol) crystal violet, and the absorbance was measured at 595 nm. Bars are the averages of measurements taken from four wells. Error bars represent the standard deviations. This experiment was performed three times with similar results. P values were determined using a two-tailed, two-sample unequal variance Student t test in GraphPad.
FIG 3.
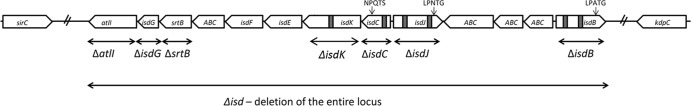
Schematic diagram of the isd locus and mutations. The open boxes denote individual genes, and the arrows indicate the direction of their transcription. Encoded NEAT motifs are shown as small black boxes. Angled dashes indicate the beginning and the end of the isd locus. Mutations are indicated by horizontal bi-headed arrows.
FIG 4.
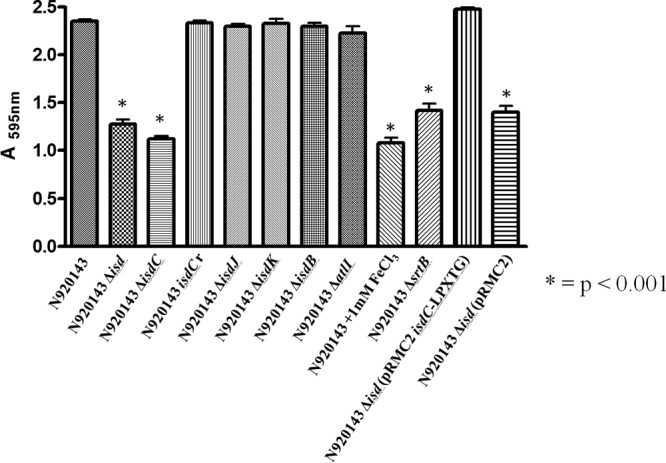
Effect of isd locus deletion on biofilm formation by S. lugdunensis in iron-limiting conditions. S. lugdunensis N920143 wild type and its deletion mutants covering the entire isd locus or single isd genes were grown overnight in RPMI. The experimental conditions for biofilm formation by the strains and biofilm detection are identical to those reported in Fig. 2. The effect of 1 mM FeCl3 on biofilm formation is also reported. This experiment was performed four times with similar results. P values were determined using a two-tailed, two-sample unequal variance Student t test in GraphPad.
IsdC is known to be surface exposed and anchored to cell wall peptidoglycan by sortase B (29). Consistent with this observation, a sortase B mutant (ΔsrtB) formed biofilm at a level comparable to that of the isdC mutant. In contrast, an atlI mutant expressed biofilm at a level comparable to the wild-type strain. Furthermore, the Δisd mutant (lacking the entire isd locus), carrying plasmid pRMC2 bearing an isdC gene engineered to express IsdC containing a sortase A recognition sequence (pRMC2isdC-LPXTG), expressed a level of biofilm similar to that of S. lugdunensis N920143 wild type. This shows that IsdC alone is necessary and sufficient to promote biofilm formation in S. lugdunensis. (Fig. 4).
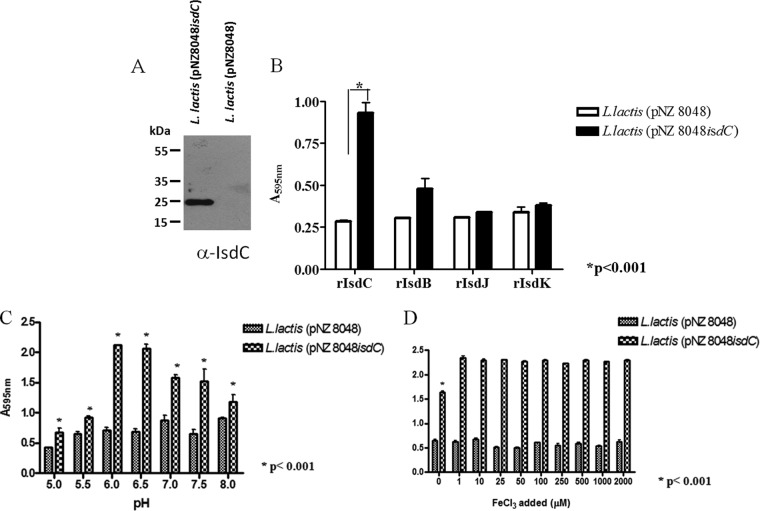
To examine further the effect of iron on biofilm development, S. lugdunensis N920143 was grown in Chelex 100-pretreated RPMI supplemented with various amounts of FeCl3 and tested for biofilm formation. The levels of IsdC detected by Western immunoblotting were the same under depleted and low-iron conditions (from 0 to 50 μM) and virtually missing in 250 to 2,000 μM FeCl3 (Fig. 5). A high level of biofilm formation was observed with bacteria growing in the low-iron environment (1 to 50 μM), whereas significantly reduced biofilm was detected either in FeCl3-depleted conditions or in the presence of high iron concentrations (≥250 μM). In conclusion, for the IsdC-dependent biofilm formation critical concentrations of FeCl3 are required.
