ABSTRACT
Tenomodulin has been recognized as a biomarker for tendon differentiation, and its gene expression is regulated by several transcription factors including Scleraxis and Mohawk. In this study, we found a novel regulatory mechanism of tenomodulin expression. Equine bone marrow-derived mesenchymal stem cells (BMSCs) in monolayer culture showed a low mRNA level of tenomodulin in comparison with the level in the tendon. When cultured in collagen gel containing a glycogen synthase kinase-3 (GSK-3) inhibitor (BIO), expression of tenomodulin in BMSCs increased up to the level in the tendon. Participation of GSK-3 in its gene expression was further demonstrated by a gene silencing experiment with small interference RNA corresponding to GSK-3, suggesting that Wnt/β-catenin signaling mediated expression of tenomodulin. These results were confirmed by nuclear translocation of β-catenin in BIO-treated BMSCs cultured in collagen gel. Under this culture condition, expression of tenomodulin-related transcription factors including Scleraxis and Mohawk was not affected, suggesting that Wnt/β-catenin signaling was independent from these transcription factors. Additionally, BIO strongly enhanced expression of type XIV collagen in collagen-embedded BMSCs up to the level in the tendon, and other tendon-related extracellular matrix components such as decorin and fibromodulin were also upregulated. Taken together, these results indicated that activation of Wnt/β-catenin signaling could induce differentiation of BMSCs into tenomodulin-expressing tendon cells in collagen gel.
Keywords: BMSCs, tendon, tenomodulin, Wnt signaling, ECM
Equine tendonitis, injury to the superficial digital flexor (SDF) tendon, commonly occurs in racehorses as a result of excessive mechanical force loaded onto the tendon tissue and is known to be an intractable disorder that tends to be recurrent. To solve such clinical problems, the technical methods for autologous implantation of bone marrow-derived mesenchymal cells (BMSCs) into the SDF tendon have been developed [11, 13, 24]. BMSCs are a population of pluripotent, non-hematopoietic marrow-derived cells maintained in adult bone marrow, and these cells can differentiate into adipocytes, osteocytes, and chondrocytes [7, 13, 21]. However, mechanism of differentiation of BMSCs into the tendon cells has not been fully evaluated.
Tendons are tough bands of connective tissue that joins bones to muscles. The main component of the tendon is collagen fiber, which mainly consists of collagen type I with minor fibrillar collagenous components, including collagen types III, V, and VI [5, 22]. In addition to these fibrillar collagens, several fibril surface-associated macromolecules are known to regulate tendon fibrillogenesis and the formation of tendon bundles. Fibril-associated collagens with interrupted triple helices (FACITs) such as collagen types XII and XIV play significant roles in tendon fibrogenesis and the ensuing formation of the extracellular matrix [2, 3, 25]. Among the small leucine-rich proteoglycans (SLRPs), lumican and fibromodulin regulate fibril diameter [10], and decorin is a key regulator of assembly of collagen fibrils in the tendon [9, 26]. Additionally, tenascin-C is known to be abundant in the developing tendon, bone and cartilage; however, its function still remains unclear. On the other hand, tenomodulin is highly expressed in the tendon and recognized as a biomarker of tendon differentiation [18, 19, 23]. Tenomodulin is a member of the type II transmembrane glycoproteins and is predominantly expressed in tendons, ligaments, and eyes. Based on the analysis of tenomodulin-null mice, this glycoprotein functions as a regulator of proliferation and density of tendon cells and is involved in collagen fibril maturation in the tendon [8].
In embryonic development, several Wnt signaling pathways have been recognized based on their functions including body axis patterning, cell fate specification, cell proliferation, and cell migration as well as determination of differentiation fate in human BMSCs [20]. In Wnt/β-catenin signaling, binding of Wnt protein to its receptor, Frizzled, induces inhibition of the β-catenin destruction complex and then promotes stabilization and nuclear translocation of β-catenin. Nuclear β-catenin forms the transcriptional complex and results in activation of the target genes. Glycogen synthase kinase-3 (GSK-3) is known as a component of the β-catenin destruction complex, and inhibition of GSK-3 results in stabilization of β-catenin and activation of sebsequent β-catenin-mediated gene expression.
In this study, we demonstrated that expression of tenomodulin in equine BMSCs cultured collagen gel was enhanced by inhibition of GSK-3 with a selective inhibitor and gene silencing with small interference RNA (siRNA). Under these culture conditions, expression of tendon-related extracellular matrix components (ECM) was also evaluated.
