Abstract
Gamma Knife Radiosurgery (GKRS) has been reported in the treatment of brainstem metastases while dose volume toxicity thresholds remain mostly undefined. A retrospective review of 52 brainstem metastases in 44 patients treated with GKRS was completed. A median dose of 18 Gy (range 10–22 Gy) was prescribed to the tumor margin (median 50 % isodose). 25 patients had undergone previous whole brain radiation therapy. Toxicity was graded by the LENT-SOMA scale. Mean and median follow-up was 10 and 6 months. Only 3 of the 44 patients are living. Multiple brain metastases were treated in 75 % of patients. Median size of lesions was 0.134 cc, (range 0.013–6.600 cc). Overall survival rate at 1 year was 32 % (95 % CI 51.0–20.1 %) with a median survival time of 6 months (95 % CI 5.0–16.5). Local control rate at 6 months and 1 year was 88 % (95 % CI 70–95 %) and 74 % (95 % CI 52–87 %). Cause of death was neurologic in 17 patients, non-neurologic in 20 patients, and unknown in four. Four patients experienced treatment related toxicities. Univariate analysis of tumor volume revealed that volume greater than 1.0 cc predicted for toxicity. A strategy of using lower marginal doses with GKRS to brain stem metastases appears to lead to a lower local control rate than seen with lesions treated within the standard dose range in other locations. Tumor size greater than 1.0 cc predicted for treatment-related toxicity.
Keywords: Gamma Knife, Brainstem, Metastasis, Toxicity, Radiosurgery
Introduction
An estimated 5 % of intracranial metastases are located in the brainstem [1]. While multiple randomized trials have demonstrated the efficacy of stereotactic radiosurgery (SRS) as a single modality treatment for patients with newly diagnosed brain metastases [2–5], brainstem metastases represent a unique clinical challenge due to potential toxicity concerns. Effective radiosurgical doses for intracranial lesions were originally derived from RTOG 90-05, a prospective study establishing toxicity thresholds based on lesion size [6]. However, this study excluded brainstem lesions. There have been several recent reports documenting the treatment of brainstem lesions with radiosurgery [7–15], though the proper dose and volume constraints remain largely undefined. Median doses used in previous reports have been lower than what has been traditionally used to treat non-brainstem metastases (e.g. 16–18 vs. 20–24 Gy). This de-escalation is due to the perceived risk of increased toxicity to critical cranial nerve nuclei and fiber tracts for this serial critical structure, and the lack of surgical salvage options should radionecrosis occur.
We present a single institution retrospective series documenting local control and toxicity rates for patients with brainstem metastases treated at our institution between 2000 and 2010 with Gamma Knife Radiosurgery (GKRS). We performed a dose volume analysis to determine any dosimetric factors that potentially affected the likelihood of local failure or toxicity.
Methods and materials
Data acquisition
This study was approved by the Wake Forest University Institutional Review Board. The Wake Forest University Medical Center Gamma Knife Program Tumor Registry was searched for all patients receiving GKRS and having a brainstem metastasis. Patients were allowed to have had previous whole brain irradiation. Between February 2000 and December 2010 a total of 52 brainstem metastases were treated with GKRS in 44 individual patients. Clinical outcome measures were determined using the patents’ electronic medical records. Dosimetric data were obtained using patients’ archived plans using the GammaPlan™ treatment planning system (Elekta AB, Stockholm, Sweden) and consisted of tumor size, prescribed dose and isodose level, number of lesions, and tumor location.