FIG 5.
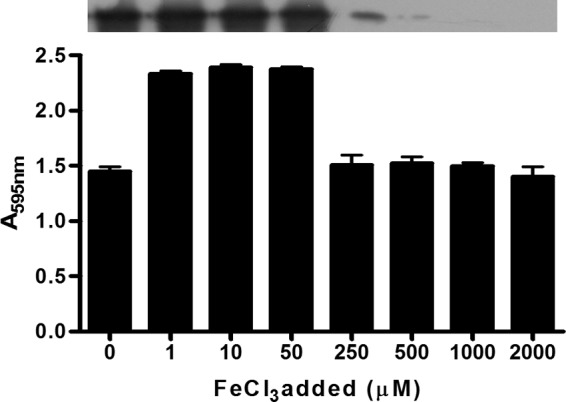
Iron-dependent biofilm formation by S. lugdunensis N920143. Bacteria grown overnight in Chelex 100-treated RPMI were diluted in the same medium supplemented with increasing amounts of FeCl3 (final concentrations from 0 to 2,000 μM), added to sterile 96-well polystyrene plates, and statically incubated at 37°C for 24 h. The experimental conditions for biofilm detection are identical to those reported in Fig. 2. The upper part of the figure shows the detection by Western blotting of IsdC protein in the lysates obtained from cultures grown overnight planktonically in different FeCl3 concentrations. This experiment was performed three times with similar results. P values were determined using a two-tailed, two-sample unequal variance Student t test in GraphPad.
We also tested the effect of pH on IsdC expression by planktonic cells of S. lugdunensis N920143 and on biofilm formation. The same level of IsdC was detected by Western immunoblotting of lysates of staphylococcal cells grown at pH 5 to 8 (Fig. 6A), whereas the biofilm level was significantly enhanced when the pH of the medium was 6.0 or 6.5 compared to 5.5, 7.0, 7.5, or 8.0 (Fig. 6B). A similar trend was found when the effect of pH on biofilm formation by the isdC mutant was examined. These conditions also promote protein-dependent biofilm formation in S. aureus (12). Together, these data indicate that different pHs do not affect IsdC expression but somehow influence the levels of biofilm formation.
FIG 6.
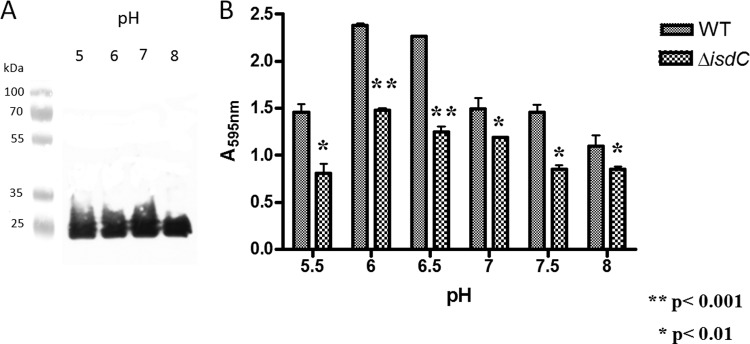
Effect of pH on the IsdC expression and biofilm formation by S. lugdunensis N920143. (A) S. lugdunensis N920143 was grown overnight in RPMI adjusted to the indicated pH and the corresponding lysates subjected to Western immunoblotting. IsdC protein transblotted onto nitrocellulose filter was detected with rabbit anti-IsdC IgG, followed by HRP-conjugated secondary goat anti-rabbit IgG. (B) S. lugdunensis N920143 and its isdC deletion mutant were grown overnight in RPMI, diluted 1:200 in the same medium adjusted to different pHs, added to sterile 96-well polystyrene plates, and statically incubated at 37°C for 24 h. Experimental conditions for biofilm detection are identical to those reported in Fig. 2. This experiment was performed three times with similar results. P values were determined using a two-tailed, two-sample unequal variance Student t test in GraphPad.
Role of IsdC in attachment and accumulation during biofilm formation.