Materials and Methods
Cell culture
BMSCs were established from eight thoroughbred racehorses (3–5 years old) as previously described [13], and their pluripotency was confirmed based on ability to differentiate into three cell lineages including osteocytes, adipocytes, and chondrocytes as previously reported [13]. All animal procedures were approved by the Ethics Committee for Laboratory Animals of the Japan Racing Association Equine Research Institute. These BMSCs were propagated with growth medium comprised of Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich Inc., St. Louis, MO, USA) supplemented with 20% fetal bovine serum (FBS, Invitrogen Corp., Carlsbad, CA, USA) and a penicillin-streptomycin-neomycin solution (Sigma-Aldrich) in an atmosphere of humidified air containing 5% CO2 at 37˚C. These BMSCs were mixed and used for the following experiments at population doubling level 4. For collagen gel culture, 5 × 105 cells suspended in 500 µl of the growth medium were mixed with 50 µl of 10 × DMEM (Biochrom, Leonorenstr, Berlin, Germany) and 450 µl of 1% collagen solution (Wako Pure Chemical Industries, Osaka, Japan), and then poured into the wells of a 24-well plate. After polymerization of collagen gel, the medium was added and cultured for 7 days, and these gels were then harvested for quantitative RT-PCR (qRT-PCR) analysis. For the experiment with a signal inhibitor, 5 μM cytochalasin D, 100 μM genistein (tyrosine kinase inhibitor), 1 μM U0126 (MEK1 inhibitor) and 10 μM BIO (GSK-3 inhibitor) were added to the culture medium at beginning of the cultivation. Cell transfection of siRNA corresponding to GSK-3α/β (Cell Signaling Technology Inc, Danvers, MA, USA) into BMSCs was performed using X-tremeGENE siRNA Transfection Reagent (Roche Diagnostics, Mannheim, Germany). Briefly, BMSCs (5 × 105 cells) were seeded into 100 mm culture dishes and cultured overnight. Two hours before transfection, the culture medium was changed to DMEM containing 5% FBS, and then the cells in each dish were transfected with a mixture of 975 µl of Opti-MEM I (Invitrogen) and 25 µl of transfection reagent containing 0.15 μM of siRNA. After culture overnight, cells were trypsinized and applied to collagen gel culture for 72 hr, total RNA was then extracted from the collagen gel.
qRT-PCR
Normal SDF tendon tissues were obtained from two adult (3-year-old) male thoroughbred racehorses. Whole tissues were powdered using a stainless ball mill under liquid nitrogen and mixed. Total RNA in tissue powder and cultured BMSCs was isolated by RNAiso Plus (Takara, Shiga, Japan) according to the manufacturer’s protocol. Complementary DNA was prepared by reverse transcription of DNase I (Roche)-treated total RNA with a One Step SYBR PrimeScript RT-PCR Kit (Takara), and then qRT-PCR was performed with a Thermal Cycler Dice Real Time System (Takara) using SYBR Premix Ex Taq II (Takara). Several matrix-related components were amplified using the specific primer sets shown in Table 1 , and nucleotide sequences of amplified DNA fragments were confirmed using an autosequencer (ABI, Life Technologies Japan, Tokyo, Japan).
Table 1. Sequences of PCR primer sets used for qRT-PCR.