Patient characteristics
The clinical characteristics of the patients in this report are detailed in Table 1. Patient factors including age, histology, RPA class, location of metastases within the brainstem defined as the midbrain bordered superiorly by the hypothalamus, pons, or medulla, total number of brain metastases and prior WBRT were determined from the electronic medical records. Median and mean follow-up for all patients was 6 and 10 months respectively with a range of 1–75 months. Imaging follow up was completed in all but three patients who died within 1 month of treatment. Multiple brain metastases were treated in 75 % of patients. The median number of additional lesions treated was 2 (range 0–39). One patient was treated on separate occasions for different brainstem lesions, but counted only once for this analysis. Among the 44 patients, 3 were treated for multiple lesions within the brainstem including patients with 2, 3, and 6 lesions. Median size of brainstem metastasis was 0.134 cc, (range 0.013–6.600 cc). A total of 19 of 44 patients had undergone previous WBRT. None of these 19 patients received radiosurgery as a planned boost. Therefore, all patients treated with radiosurgery were as a result of WBRT failing to achieve local control.
Table 1.
Patient and treatment details
| Patient characteristics | |
|---|---|
| Patients | 44 |
| Median age (range) | 57 (30–85) |
| Gender | |
| Female | 25 |
| Male | 19 |
| RPA | |
| Class I | 5 |
| Class II | 37 |
| Class III | 1 |
| Median KPS (range) | 80 (60–80) |
| Tumor Characteristics | |
| Number brainstem lesions | 52 |
| Number of lesions treated at GKRS | |
| Single brainstem lesion | 11 |
| Brainstem and ≥1 synchronous non-brainstem lesion (median and range of additional lesions treated) | 33 (2, 1–39) |
| Median size (range) | 0.134 cc (0.013–6.6 cc) |
| 0–0.50 cc | 41 |
| 0.51–1.00 cc | 1 |
| 1.10–1.50 cc | 4 |
| 1.51–2.00 cc | 2 |
| > 2.00 cc | 4 |
| Location | |
| Midbrain | 9 (3 spanned midbrain and pons) |
| Pons | 28 |
| Medulla | 4 |
| Histology | |
| Non-small cell lung cancer | 11 |
| Small cell lung cancer | 6 |
| Breast | 15 |
| Renal cell | 4 |
| Melanoma | 4 |
| Ovarian | 2 |
| Colorectal | 1 |
| Unknown | 1 |
| Treatment details | |
| Median dose (range) | 18 Gy (14–22 Gy) |
| Prior WBRT | 25 |
Radiosurgical treatment
The Leksell Perfexion Gamma Knife™ was used for treatment delivery in patients treated after May 2009 and prior to this date Leksell Gamma Knife Models B/C were used (all models, Elekta AB, Stockholm, Sweden). On the day of treatment, the neurosurgeon placed a stereotactic headframe. A multi-sequence contrast-enhanced stereo-tactic magnetic resonance image (MRI) was then obtained. A 1.5 Tesla MRI was used prior to 2005, after which this unit was replaced by a 3.0 T unit (both devices, GE Healthcare, Waukesha, WI, USA). The GammaPlan system was then used for target delineation and treatment planning. The target included the enhancing tumor volume on SPGR and T1 post contrast MRI sequences. No clinical tumor margin was used.
Both the neurosurgeon and the treating radiation oncologist reviewed each volume. A median dose of 18 Gy (range 10–22 Gy) was prescribed to the median 50 % isodose line conforming to the tumor margin while maintaining greater than 99 % tumor coverage. A dose volume histogram (DVH) was created for individual lesions. The DVH was reviewed evaluating the tumor coverage as well as the total treatment volume. The prescribed dose was based on the discretion of the treating radiation oncologist, and while larger tumors were generally treated with doses below 18 Gy, there was a heterogeneity of doses prescribed based on six different radiation oncologists that treated patients during this 10 year period. An illustration of a representative patient treated with 17 Gy to the 50 % isodose line and subsequent 6 month follow up scan is shown in Fig. 1.
Fig. 1.