Biofilm formation in static growth conditions occurs in several stages, beginning with attachment of individual cells to a surface, followed by growth and the accumulation of cells in a multilayered complex held together by proteins or polysaccharide. In our experiments, bacteria must attach to the surface of a polystyrene dish. To test attachment, bacterial cells were incubated in dishes, washed, and immobilized in molten agar before incubation overnight to allow colonies to develop. For the wild-type strain N920143 73% of the added cells attached, whereas for the isdC mutant only 36.7% attached. The reverted control isdCr strain adhered at a level similar to that of the wild type. To investigate whether IsdC contributes to cell-cell aggregation and thus possibly to biofilm accumulation, suspensions of RPMI-grown bacteria were allowed to settle for up to 24 h. The density of cells at the top of the suspension was measured periodically. The density of the isdC mutant was significantly lower than that of the wild type or the restored mutant after 6 and 8 h, but the densities were the same after 24 h. This indicates that IsdC is involved both in the initial attachment of bacterial cells and also in the accumulation phase.
IsdC forms homodimers.
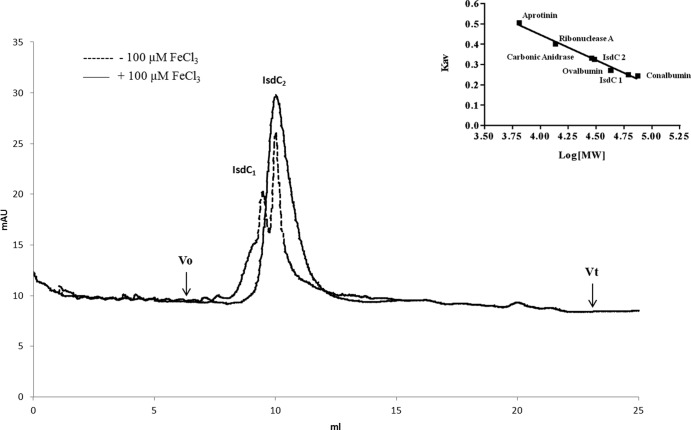
To test the hypothesis that the ability of IsdC to promote the accumulation phase of biofilm formation by iron-starved bacteria could be due to a homophilic interaction between IsdC proteins attached to adjacent cells, the ability of the protein to dimerize in solution was examined. A preparation of purified recombinant IsdC was subjected to gel filtration chromatography on a Superose 12 HR column equilibrated with or without FeCl3 (Fig. 7). Two major peaks were eluted from the column equilibrated with 100 μM FeCl3, an early peak corresponding to IsdC dimers (Mr = 59 kDa) and a retarded peak with a molecular mass of 29.5 kDa which corresponds to the IsdC monomer. Conversely, a single peak of 29.5 kDa was obtained from the column equilibrated and eluted with PBS only. Thus, an equilibrium exists between the monomeric and dimeric forms of IsdC in solution.
FIG 7.
Analysis of IsdC interactions by size exclusion chromatography. A 100-μl sample of IsdC at a concentration of 100 μM was loaded onto a gel filtration column of Superose 12 HR and eluted with one column volume (Vt = 24 ml) of PBS with or without 100 μM FeCl3 at a flow rate of 0.5 ml/min. Molecular weight determination was carried out by comparing elution volumes of the two peaks marked as IsdC1 and IsdC2, with the values obtained for several known calibration standards. The Mr of IsdC1 or IsdC2 was determined from the calibration curve (plot of Kav versus log Mr) once their Kav values were calculated from the measured elution volumes [Kav = (elution volume − void volume)/(column volume − void volume)] (inset).
To determine whether IsdC protein that is attached to the surface of a bacterial cell could interact with other IsdC molecules, the isdC gene was cloned into the nisin-inducible L. lactis expression vector pNZ8048. The sortase B cleavage motif NPQTS was changed to LPQTG in order to provide a substrate for sortase A in L. lactis. After induction, expression of IsdC was detected in the cell wall fraction derived from protoplasts of L. lactis by Western immunoblotting (Fig. 8A), indicating that IsdC was sorted to the cell wall. When the ability of L. lactis expressing IsdC to adhere to immobilized Isd proteins was tested, L. lactis(pNZ8048isdC) adhered to immobilized IsdC at a 4-fold-higher level than the control strain bearing the empty vector but not significantly to IsdB, IsdJ, or IsdK. A slight promotion of adherence to IsdB was noted, but this was not statistically significant. These data are consistent with the conclusion that IsdC homophilic interactions contribute to the accumulation phase of S. lugdunensis biofilm formation under iron-restricted conditions (Fig. 8B). As reported for biofilm formation by S. lugdunensis N920143 (Fig. 6B), the interaction of L. lactis(pNZ8048isdC) with immobilized IsdC was enhanced at pH 6.0 and 6.5 and decreased at pH 5.0, 5.5, 7.0, 7.5, and 8.0 (Fig. 8C). Consistent with low-iron-induced biofilm formation (Fig. 5), we also found that attachment of L. lactis(pNZ8048isdC) to surface-coated IsdC requires the presence of FeCl3 (≥1 μM). Significantly reduced levels of IsdC homophilic interactions were observed when bacterial attachment was performed in iron-depleted conditions (Fig. 8D).