| Gene | Sequence | Sequence | Anealing temp | Size |
|---|---|---|---|---|
| GAPDH | U: 5´- GTGTCCCCACCCCTAACG -3´ | L: 5´- AGTGTAGCCCAGGATGCC -3´ | 55.1 | 131 |
| Scleraxis | U: 5´- CCCCCACGGACCTGACTC -3´ | L: 5´- GGTAGGAAGCCAGCACGG -3´ | 59.6 | 167 |
| Mohawk | U: 5´- TAATCCCGTTCACCATCC -3´ | L: 5´- CTTTGCCTTGTCTTTCCC -3´ | 52.7 | 195 |
| Tenomodulin | U: 5´- GGCGGGTTATCTGTCGTG -3´ | L: 5´- TACCAGGAGCCAAATGCC -3´ | 53.0 | 169 |
| NKD1 | U: 5´- CACAGGAAGCCCAAGCAC -3´ | L: 5´- TTCGTGGTGGTGGTGATG -3´ | 59.2 | 183 |
| Col1a2 | U: 5´- GTGGAAAAGGTGAACAGG -3´ | L: 5´- CAATAGGACCAGCAGGAC -3´ | 55.3 | 198 |
| Col3a1 | U: 5´- CCTGGTTACTGCTTGCTC -3´ | L: 5´- GAATCTCTGGGTTGGGAC -3´ | 52.2 | 213 |
| Col12a1 | U: 5´- TTTTATGTGAGCCGACTG -3´ | L: 5´- TACTCATCTTCCACCACG -3´ | 49.1 | 98 |
| Col14a1 | U: 5´- CTGGACGATGGAAGTGAG -3´ | L: 5´- GTGACCCTGAACTGCTGC -3´ | 52.8 | 215 |
| Decorin | U: 5´- TTATCAAAGTGCCTGGTG -3´ | L: 5´- CATAGACACATCGGAAGG -3´ | 51.1 | 204 |
| Fibromodulin | U: 5´- GCTTCTGCTGAGGGACAC -3´ | L: 5´- GATTTCTGGGGTTGGGAC -3´ | 52.1 | 91 |
| Lumican | U: 5´- ATTTCATCACAAGCACAG -3´ | L: 5´- TGACTTCCATACAACCAG -3´ | 45.6 | 131 |
| Tenascin-C | U: 5´- GAACACGGTGGAGTATGC -3´ | L: 5´- TTGGTAGTGATGGCTGAG -3´ | 52.1 | 105 |
Histological analysis
Collagen gels containing BMSCs were fixed with 4% paraformaldehyde for 6 hr, dehydrated with graded ethanol, immersed in xylene, and then embedded in paraffin. Sections 4 μm thick were deparaffinized and stained with hematoxylin and eosin. Immunofluorescent microscopy was performed to analyze the localization of β-catenin protein. Deparaffinized sections were incubated with an anti-β-catenin polyclonal antibody (Sigma-Aldrich) at 4˚C overnight, followed by incubation with tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC)-conjugated secondary antibody (Chemicon International Inc., Temecula, CA, USA) and DAPI for one hour at room temperature. Resulting slides were mounted with aqueous mounting medium (Mount-Quick Aqueous, Daido Sangyo Co., Ltd., Tokyo, Japan), and images were acquired with an LSM710 confocal laser-scanning microscope (Carl Zeiss Microscopy GmbH, Munich, Germany).
Statistical analysis
The results are shown as the mean ± SD of a representative experiment performed in triplicate. The statistical significance of differences between the values of the respective experimental groups and controls was determined by Mann-Whitney’s U test, and values of P<0.05 were considered significant.
Results
Comparison of tendon-related components between tendon and monolayer BMSCs by qRT-PCR analysis
Levels of mRNA corresponding to tendon-related components were compared between the tendon and BMSCs by qRT-PCR analysis (Table 2). The level of tenomodulin mRNA of BMSCs in monolayer culture was about 1/10 of the level in the tendon. Among major tendon collagens, no significant difference in mRNA levels corresponding to collagen type I α2 chain (Col1a2) and type III α1 chain (Col3a1) was observed between the tendon and monolayer BMSCs, while the level of the type XII α1 chain (Col12a1) in BMSCs was rather higher than that in the tendon. On the other hand, the level of mRNA corresponding to the type XIV collagen α1 chain (Col14a1) in BMSCs was about 1/50 of the level in the tendon. Among other tendon-related ECM components, the mRNA levels corresponding to decorin and fibromodulin in BMSCs showed about 1/40 and 1/20 of the level of the tendon, respectively, while there was no significant difference in mRNA level of lumican and tenascin-C. These results indicated that Col14a1, decorin, and fibromodulin showed lower expression in monolayer BMSCs than in the tendon, suggesting that these components could be additional differentiation markers of tendon.