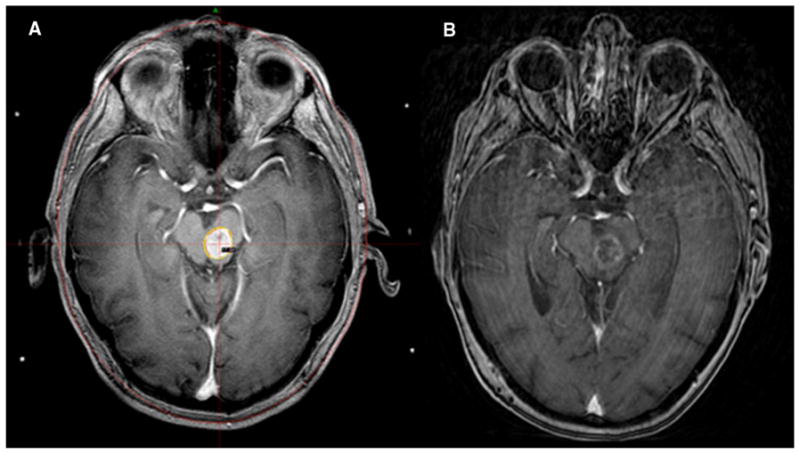
Patient details for a 73 year old male with non-small cell lung cancer who suffered grade III toxicity. a Treatment planning MRI with spoiled gradient recalled acquisition in steady state (SPGR) sequence using GammaPlan software. Brainstem lesion contoured with plan delivering17 Gy prescribed to the 50 % isodose line for a 1.4 cc lesion located in the pons and midbrain. b Contrasted MRI with SPGR sequence 6 months post GKRS revealing response to therapy with diminished enhancement and decrease in tumor size
Outcome assessment
Electronic medical records were used to assess for clinical and imaging outcomes. Patients were followed with serial imaging and clinically. MRI scans were reviewed and measured longitudinally in the largest axial, sagittal, and coronal slices and compared to the planning MRI from the date of gamma knife delivery. For patients with enlargement on any slice, the MRI was imported into the GammaPlan system and contoured on serial axial slices for volume generation and then compared to the treatment volume from the gamma knife treatment day. Patients were considered to have failed locally after evidence of tumor volume enlargement by at least 25 %. Because of the possibility of radionecrosis, local failures were generally followed with serial imaging and treated conservatively with either steroids or a combination of vitamin E and pentoxifylline prior to determination of a treatment failure. Distant failures included new intracranial lesions elsewhere in the brain. Patients were screened for rates of salvage WBRT or for repeat GKRS procedures. Toxicity was graded by the LENT-SOMA scale. Cause of death was recorded for each patient and scored as neurologic, non-neurologic, or unknown. Neurologic death, as originally defined by Patchell et al. [16] included patients with progressive neurologic dysfunction with controlled extracranial disease, or patients with severe neurologic dysfunction who died of intercurrent illness.
Statistical analysis
Kaplan–Meier analysis was performed to determine local control and overall survival for the patient population. Time to local or distant failure was recorded from date of GKRS. Survival was similarly recorded from date of GKRS or censored at time of last follow up. Time to toxicity event was analyzed via univariate Cox proportional hazards regression to investigate tumor size’s effect on toxicity. Cox proportional hazards regressions were also performed to investigate the univariate effect of tumor size, dose, number of lesions, and tumor location on local control, and overall survival. Wald type p-values were examined to assess statistical significance. All statistical analyses were performed in SAS, version 9.2 (SAS Institute, Inc.), and Stata, version 10.1 (StataCorp LP).