FIG 8.
Homophilic and heterophilic interactions of IsdC expressed by L. lactis with surface-coated Isd proteins. (A) Validation of IsdC expression by L. lactis. The expression of IsdC by L. lactis(pNZ8084isdC) and L. lactis(pNZ8084) was demonstrated by Western immunoblotting. IsdC protein in the solubilized cell wall lysates was detected with rabbit anti-IsdC IgG, followed by HRP-conjugated secondary goat anti-rabbit IgG. (B) Adhesion of L. lactis expressing IsdC to Isd proteins. Microtiter wells were coated with recombinant IsdB, IsdC, IsdK, and IsdJ in a bicarbonate buffer overnight (1 μg/well). L. lactis(pNZ8084isdC) and L. lactis(pNZ8084) (5 × 108 cells/well) were added to the wells, and the mixtures were incubated for 1 h. After being washed with PBS, adherent cells were fixed, stained with crystal violet, and measured at 595 nm. (C) Effect of pH on the adherence of L. lactis(pNZ8084isdC) and L. lactis(pNZ8084) to immobilized IsdC protein. Bacterial cells (5 × 108 cells/well) suspended in growth medium were adjusted to the indicated pHs and added to the wells. (D) Effect of iron concentrations on the adherence of L. lactis. Bacterial cells (5 × 108 cells/well) suspended in growth medium were supplemented with increasing amounts of FeCl3 and added to the wells. Incubation of the mixtures as described in panels C and D was carried out for 1 h, and detection of attached bacteria was performed as reported for panel B. The results shown in panels B, C, and D are mean values of triplicate samples. Error bars show the standard deviations. P values were determined using a two-tailed, two-sample unequal variance Student t test in GraphPad.
Inhibition by recombinant Isd proteins and antibodies.
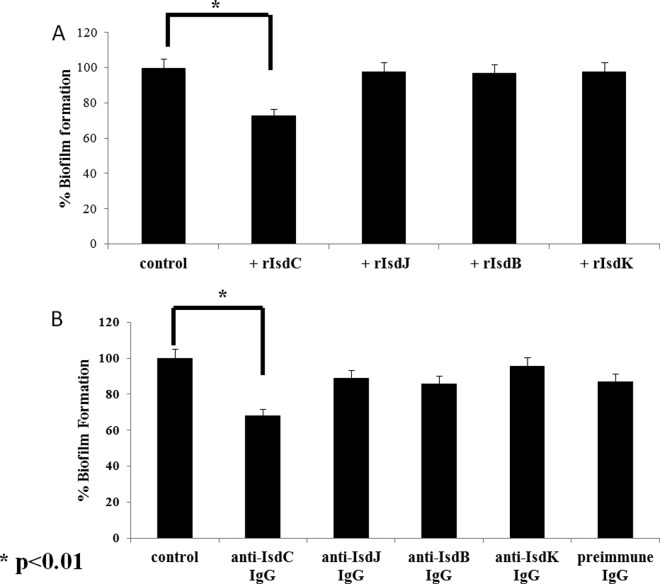
The soluble recombinant Isd proteins or specific antisera raised against each of the Isd proteins were added to the RPMI0.3%glucose–2 mM glutamine medium at the same time as the inoculum and incubated for 24 h before the density of biofilm formed was measured. Only IsdC protein or anti-IsdC serum caused a significant (∼ 35%) reduction in biofilm formation (Fig. 9). Although complete inhibition was not achieved, we can be confident of the specificity of the effect, which is consistent with an important role in biofilm formation.
FIG 9.