Table 2. Comparison of mRNA level between the tenodon and monolayer BMSC by qRT-PCR analysis.
| Gene | Tenodon | Monolayer BMSC |
|---|---|---|
| Tenomodulin | 0.00057 ± 0.00012* | 0.00006 ± 0.00001 |
| Col1a2 | 0.11810 ± 0.03612 | 0.15139 ± 0.02522 |
| Col3a1 | 0.00880 ± 0.00251 | 0.13373 ± 0.02121 |
| Col12a1 | 0.07856 ± 0.01431 | 0.24827 ± 0.03142 |
| Col14a1 | 0.01458 ± 0.00373* | 0.00003 ± 0.00001 |
| Decorin | 29.65080 ± 2.85643* | 0.70031 ± 0.14381 |
| Fibromodulin | 0.11311 ± 0.02413* | 0.00599 ± 0.00143 |
| Lumican | 1.16473 ± 0.28143 | 0.94606 ± 0.14877 |
| Tenascin-C | 0.01858 ± 0.00143 | 0.01010 ± 0.00131 |
The values are expressed as means ± SD obtained from three independent experiments. * P<0.05 vs. monolayer BMSC.
Regulation of tenomodulin expression via canonical Wnt/β-catenin signaling
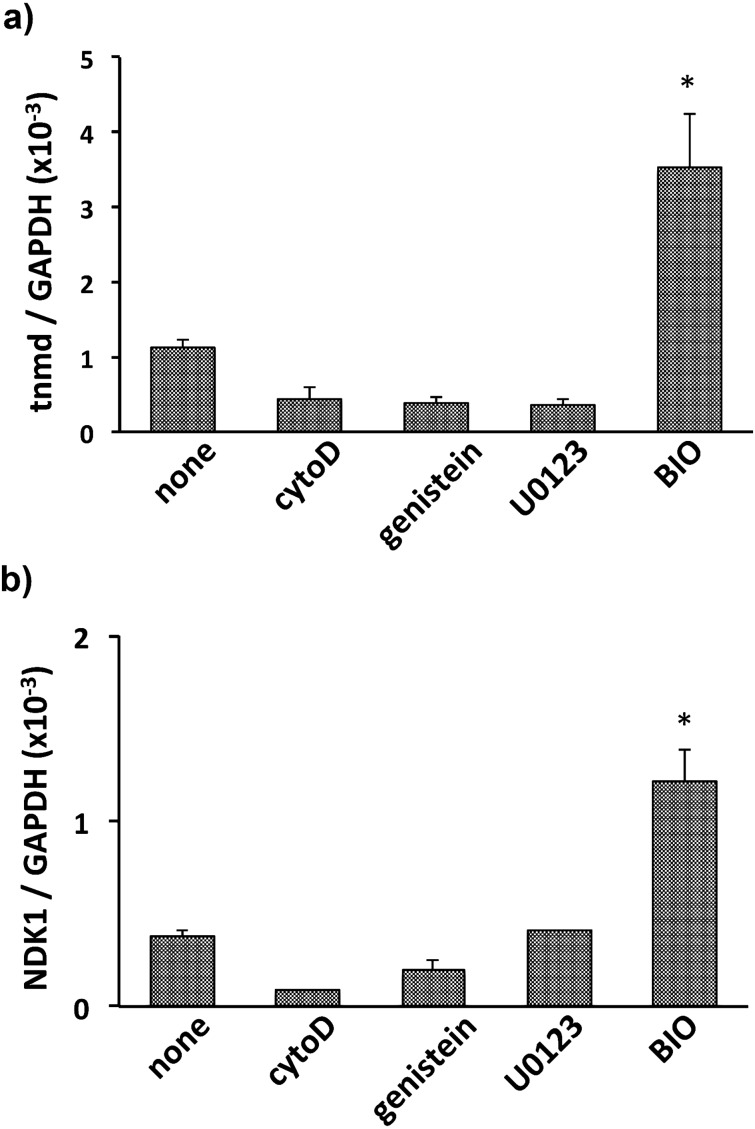
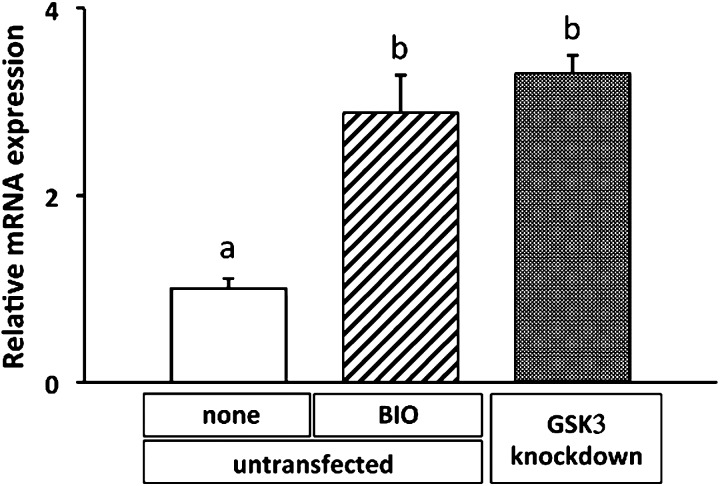
To evaluate the effect of the GSK-3 inhibitor on expression of tenomodulin, BMSCs were cultured in collagen gel with BIO. As a result, BIO intensely enhanced expression of tenomodulin mRNA, whereas other signal inhibitors including cytochalasin D, genistein, and U0126 did not affect its mRNA level (Fig. 1a). These results were supported by BIO-mediated induction of naked cuticle 1 homolog (NKD1), which is a well-known downstream target of Wnt signaling (Fig. 1b). Participation of Wnt/β-catenin signaling in tenomodulin expression was further confirmed by a gene silencing experiment for GSK-3α/β. While the mRNA level of tenomodulin in BIO-treated BMSCs was upregulated by approximately 3-fold within 72 hr compared with control BMSCs, the mRNA level of GSK-3-knockdown BMSCs similarly increased when the cells were cultured in collagen gel without BIO (Fig. 2). These results demonstrated that inhibition of GSK-3 brought upregulation of tenomodulin. Next, to