Results
Overall survival
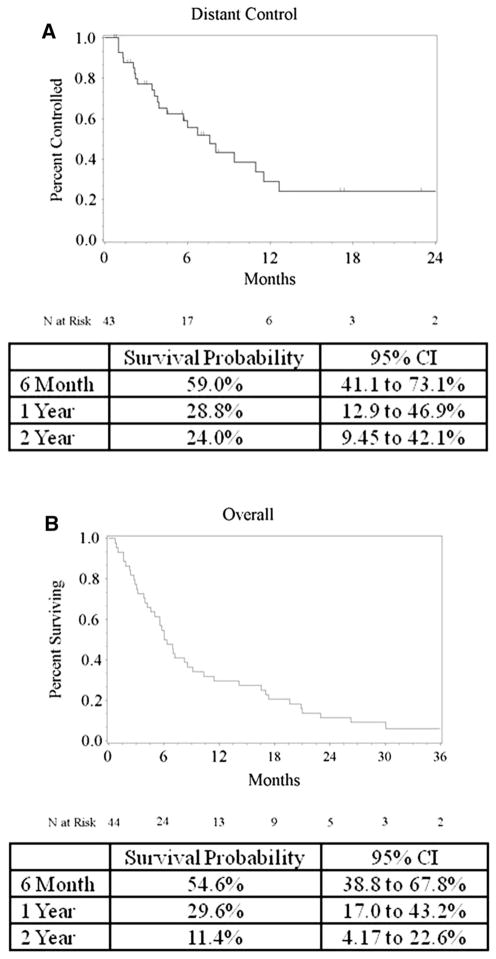
Kaplan–Meier analysis was used to estimate overall survival (Fig. 2). Overall survival rate at 1 year was 30 % (95 % CI 17.0–43.2 %) for the entire population with a median survival time of 6 months (95 % CI 4.1–9.1 months). Cause of death was neurologic in 17 patients, non-neurologic in 20 patients, and unknown in four. Three patients remain alive at time of analysis. No statistical relationship was found between tumor size, number of treated lesions, tumor location, or tumor dose predicting for overall survival, or cause of death. Among the patients who experienced local failure, five died a neurologic death, one a non-neurologic death, one unknown, and the final patient remained alive at time of this analysis.
Fig. 2.
Kaplan–Meier plot of distant control (a) and overall survival (b)
Local control
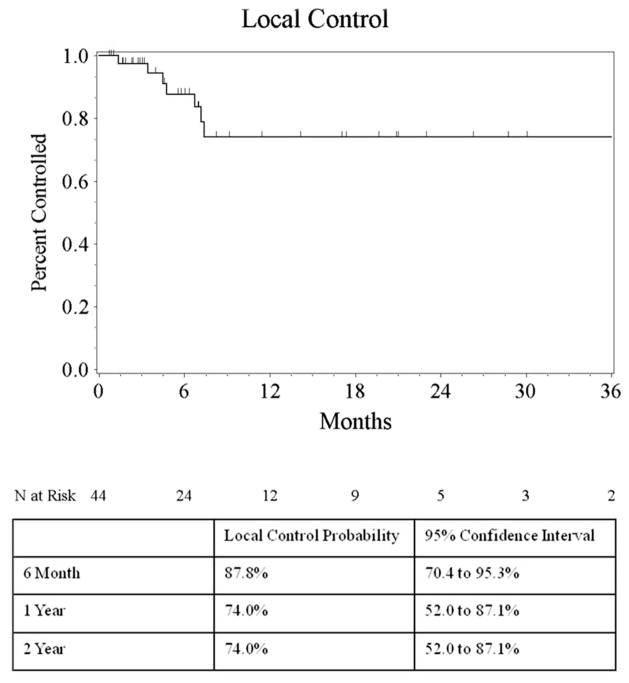
Eight of 44 patients experienced a local failure. Kaplan–Meier estimate of local control rate at 6 months and 1 year was 88 % (95 % CI 70–95 %) and 74 % (95 % CI 52–87 %). Kaplan–Meier curve for local control is depicted in Fig. 3. The mean size of the lesions having failed was 0.499 cc (range 0.013–1.7), with only one of these lesions measuring over 1.0 cc. However, likely a result of the smaller sample size, this difference failed to show statistical significance when evaluated on univariate analysis for local control (T test, p = 0.30) Treatment doses for these lesions ranged from 10 to 22. No statistically significant relationship on univariate analysis was found between size, dose, histology and local control. Six of the eight patients who failed locally had received WBRT prior to their GKRS. The single patient who had not received WBRT experienced simultaneous local and distant failure and received salvage WBRT 5 months after GKRS.