Effect of soluble Isd proteins or anti-Isd IgG on the biofilm formation by S. lugdunensis 920143. Overnight cultures of S. lugdunensis 920143 grown in RPMI were diluted into fresh RPMI-glucose, and 200-μl portions of the mixtures were allowed to form biofilm for 24 h in the presence of 5 μM Isd proteins (A) or 2 μg of preimmune or immune IgG/ml raised against each Isd protein (B). Adherent cells were stained with crystal violet, and the absorbance was measured at 595 nm. Bars are the averages of measurements taken from four wells. Biofilm values are expressed as the percentage of the biofilm level obtained from cultures grown in the absence of any potential inhibitor. Error bars represent the standard deviations. This experiment was performed three times with similar results. P values were determined using a two-tailed, two-sample unequal variance Student t test in GraphPad.
DISCUSSION
The identification of surface proteins that are able to induce biofilm development in the absence of polysaccharides is one of the most unexpected results obtained recently in the field of staphylococcal biofilm studies. The existence of alternative mechanisms promoting biofilm development suggests that different staphylococcal strains might involve a specific component to form a particular type of biofilm matrix. S. aureus can opt for at least five different surface proteins, Bap (8), protein A (11), SasG (9), SasC (10), and FnBPs (12, 13) to achieve protein-mediated biofilm. Moreover, functional amyloids composed of phenol soluble modulins stabilize S. aureus biofilms (43). A similar proteinaceous biofilm has been demonstrated with S. epidermidis based on the expression of proteins such as Aap (14) or SesC (16, 44). Furthermore, the same staphylococcal strain can switch between a proteinaceous or polysaccharide-based biofilm, depending on the growth conditions (45).
Investigating biofilm production by a collection of strains of S. lugdunensis, we confirmed the proteinaceous nature of biofilms in iron-rich (TSB) medium (24) and demonstrated for the first time that biofilm formed in iron-restricted conditions (RPMI) was also protein dependent. We found that proteins expressed by the isd locus are involved in biofilm development, and we identified the IsdC protein as the main factor responsible of cell-to-cell interactions and biofilm formation in iron-restricted conditions. IsdC contributed both to the attachment of cells to a polystyrene surface and to the accumulation phase. Thus, IsdC can be added to the growing list of proteinaceous factors involved in staphylococcal biofilm formation.
Surprisingly, for maximal biofilm formation, both IsdC expression and iron-restricted conditions were required, whereas S. lugdunensis grown in completely iron-depleted conditions (Chelex-treated RPMI) or in RPMI supplemented with excess amounts of FeCl3 (≥250 μM) developed a significantly reduced biofilm. Thus, iron plays a dual role in IsdC-dependent biofilm formation. Iron restriction is required to induce IsdC expression, and a low level of iron is required to support the formation of biofilm, whereas high levels (>250 μM) are inhibitory. Given that S. lugdunensis biofilm is dependent on IsdC and that IsdC has been demonstrated to bind heme (29), it is also possible that IsdC-associated heme is involved in biofilm formation, although it cannot be excluded that the requirement for iron could be unrelated to heme binding. The fact that IsdC is only expressed under iron-restricted conditions and the ability of S. lugdunensis strains also to form proteinaceous biofilm under iron-replete conditions implies that biofilm formation in TSB is promoted by proteinaceous factors distinct from those involved in RPMI growth medium. It would be interesting to determine whether the protein(s) involved are anchored by sortase A.
The observation that IsdC confers primary attachment to polystyrene and production of cell aggregates in the accumulation phase of biofilm, parallels the behavior observed for Bap (8) and SasC (10) of S. aureus. The aggregation activity observed for IsdC is also promoted by staphylococcal proteins such as Aap (14), SasG (9), and FnBPs (13).
The structural characterization of Aap and SasG in biofilm development has been recently defined (46, 47). Aap and SasG comprise an N-terminal A region and repeated B domains toward the C terminus. Proteolytic removal of the A domain of Aap or limited partial cleavage within the B domains of SasG is required for biofilm formation. This presumably reduces steric hindrance by the N-terminal region and allows the B repeats from opposing cells to engage. The length of the B region was shown to be critical for biofilm development because only SasG (or Aap) constructs with five or more B-repeats supported biofilm formation, while constructs with four or fewer B-repeats did not. Structural studies revealed that Aap B-repeats of the appropriate length adopt an elongated, rope-like structure coordinated by zinc ions and wrap around one another in an antiparallel fashion to form bundles of fibers that establish homophilic interactions and have the potential to interconnect neighbor cells (46, 47).
Homophilic protein-protein interactions may also be operational in the biofilm promoted by IsdC. Indeed, IsdC forms dimers in solution in the presence of FeCl3 and immobilized recombinant IsdC specifically interacts with IsdC expressed on the surface of L. lactis. Moreover, as reported for biofilm formation by S. lugdunensis, this interaction is dependent on the pH, suggesting that pH-induced changes in the conformation of IsdC contribute to both biofilm formation and homophilic interactions.