evaluate nuclear translocation of β-catenin, histological examination was performed. The cells in the control gel showed a fibroblastic shape, and assemblies of collagen fiber were observed around the cells (Fig. 3a, arrows), whereas BIO-treated cells had a large round shape with very thin cytoplasm (Fig. 3b, arrowheads). Immunocytochemical staining of β-catenin demonstrated nuclear translocation of β-catenin in BIO-treated BMSCs (Fig. 3d), while β-catenin was located in the cytoplasm in untreated cells (Fig. 3c). These results demonstrated that Wnt/β-catenin signaling regulated expression of tenomodulin in BMSCs.
Fig. 1.
qRT-PCR analysis of tenomodulin (a) and NKD1 (b) in BMSCs cultured in collagen gel with cytochalasin D (cytoD), genistein, U0126, or BIO, respectively. The values are expressed relative to the control (mean ± SD) from three independent experiments (* P<0.05).
Fig. 2.
Effect of siRNA corresponding to GSK-3α/β on expression of tenomodulin mRNA. qRT-PCR analysis showed the level in untreated BMSCs without BIO (non), with BIO, or with GSK-3α/β-knockdown BMSCs cultured for 72 hr in collagen gel. The values are expressed relative to the control (mean ± SD) from three independent experiments. Different superscript letters indicate significant differences (P<0.05).
Fig. 3.
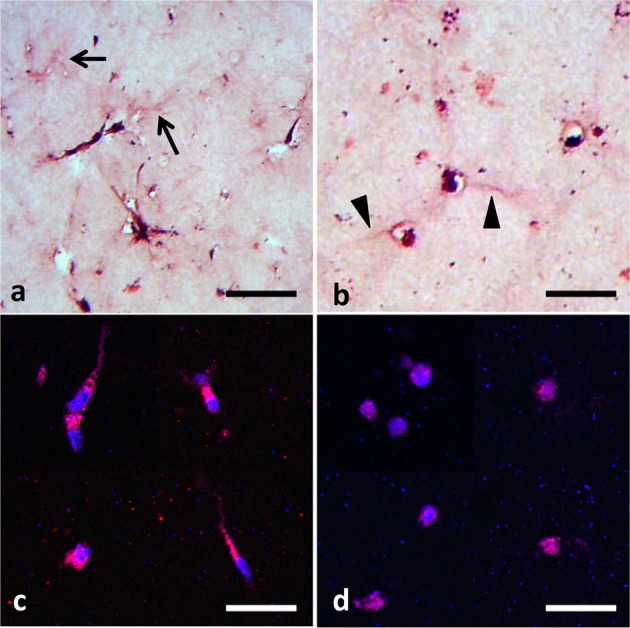
Histology of BMSCs embedded in collagen gel cultured for 7 days. HE staining of BMSCs cultured in collagen gel without (a) or with BIO (b). Arrows indicate the assembly of collagen fibers observed in the control gel, and arrowheads indicate very thin cytoplasm of round shaped BMSCs formed in BIO-supplemented gel. Immunohistochemical staining of β-catenin (red) in BMSCs cultured for 7 days in collagen gel without (c) or with BIO (d). Nuclear stainings were performed with DAPI (blue; bars=20 μm).