Fig. 3.
Kaplan–Meier plot of local control
Distant brain failure
Distant failures were noted in 23 patients. Five of these patients were noted to have leptomeningeal failure, and most were referred to comfort care as a result. Six patients received a second course of GKRS, and two patients received a third course. Only 2 of the 23 patients who suffered distant failures were treated with WBRT at failure, which can be attributed to the fact that many of these patients had been treated previously with WBRT.
Toxicity
Four patients experienced treatment toxicities related to their brainstem metastasis. Toxicity details are summarized in Table 2. Of these, one patient had grade 2 toxicity and three patients had grade 3 toxicities. No toxic deaths occurred. None of the 4 patients with toxicity had received prior WBRT. This is in comparison to 19 of 40 patients (45 %) who did not experience toxicity. Uni-variate cox proportional hazards regression found increasing tumor volume to be correlated with an increased hazard for toxicity (Hazard Ratio 1.63 per cc, p = 0.01, 95 % CI 1.11–2.40). Freedom from complication rates at 6 months and 1 year was 100 and 100 % compared to 63 and 31 % for tumor volume less than 1 cc and greater than 1 cc respectively (Log-Rank Test, p < 0.001). Other factors examined, such as prior WBRT, marginal dose, histology and location, age, gender and extent of extracranial metastatic disease failed to predict for a toxicity event on univariate analysis and therefore were eliminated from the multivariate analysis. A single patient who developed persistent symptomatic radiation injury (suspected radionecrosis) within a lesion in the pons was treated with bevacizumab as part of his chemotherapy regimen for colon cancer. After a single cycle of bevacizumab, he experienced near complete response of his severe diplopia and imaging resolution of the treatment related edema.
Table 2.
Toxicity details
| Patient/KPS/RPA | Age/gender | Histology/number of additional lesions treated | Location/size (cc) | GK dose/prior WBRT | Local failure/neurologic Death | Toxicity | Management |
|---|---|---|---|---|---|---|---|
| 1 90/II | 73/male | Non-small cell lung cancer/1 | Pons and midbrain/1.4 | 17 Gy/no | No/yes | Grade III. severe diplopia and dysphagia | Long term steroids provided minimal relief |
| 2 70/II | 57/female | Ovarian/1 | Midbrain/1.8 | 18 Gy/no | No/no | Grade III. Severe diplopia requiring continuous closure of left eye | Steroids provided little relief. She was unable to discontinue their use. She was offered tarsorrhaphy although she refused |
| 3 70/II | 59/male | Adenocarcinoma of unknown primary/0 | Pons/3.2 | 18 Gy/no | No/yes | Grade III. Suspected Radiation Necrosis. Mild ataxia and diplopia | Bevacizumab, complete resolution of symptoms |
| 4 80/II | 57/male | Non-small cell lung cancer/0 | Pons/3.5 | 18 Gy/no | No/no | Grade II. suspected radiation necrosis and ataxia | Low dose steroids provided minimal relief |
Discussion
The present analysis represents one of the largest published series on brainstem metastases treated with radiosurgery. The data from our analysis supports GKRS as a safe and effective method for patients with lesions less than 1.0 cc in total volume. Our findings are consistent with other series specifically addressing SRS for brainstem lesions with comparable size tumors and comparable radiation doses. The 9 previously reported series of patients with brainstem metastases are summarized in table 3 [7–15].
Table 3.