Due to the absence of tandem repeats in IsdC, alternative structures/mechanisms should be envisaged in IsdC-mediated biofilm formation. For example, the N-terminal subdomain N2N3 of the A region of the fibronectin-binding protein FnBPA and the N-terminal domain of SasC, but not their repetitive regions, mediate biofilm formation in S. aureus (10, 13). Thus, there are two different scenarios: one involving Aap and SasG and requiring a Zn-dependent interaction between repeats and a second wherein a direct Zn-dependent binding of the A domain of FnBPA on one cell to the A domain on another one is needed. The possibility that the A domain of FnBPA bound to a different ligand on the adjacent cell surface cannot be excluded. However, this was not backed up by in vitro studies with recombinant proteins as in the case of IsdC.
Although in vitro evidence indicates the involvement of homophilic interactions, it cannot be excluded that IsdC in vivo might establish heterophilic interactions with proteinaceous or nonproteinaceous components expressed on the surface or localized in the extracellular matrix of staphylococcal cells. Moreover, due to the reduced but not abolished ability of the Δisd mutant of S. lugdunensis strains to develop biofilms and the partial inhibition by soluble IsdC protein or anti-IsdC serum, it is plausible that additional, as-yet-undefined factors may contribute. The residual biofilm in the Δisd mutant and ΔisdC mutant was the same and appears to be proteinaceous (data not shown). It would be interesting to determine whether sortase A-anchored proteins (other than IsdB or IsdJ) could play a role in this process. This would require construction of a double Δisd srtA mutant.
Several questions on the role of IsdC in biofilm formation remain unanswered. For example, it should be of interest to investigate the molecular details of IsdC-mediated biofilm formation and whether the putative heme-binding NEAT domain is involved. This would require the production of recombinant mutant proteins and assessment of their ability to form dimers. Mutant strains with an altered potential to bind heme could also be constructed and concurrent evaluation of their capability to form biofilm performed. In connection with this, it could be also interesting to investigate the reasons why only IsdC, but not IsdB, IsdJ, or IsdK, is involved in biofilm formation, despite the similarity between their NEAT domains (29).
S. lugdunensis is an important cause of infections associated with indwelling medical devices. In general, device-related staphylococcal infections, including those associated with S. lugdunensis infections, are difficult to treat because staphylococci form biofilm and are protected from antimicrobial agents and the host's immune system. As a consequence, device removal can be required to resolve the infection. Thus, it will be of interest to determine whether IsdC-promoted biofilm contributes to virulence in animal models of foreign body infection. This is particularly important if one considers that due to the very low concentrations of free iron in the body fluids, IsdC should be expressed on the surfaces of S. lugdunensis cells and could play a role in biofilm formation under these conditions. We provide here initial clues about a protein that could be targeted to prevent developing of biofilm under conditions resembling those present in the body fluids.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge funding from Fondazione CARIPLO (Grant Vaccines 2009-3546) to P.S. Research in Dublin was supported by the IRCSET Embark Scholarship and by Science Foundation Ireland (Programme Investigator grant 08/IN.1/B1854).
Footnotes
Published ahead of print 31 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01542-14.