Induction of tendon-related components in BMSCs cultured in collagen gel by activation of Wnt/β-catenin signaling
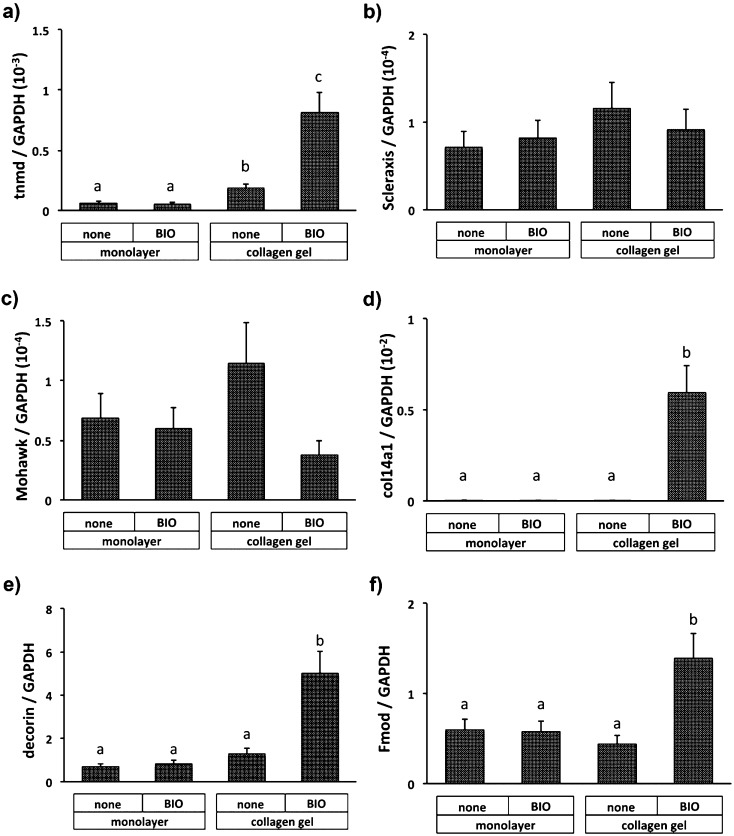
The effect of BIO on tenomodulin expression was not observed in monolayer culture. Tenomodulin expression was slightly enhanced when cultured in collagen gel and was augmented by Wnt/β-catenin activation (Fig. 4a). Regarding transcription factors, which are thought to regulate tenomodulin expression, both Scleraxis (Fig. 4b) and Mohawk (Fig. 4c) were not apparently affected by addition of BIO in both culture conditions. Whereas BIO did not affect expression of Col14a1 (Fig. 4d), decorin (Fig. 4e), and fibromodulin (Fig. 4f) in monolayer BMSCs, thier expressions were significantly enhanced in BIO-containing collagen gel. The value of Col14a1 relative to GAPDH in monolayer BMSCs was upregulated by 160-fold by addition of BIO to collagen gel, while the relative values for decorin and tenomodulin increased by 7-fold and 2-fold compared with the values in monolayer, respectively.
Fig. 4.
qRT-PCR analysis of mRNA levels of tenomodulin (tnmd; a), Scleraxis (b), Mohawk (c), type XIV collagen (col14a1; d), decorin (e) and fibromodulin (Fmod; f) in monolayer culture or collagen gel with or without BIO (non). The values are expressed relative to the control (mean ± SD) from three independent experiments. Different superscript letters indicate significant differences (P<0.05).