Published Brainstem Metastases Series
| Series | Dates | Patients (lesions) | Follow up (months) | Median dose/range (Gy) | Size/range (cc) | Local control | Median survival (months) | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Huang et al. [9] | 1988–1998 | 26 (27) | 9.5 | 16/12–20 | 1.1/0.3–9.7 | 95 % at last follow up | 9 | None reported |
| Shuto et al. [10] | 1992–2001 | 25 (31) | 5.2 | 13/8–18 | 2.1/0.02–12 | 77 % at last follow up | 4.9 | 2 with undefined radiation induced injury |
| Fuentes et al. [11] | 1992–2001 | 28 (28) | 11 | 19.6/11–30 | 2.1/0.4–11 | 92 % at last follow up | 12 | 3 had worsening of neurological symptoms transiently. |
| Yen et al. [12] | 1989–2005 | 53 (53) | 9.8 | 17.6/9–25 | 2.8/0.5–21 | 86 % at last follow upa | 11 | None reported |
| Hussain et al. [13] | 1991–2004 | 22 (22) | 8.5 | 16/14–23 | 0.9/0.1–3.3 | 100 % at last follow up | 8.5 | 1 developed hemiparesis after 3 months |
| Kased et al. [14] | 1991–2005 | 42 (44) | 6.9 | 16/10–19.8 | 0.26 0.015–2.8 | 77 % at 1 year | 9 | 4 patients all with tumors > 1 cc |
| Lorenzoni et al. [15] | 1999–2006 | 25 (27) | 10.5 | 20/15–24 | Mean 0.6/0.013–3.6 | 95 % at last follow up | 11.1 | None reported |
| Koyfman et al. [7] | 1997–2007 | 43 (43) | 5.3 | 15/9.6–24 | 0.37/0.01–8.8 | 85 % at 1 year | 5.8 | None reported |
| Hatiboglu et al. [8] | 1994–2007 | 60 (60) | 12.8 | 15/8–18 | 1.0/0.1–8.7 | 35 % at 1 year | 4.0 | 12 patients including 4 with hemiparesis and 2 with hemorrhage |
| Present | 2000–2010 | 44 (52) | 6.0 | 18/10–22 | 0.134, 0.013–6.600 | 64 % at 1 year | 6.0 | 4 patients all with tumors > 1 cc |
No local control data reported for 16 of the 53 patients
In the first series published on brainstem metastases in 1999, Huang et al. [9] from the University of Pittsburgh reported on 26 patients treated with a median marginal dose of 16 Gy (range 12–20 Gy) achieving an actuarial local control rate of 95 % and a median survival of 11 months. More recently, in 2010, Koyfman et al. [7] reported on 43 patients treated for brainstem metastases between 1997 and 2007 at the Cleveland Clinic. With a lower median dose of 15 Gy, local control at 1 year was 85 %, with a 1 year survival rate of 32 % and median survival of 5.8 months. Median tumor volume was 0.37 cc (range of 0.01–8.8 cc). Despite larger lesions being treated, no grade 3 or 4 toxicities were reported yet radiation necrosis was documented on imaging in two patients. However, smaller tumor volume did predict for improved survival with a hazard ratio of 1.23 on multivariate analysis, p = 0.001.
Although multiple series have reported toxicities with rates ranging from 0 to 20 %, only one study found size to predict for toxicity similar to the outcomes reported in the present series. Kasad et al. published outcomes on 42 brainstem metastases patients treated from 1991 to 2005 [14]. Local control was achieved in 90 and 77 % at 6 months and 1 year with a median survival of 9 months. Investigators found tumor size greater than 0.26 cc to predict for a brainstem complication. Although 0.26 cc was found to predict for toxicity, all four patients with toxicity were found to have tumors greater than 1 cc. Based on this finding, they reported a freedom from complication rate at 6 months and 1 year for tumor volume less than 1 cc to be 100 % compared to 40 and 60 % for tumors greater than 1 cc at 6 months and 1 year respectively (p < 0.001). A comparative analysis among our patients showed a freedom from complication rate at 6 months and 1 year of 100 % compared to 63 and 31 % (p < 0.001). The four toxicities included brainstem necrosis resulting in ataxia, disequilibrium, and left facial numbness, a second patient with disequilibrium alone, a third patient with hemiparesis, and a fourth patient with right facial numbness and what was described as mild right hemiparesis.