REFERENCES
- 1.Götz F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367–1378. 10.1046/j.1365-2958.2002.02827.x [DOI] [PubMed] [Google Scholar]
- 2.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. 10.1016/S0140-6736(01)05321-1 [DOI] [PubMed] [Google Scholar]
- 3.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 6:269–275. 10.1046/j.1462-5822.2004.00367.x [DOI] [PubMed] [Google Scholar]
- 4.Bose JL, Lehman MK, Fey PD, Bayles KW. 2012. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7:e42244. 10.1371/journal.pone.0042244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. 2011. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect. Immun. 79:1153–1165. 10.1128/IAI.00364-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaudaux PE, François P, Proctor RA, McDevitt D, Foster TJ, Albrecht RM, Lew DP, Wabers H, Cooper SL. 1995. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect. Immun. 63:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083–1091. 10.1111/j.1365-2958.1996.tb02548.x [DOI] [PubMed] [Google Scholar]
- 8.Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penadés JR. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888–2896. 10.1128/JB.183.9.2888-2896.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geoghegan JA, Corrigan RM, Gruszka DT, Speziale P, O'Gara JP, Potts JR, Foster TJ. 2010. Role of surface protein SasG in biofilm formation by Staphylococcus aureus. J. Bacteriol. 192:5663–5673. 10.1128/JB.00628-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder K, Jularic M, Horsburgh SM, Hirschhausen N, Neumann C, Bertling A, Schulte A, Foster S, Kehrel BE, Peters G, Heilmann C. 2009. Molecular characterization of a novel Staphylococcus aureus surface protein (SasC) involved in cell aggregation and biofilm accumulation. PLoS One 4:e7567. 10.1371/journal.pone.0007567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penadés JR, Lasa I. 2009. Protein A-mediated multicellular behavior in Staphylococcus aureus. J. Bacteriol. 191:832–843. 10.1128/JB.01222-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O'Gara JP. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190:3835–3850. 10.1128/JB.00167-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geoghegan JA, Monk IR, O'Gara JP, Foster TJ. 2013. Subdomains N2N3 of fibronectin binding protein A mediate Staphylococcus aureus biofilm formation and adherence to fibrinogen using distinct mechanisms. J. Bacteriol. 195:2675–2683. 10.1128/JB.02128-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohde H, Burdelski C, Bartscht K, Hussain M, Buck F, Horstkotte MA, Knobloch JK, Heilmann C, Herrmann M, Mack D. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55:1883–1895. 10.1111/j.1365-2958.2005.04515.x [DOI] [PubMed] [Google Scholar]
- 15.Banner MA, Cunniffe JG, Macintosh RL, Foster TJ, Rohde H, Mack D, Hoyes E, Derrick J, Upton M, Handley PS. 2007. Localized tufts of fibrils on Staphylococcus epidermidis NCTC 11047 are comprised of the accumulation-associated protein. J. Bacteriol. 189:2793–2804. 10.1128/JB.00952-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahrooei M, Hira V, Stijlemans B, Merckx R, Hermans PW, Van Eldere J. 2009. Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein. Infect. Immun. 77:3670–3678. 10.1128/IAI.01464-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PAD, Nervi C, Fleurette J. 1988. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int. J. Syst. Bacteriol. 38:168–172. 10.1099/00207713-38-2-168 [DOI] [Google Scholar]
- 18.Vandenesch F, Etienne J, Reverdy ME, Eykyn SJ. 1993. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin. Infect. Dis. 17:871–876 [DOI] [PubMed] [Google Scholar]
- 19.Patel R, Piper KE, Rouse MS, Uhl JR, Cockerill FR, III, Steckelberg JM. 2000. Frequency of isolation of Staphylococcus lugdunensis among staphylococcal isolates causing endocarditis: a 20-year experience. J. Clin. Microbiol. 38:4262–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anguera I, Del Río A, Miró JM, Matínez-Lacasa X, Marco F, Gumá JR, Quaglio G, Claramonte X, Moreno A, Mestres CA, Mauri E, Azqueta M, Benito N, García-de la María C, Almela M, Jiménez-Expósito, Sued MJO, De Lazzari E, Gatell JM. 2005. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart 91:e10. 10.1136/hrt.2004.040659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebright JR, Penugonda N, Brown W. 2004. Clinical experience with Staphylococcus lugdunensis bacteremia: a retrospective analysis. Diagn. Microbiol. Infect. Dis. 48:17–21. 10.1016/j.diagmicrobio.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 22.Sampathkumar P, Osmon DR, Cockerill FR. 2000. Prosthetic joint infection due to Staphylococcus lugdunensis. Mayo Clin. Proc. 75:511–512. 10.1016/S0025-6196(11)64220-1 [DOI] [PubMed] [Google Scholar]
- 23.Sandoe JA, Longshaw CM. 2001. Ventriculoperitoneal shunt infection caused by Staphylococcus lugdunensis. Clin. Microbiol. Infect. 7:385–387. 10.1046/j.1198-743x.2001.00268.x [DOI] [PubMed] [Google Scholar]