Discussion
Tenomodulin has been recognized as the marker of tendon differentiation, however, the mechanism of its transcriptional regulation remains unclear. Two DNA-binding proteins have been identified as being involved in vertebrate tendon formation, the basic helix-loop-helix transcription factor, Scleraxis [17], and the Mohawk homeobox [12, 15]. Scleraxis-null mice displayed decreased expression of type I collagen as well as tenomodulin on embryonic day 16.5 [15]. On the other hand, analysis of Mohawk-null mice indicated that this homeobox regulated tenomodulin expression in the embryo [12], while expression of type I collagen was regulated in the adult stage [15]. Additionally, mice with a mutation in the early growth response transcription factors, Egr1, showed downregulation of Scleraxis, Mohawk and tenomodulin as well as type XIV collagen [14]. In vitro studies demonstrated that Smad3, the mediator of TGFβ signaling, binds both Scleraxis and Mohawk and that loss of Smad3 results in reduced protein expression of the matrix components including type I collagen and tenascin-C [4]. Furthermore, Scleraxis could also induce to differentiation of BMSCs into the lineage of tendon cells. It was also reported that forced expression of Scleraxis induced human BMSCs to express tendon-related ECM components in addition to tenomodulin [1] and that the combination of forced expression of Scleraxis and mechanical stress converted human ES cells to tendon cells [6]. These previous studies demonstrated that tenomodulin is regulated several transcription factors; however, there is no information concerning external factors that directly upregulate tendon-related differentiation markers.
In this study, we found that a selective inhibitor of GSK-3, BIO, increased the mRNA level of tenomodulin and nuclear translocation of β-catenin in BMSCs cultured in collagen gel. While the level of tenomodulin mRNA in monolayer BMSCs was about 1/10 of that in the tendon, the mRNA level was slightly increased in collagen gel culture and further enhanced in the presence of BIO. Collagen gel culture is known to produce an artificially created environment in which biological cells are permitted to express specific phenotypes. It was recently reported that collagen lattice stimulated human BMSCs and increased the nuclear β-catenin protein level [16]. Our results also indicated slight upregulation of tenomodulin in collagen gel without BIO (Fig. 4a). Taking these findings into account, equine BMSCs may also upregulate β-catenin in collagen gel, and so further stabilization of β-catenin by BIO could contribute to upregulation of tenomodulin in BMSCs.
Next, whether inhibition of GSK-3 affects expression of tenomodulin-regulating transcription factors including Scleraxis and Mohawk was evaluated. The results showed that theses transcription factors did not significantly changed under several culture conditions, suggesting a new regulation pathway for expression of tenomodulin via the Wnt/β-catenin signaling pathway. Regarding the tendon-related ECM components, the mRNA level of Col14a1, decorin, and fibromodulin were quite low in monolayer BMSCs, as shown in Table 2, as compared with the levels in the tendon. Addition of BIO significantly increased the mRNA levels of these components in collagen gel compared with those in monolayer culture. The level for Col14a1 relative to GAPDH in monolayer BMSCs was less than 1/100 of that in the tendon, and BIO increased the level up to that in the tendon in BMSCs culture in collagen gel (Fig. 4d). On the other hand, BIO increased expression of decorin and fibromodulin by 3- to 4-fold in comparison with the expression in monolayer culture. These results indicated that Wnt/β-catenin signaling also stimulated expression of tendon-related ECM components in addition to tenomodulin. Taking the above into account, activation of Wnt/β-catenin signaling shifted BMSCs cultured in collagen gel to tenomodulin-expressing tendon cells, but the tendon-related ECM components, except for Col14a1 were not fully upregulated to the level of the tendon. Thus, further studies to develop a culture system to enhance expression of these tendon-related ECM components will be needed.