In the present series, toxicity occurred in 9 % of patients. However, the toxicity rate for lesions greater than 1 cc was 40 % with four of ten patients suffering grade 2–3 toxicity. It is likely that the increased rate of toxicity seen in the current series is due to the larger size of brainstem lesions treated in the current study. When the results of the current series are considered with data published by Kased et al. [14]., it would appear that a threshold of 1 cc is the size at which brainstem lesions yield significant rates of toxicity with single fraction radiosurgery. Currently, our approach to lesions greater than 1 cc is to treat with one of two approaches. For patients with 1–4 lesions who have not received prior WBRT, we consider either (1) hypofractionated stereotactic radiation with a typical dose range of 21–30 Gy in 3–5 fractions or, (2) WBRT. For those who have received prior WBRT, the hypofractionated approach is generally favored.
In the current study, the local control rate at 6 months and 1 year was 88 and 74 %, respectively. This is somewhat lower than expected for other metastases of comparable size that is treated with single fraction radiosurgery. Furthermore, 6 of the 8 patients experiencing local failure after radiosurgery had received prior WBRT. We acknowledge more aggressive phenotypes may have been selected in this scenario. The lack of a dose response detected in this study is likely due to the small number of events. Among previously published series, local control has ranged from 62 to 100 %. Previous series have documented that brain metastases as a whole experience a dose response and that doses below 20–22 Gy will more often lead to local failures in patients with prolonged survival [17–19].
Dose selection for intracranial metastases in general is largely based on RTOG 9005, a prospective trial among patients previously treated with WBRT with the primary objective of establishing a maximum tolerated dose for single fraction radiosurgery [6]. Subsequent phase III protocols employed these dosing schemas for brain metastases based on target size and have established current dosing guidelines [9, 20, 21]. Lesions within the brainstem have generally been treated with as much as a 20 % dose reduction because of a concern for increased toxicity. No prospective data exists to establish whether the toxicity concern is valid, and if this concern justifies potentially sacrificing local control. The modestly higher rate of local failure rate in brainstem metastases must be weighed against toxicity, and the shortened survival time of this population. Median survival in the current series was 6.1 months with an overall survival rate at 6 months and 1 year of 55 and 30 %, respectively. Median survival has ranged from 4.9 to 11.1 months in previous reports and the range can likely be attributed to referral and patient selection bias at each institution.
This study was limited by the fact that it was retrospective in nature, and thus limited to hypothesis-generation. Furthermore, the 1 cc volume threshold seen in the multivariate analysis was based on 4 toxicity events. As such, this data likely requires validation from an independent dataset. In spite of the limitations, the present study represents the first attempt to determine the threshold volume for treatment of brainstem lesions with single fraction radiosurgery.
Conclusion
GKRS remains a safe and effective approach for patients treated upfront or after WBRT for brainstem metastases. Lesions over 1 cc treated with GKRS may carry a higher risk of toxicity, though these findings remain to be validated in a prospective setting.
Footnotes
Portions of this manuscript were presented as a Poster Presentation at the 53rd Annual Meeting of American Society for Therapeutic Radiation Oncology, Miami, 10/2/2011-10/6/11.
Conflict of interest : There are no conflicts of interest to disclose.