- 24.Frank KL, Patel R. 2007. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect. Immun. 75:4728–4742. 10.1128/IAI.00640-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geoghegan JA, Ganesh VK, Smeds E, Liang X, Höök M, Foster TJ. 2010. Molecular characterization of the interaction of staphylococcal microbial surface components recognizing adhesive matrix molecules (MSCRAMM) ClfA and Fbl with fibrinogen. J. Biol. Chem. 285:6208–6216. 10.1074/jbc.M109.062208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabados F, Marlinghaus L, Korte M, Neumann S, Kaase M, Gatermann SG. 2011. Fbl is not involved in the invasion of eukaryotic epithelial and endothelial cells by Staphylococcus lugdunensis. FEMS Microbiol. Lett. 324:48–55. 10.1111/j.1574-6968.2011.02382.x [DOI] [PubMed] [Google Scholar]
- 27.Nilsson M, Bjerketorp J, Wiebensjö A, Ljungh A, Frykberg L, Guss B. 2004. A von Willebrand factor-binding protein from Staphylococcus lugdunensis. FEMS Microbiol. Lett. 234:155–161. 10.1111/j.1574-6968.2004.tb09527.x [DOI] [PubMed] [Google Scholar]
- 28.Heilbronner S, Holden MT, van Tonder A, Geoghegan JA, Foster TJ, Parkhill J, Bentley SD. 2011. Genome sequence of Staphylococcus lugdunensis N920143 allows identification of putative colonization and virulence factors. FEMS Microbiol. Lett. 322:60–67. 10.1111/j.1574-6968.2011.02339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zapotoczna M, Heilbronner S, Speziale P, Foster TJ. 2012. Iron-regulated surface determinant (Isd) proteins of Staphylococcus lugdunensis. J. Bacteriol. 194:6453–6467. 10.1128/JB.01195-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haley KP, Janson EM, Heilbronner S, Foster TJ, Skaar EP. 2011. Staphylococcus lugdunensis IsdG liberates iron from host heme. J. Bacteriol. 193:4749–4757. 10.1128/JB.00436-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, Tarkowski A, Foster SJ. 2007. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1:199–212. 10.1016/j.chom.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 32.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller E, Takeda S, Shiro H, Goldmann D, Pier GB. 1993. Occurrence of capsular polysaccharide/adhesin among clinical isolates of coagulase-negative staphylococci. J. Infect. Dis. 168:1211–1218. 10.1093/infdis/168.5.1211 [DOI] [PubMed] [Google Scholar]
- 34.Heilbronner S, Hanses F, Monk IR, Speziale P, Foster TJ. 2013. Sortase A promotes virulence in experimental Staphylococcus lugdunensis endocarditis. Microbiology 159:2141–2152 [DOI] [PubMed] [Google Scholar]
- 35.Corrigan RM, Foster TJ. 2009. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 61:126–129. 10.1016/j.plasmid.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 36.Li MZ, Elledge SJ. 2007. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 4:251–256. 10.1038/nmeth1010 [DOI] [PubMed] [Google Scholar]
- 37.Li MZ, Elledge SJ. 2012. SLIC: a method for sequence- and ligation-independent cloning. Methods Mol. Biol. 852:51–59. 10.1007/978-1-61779-564-0_5 [DOI] [PubMed] [Google Scholar]
- 38.Bahey-El-Din M, Griffin BT, Gahan CG. 2008. Nisin inducible production of listeriolysin O in Lactococcus lactis NZ9000. Microb. Cell Fact 7:24. 10.1186/1475-2859-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk IR, Casey PG, Hill C, Gahan CG. 2010. Directed evolution and targeted mutagenesis to murinize Listeria monocytogenes internalin A for enhanced infectivity in the murine oral infection model. BMC Microbiol. 10:318. 10.1186/1471-2180-10-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaignon P, Sadovskaya I, Ragunah Ch Ramasubbu N, Kaplan JB, Jabbouri S. 2007. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 75:125–132. 10.1007/s00253-006-0790-y [DOI] [PubMed] [Google Scholar]
- 42.Izano EA, Amarante MA, Kher WB, Kaplan JB. 2008. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 74:470–476. 10.1128/AEM.02073-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. 2012. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 8:e1002744. 10.1371/journal.ppat.1002744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahrooei M, Hira V, Khodaparast L, Khodaparast L, Stijlemans B, Kucharíková S, Burghout P, Hermans PW, Van Eldere J. 2012. Vaccination with SesC decreases Staphylococcus epidermidis biofilm formation. Infect. Immun. 80:3660–3668. 10.1128/IAI.00104-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vergara-Irigaray M, Valle J, Merino N, Latasa C, García B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penadés JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect. Immun. 77:3978–3991. 10.1128/IAI.00616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conrady DG, Wilson JJ, Herr AB. 2013. Structural basis for Zn2+-dependent intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. U. S. A. 110:E202–E211. 10.1073/pnas.1208134110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruszka DT, Wojdyla JA, Bingham RJ, Turkenburg JP, Manfield IW, Steward A, Leech AP, Geoghegan JA, Foster TJ, Clarke J, Potts JR. 2012. Staphylococcal biofilm-forming protein has a contiguous rod-like structure. Proc. Natl. Acad. Sci. U. S. A. 109:E1011–E1018. 10.1073/pnas.1119456109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.