References
- 1.Alberton P., Popov C., Prägert M., Kohler J., Shukunami C., Schieker M., Docheva D.2012. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 21: 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansorge H.L., Meng X., Zhang G., Veit G., Sun M., Klement J.F., Beason D.P., Soslowsky L.J., Koch M., Birk D.E.2009. Type XIV collagen regulates fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE. J. Biol. Chem. 284: 8427–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai K., Kasashima Y., Kobayashi A., Kuwano A., Yoshihara T.2002. TGF-beta alters collagen XII and XIV mRNA levels in cultured equine tenocytes. Matrix Biol. 21: 243–250 [DOI] [PubMed] [Google Scholar]
- 4.Berthet E., Chen C., Butcher K., Schneider R.A., Alliston T., Amirtharajah M.2013. Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization. J. Orthop. Res. 31: 1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho H.F., Felisbino S.L., Keene D.R., Vogel K.G.2006. Identification, content, and distribution of type VI collagen in bovine tendons. Cell Tissue Res. 325: 315–324 [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Yin Z., Chen J.L., Shen W.L., Liu H.H., Tang Q.M., Fang Z., Lu L.R., Ji J., Ouyang H.W.2012. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci. Rep. 2: 977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Schauwer C., Meyer E., Van de Walle G.R., Van Soom A.2011. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology 75: 1431–1443 [DOI] [PubMed] [Google Scholar]
- 8.Docheva D., Hunziker E.B., Fässler R., Brandau O.2005. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell Biol. 25: 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunkman A.A., Buckley M.R., Mienaltowski M.J., Adams S.M., Thomas S.J., Satchell L., Kumar A., Pathmanathan L., Beason D.P., Iozzo R.V., Birk D.E., Soslowsky L.J.2013. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 32: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezura Y., Chakravarti S., Oldberg A., Chervoneva I., Birk D.E.2000. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 151: 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortier L.A., Smith R.K.2008. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet. Clin. North Am. Equine Pract. 24: 191–201 [DOI] [PubMed] [Google Scholar]
- 12.Ito Y., Toriuchi N., Yoshitaka T., Ueno-Kudoh H., Sato T., Yokoyama S., Nishida K., Akimoto T., Takahashi M., Miyaki S., Asahara H.2010. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 107: 10538–10542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasashima Y., Ueno T., Tomita A., Goodship A.E., Smith R.K.2011. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Vet. J. 43: 288–294 [DOI] [PubMed] [Google Scholar]
- 14.Lejard V., Blais F., Guerquin M.J., Bonnet A., Bonnin M.A., Havis E., Malbouyres M., Bidaud C.B., Maro G., Gilardi-Hebenstreit P., Rossert J., Ruggiero F., Duprez D.2011. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J. Biol. Chem. 286: 5855–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W., Watson S.S., Lan Y., Keene D.R., Ovitt C.E., Liu H., Schweitzer R., Jiang R.2010. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell Biol. 30: 4797–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauney J., Volloch V.2009. Collagen I matrix contributes to determination of adult human stem cell lineage via differential, structural conformation-specific elicitation of cellular stress response. Matrix Biol. 28: 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murchison N.D., Price B.A., Conner D.A., Keene D.R., Olson E.N., Tabin C.J., Schweitzer R.2007. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134: 2697–2708 [DOI] [PubMed] [Google Scholar]
- 18.Oshima Y., Sato K., Tashiro F., Miyazaki J., Nishida K., Hiraki Y., Tano Y., Shukunami C.2004. Anti-angiogenic action of the C-terminal domain of tenomodulin that shares homology with chondromodulin-I. J. Cell Sci. 117: 2731–2744 [DOI] [PubMed] [Google Scholar]
- 19.Oshima Y., Shukunami C., Honda J., Nishida K., Tashiro F., Miyazaki J., Hiraki Y., Tano Y.2003. Expression and localization of tenomodulin, a transmembrane type chondromodulin-I-related angiogenesis inhibitor, in mouse eyes. Invest. Ophthalmol. Vis. Sci. 44: 1814–1823 [DOI] [PubMed] [Google Scholar]
- 20.Qiu W., Chen L., Kassem M.2011. Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochem. Biophys. Res. Commun. 413: 98–104 [DOI] [PubMed] [Google Scholar]
- 21.Ranera B., Lyahyai J., Romero A., Vázquez F.J., Remacha A.R., Bernal M.L., Zaragoza P., Rodellar C., Martín-Burriel I.2011. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 144: 147–154 [DOI] [PubMed] [Google Scholar]
- 22.Roulet M., Ruggiero F., Karsenty G., LeGuellec D.2007. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: a clue for understanding collagen V function in developing connective tissues. Cell Tissue Res. 327: 323–332 [DOI] [PubMed] [Google Scholar]
- 23.Shukunami C., Oshima Y., Hiraki Y.2001. Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochem. Biophys. Res. Commun. 280: 1323–1327 [DOI] [PubMed] [Google Scholar]
- 24.Smith R.K., Korda M., Blunn G.W., Goodship A.E.2003. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 35: 99–102 [DOI] [PubMed] [Google Scholar]
- 25.Young B.B., Gordon M.K., Birk D.E.2000. Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev. Dyn. 217: 430–439 [DOI] [PubMed] [Google Scholar]
- 26.Zhang G., Ezura Y., Chervoneva I., Robinson P.S., Beason D.P., Carine E.T., Soslowsky L.J., Iozzo R.V., Birk D.E.2006. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell Biochem. 98: 1436–1449 [DOI] [PubMed] [Google Scholar]