Contributor Information
Jeremy M. Kilburn, Email: jkilburn@wakehealth.edu, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
Thomas L. Ellis, Department of Neurosurgery, Wake Forest School of Medicine, Winston-Salem, NC, USA
James F. Lovato, Division of Public Health Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA
James J. Urbanic, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
J. Daniel Bourland, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA.
Michael T. Munley, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
Allan F. deGuzman, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
Kevin P. McMullen, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
Edward G. Shaw, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
Stephen B. Tatter, Department of Neurosurgery, Wake Forest School of Medicine, Winston-Salem, NC, USA
Michael D. Chan, Department of Radiation Oncology, Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27157, USA
References
- 1.Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45(7):741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K, Nakagawa K, Kobashi G, Shirato H. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68(5):1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 7.Koyfman SA, Tendulkar RD, Chao ST, Vogelbaum MA, Barnett GH, Angelov L, Weil RJ, Neyman G, Reddy CA, Suh JH. Stereotactic radiosurgery for single brainstem metastases: the cleveland clinic experience. Int J Radiat Oncol Biol Phys. 2010;78(2):409–414. doi: 10.1016/j.ijrobp.2009.07.1750. [DOI] [PubMed] [Google Scholar]
- 8.Hatiboglu MA, Chang EL, Suki D, Sawaya R, Wildrick DM, Weinberg JS. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011;69(4):796–806. doi: 10.1227/NEU.0b013e31821d31de. [DOI] [PubMed] [Google Scholar]
- 9.Huang CF, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91(4):563–568. doi: 10.3171/jns.1999.91.4.0563. [DOI] [PubMed] [Google Scholar]
- 10.Shuto T, Fujino H, Asada H, Inomori S, Nagano H. Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien) 2003;145(9):755–760. doi: 10.1007/s00701-003-0034-1. [DOI] [PubMed] [Google Scholar]
- 11.Fuentes S, Delsanti C, Metellus P, Peragut JC, Grisoli F, Regis J. Brainstem metastases: management using gamma knife radiosurgery. Neurosurgery. 2006;58(1):37–42. doi: 10.1227/01.neu.0000190655.95669.5c. (discussion 37–42) [DOI] [PubMed] [Google Scholar]
- 12.Yen CP, Sheehan J, Patterson G, Steiner L. Gamma knife surgery for metastatic brainstem tumors. J Neurosurg. 2006;105(2):213–219. doi: 10.3171/jns.2006.105.2.213. [DOI] [PubMed] [Google Scholar]
- 13.Hussain A, Brown PD, Stafford SL, Pollock BE. Stereo-tactic radiosurgery for brainstem metastases: survival, tumor control, and patient outcomes. Int J Radiat Oncol Biol Phys. 2007;67(2):521–524. doi: 10.1016/j.ijrobp.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 14.Kased N, Huang K, Nakamura JL, Sahgal A, Larson DA, McDermott MW, Sneed PK. Gamma knife radiosurgery for brainstem metastases: the UCSF experience. J Neurooncol. 2008;86(2):195–205. doi: 10.1007/s11060-007-9458-4. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzoni JG, Devriendt D, Massager N, Desmedt F, Simon S, Van Houtte P, Brotchi J, Levivier M. Brain stem metastases treated with radiosurgery: prognostic factors of survival and life expectancy estimation. Surg Neurol. 2009;71(2):188–195. doi: 10.1016/j.surneu.2008.01.029. (discussion 195, 195-6) [DOI] [PubMed] [Google Scholar]
- 16.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 17.Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104(6):907–912. doi: 10.3171/jns.2006.104.6.907. [DOI] [PubMed] [Google Scholar]
- 18.Chao ST, Barnett GH, Vogelbaum MA, Angelov L, Weil RJ, Neyman G, Reuther AM, Suh JH. Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer. 2008;113(8):2198–2204. doi: 10.1002/cncr.23821. [DOI] [PubMed] [Google Scholar]
- 19.Shiau CY, Sneed PK, Shu HK, Lamborn KR, McDermott MW, Chang S, Nowak P, Petti PL, Smith V, Verhey LJ, Ho M, Park E, Wara WM, Gutin PH, Larson DA. Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys. 1997;37(2):375–383. doi: 10.1016/s0360-3016(96)00497-x. [DOI] [PubMed] [Google Scholar]
- 20.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ., Jr Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 21.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45(2):427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]