Abstract
Progressive loss of functioning nephrons, secondary to age-related glomerular disease, can impair the ability of the kidneys to effectively clear metabolic wastes and toxicants from blood. Additionally, as renal mass is diminished, cellular hypertrophy occurs in functional nephrons that remain. We hypothesize that these nephrons are exposed to greater levels of nephrotoxicants, such as inorganic mercury (Hg2+), and thus are at an increased risk of becoming intoxicated by these compounds. The purpose of the present study was to characterize the effects of aging on the disposition and renal toxicity of Hg2+ in young adult and aged Wistar rats. Paired groups of animals were injected (i.v.) with either a 0.5 μmol • kg−1 non-nephrotoxic or a 2.5 μmol • kg−1 nephrotoxic dose of mercuric chloride (HgCl2). Plasma creatinine and renal biomarkers of proximal tubular injury were greater in both groups of aged rats than in the corresponding groups of young adult rats. Histologically, evidence of glomerular sclerosis, tubular atrophy, interstitial inflammation and fibrosis were significant features of kidneys from aged animals. In addition, proximal tubular necrosis, especially along the straight segments in the inner cortex and outer stripe of the outer medulla was a prominent feature in the renal sections from both aged and young rats treated with the nephrotoxic dose of HgCl2. Our findings indicate 1) that overall renal function is significantly impaired in aged rats, resulting in chronic renal insufficiency and 2) the disposition of HgCl2 in aging rats is significantly altered compared to that of young rats.
Keywords: kidney, mercury, aging, toxicology
1. Introduction
Aging often leads to significant structural and physiological changes in the kidneys, with the glomerulus being the primary target of the aging process. Age-related glomerular changes are characterized by thickened glomerular basement membranes, expanded mesangial matrices, shrinkage and occlusion of the glomerular capillaries, and eventual glomerulosclerosis (Choudhury 2004; Lopez-Novoa 2008; Zhou 2008; Zhou et al. 2008). These changes ultimately lead to atrophy and death of nephrons associated with diseased glomeruli. As the number of functional nephrons decreases, vascular, glomerular, and tubular changes occur in the remaining functional nephrons in an attempt to compensate for the reduction in renal function caused by the diseased nephrons (Lopez-Novoa 2008; Zatz and Fujihara 1994). These compensatory changes result in glomerular hypertrophy, hyperperfusion, and hyperfiltration and lead to increases in single nephron glomerular filtration rates (SNGFR) and intraglomerular shearing forces, which predispose affected glomeruli to sclerotic changes (Anderson and Brenner 1986; Fogo 2000; Lopez-Novoa 2008; Weinstein and Anderson 2010). Indeed, a positive correlation between glomerular hypertrophy and the development of glomerulosclerosis has been demonstrated in aging mice (Ferder et al. 1994). In addition, proliferation of mesangial cells and expansion of mesangial matrix, both of which may be frequently associated with glomerular hypertrophy, appear to precede and contribute to the development of glomerulosclerosis (Floege et al. 1992). Age-related changes have also been observed in epithelial cells of renal tubules and the interstitium. These changes include atrophy and degeneration, formation of diverticula, irregular thickening of the tubular basal lamina and tubulointerstitial fibrosis, which can be associated with interstitial inflammation, fibroblast activation, increased deposition of collagen, and nephrocalcinosis (Abrass et al. 1995; Ding et al. 2001; Thomas et al. 1998; Zhou et al. 2008). It should be noted that these changes are considered to be pathological and they are distinctly different from the compensatory changes that occur following the loss of renal mass that occurs with uninephrectomy (Hayslett et al. 1968).
As the number of functioning nephrons diminishes in aged kidneys, the remaining functional nephrons undergo compensatory changes in order to maintain normal renal function (Fine LG 1992; Fine and Norman 1989). These changes may consequently lead to increased exposure of renal tubular epithelial cells to metabolic wastes and xenobiotics, including nephrotoxicants. In addition, these substances may be taken up more readily by hypertrophied and hypermetabolic proximal tubular cells because of compensatory increases in blood flow within glomeruli and expression of certain cellular transport mechanisms along the proximal tubule. The increased exposure to, and probable increase in uptake of available nephrotoxicants likely enhances the risk of hypertrophied tubular cells being affected adversely by these substances (Lopez-Novoa 2008). These adverse effects could conceivably lead to additional cell death along the nephron and possibly additional glomerulosclerosis, both of which would further reduce functional renal mass.
Mercury is a nephrotoxic metal that is found ubiquitously in many environmental and certain occupational settings. Most often, humans are exposed to organic forms of mercury such as methylmercury (CH3Hg+) through consumption of contaminated fish. Once ingested, a fraction of CH3Hg+ is oxidized to form inorganic mercury (Hg2+). Although both forms of mercury have been shown to accumulate in the kidney, this organ appears to be a preferential site of Hg2+ accumulation. Indeed, renal accumulation of Hg2+ occurs rapidly with as much as 40% of a nontoxic dose present in the kidneys within a few hours after exposure (Zalups 1993a). The vast majority of this Hg2+ accumulates in the epithelial cells of the proximal tubule (Zalups 2000).
As the kidney is the primary target of Hg2+ accumulation and intoxication, elderly and aged individuals with reduced renal mass may be at higher risk of being intoxicated by this metal. Exposure to toxicants such as Hg2+ may enhance morbidity and even mortality. Owing to the prevalence of Hg2+ and other toxicants in our environment and the increased life-span of humans, it is important to human health that we have a thorough understanding of the way in which mercuric ions and other environmentally and occupationally relevant nephrotoxicants are handled by the aged kidney. Our laboratory has previously characterized the handling of Hg2+ in a non-pathological model of reduced renal mass (e.g., uninephrectomy) (Zalups 1993b; Zalups 1997), but to our knowledge, no one has examined the handling of Hg2+ in a model of aging, which is characterized by pathological changes. Therefore, the purpose of the present study was to characterize the effects of aging on the corporal disposition and renal toxicity of Hg2+ in young adult and two-year old rats, and to test the hypothesis that remaining functioning nephrons in aged kidneys are at increased risk of being intoxicated by nephrotoxic compounds, such as Hg2+. This study represents the first report of Hg2+ disposition and intoxication in a model of aging. The findings from this study will provide important information regarding the susceptibility of aging kidneys to nephrotoxicants such as Hg2+.
2. Methods
2.1 Animals
Male Wistar rats were obtained from our breeding colony housed in the Mercer University School of Medicine animal facility. “Young” rats were used at an age of eight weeks while “Aged” rats were approximately 20 months of age. Mean body weights for each group of animals are listed in Table 1. Animals were provided a commercial laboratory diet (Teklad Global Soy Protein Free Extruded Rodent Diet, Harlan Laboratories) and water ad libitum throughout all aspects of the present study. All procedures involving animals were reviewed and approved by the Mercer University Institutional Animal Care and Use Committee. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Table 1.
Body weights, total renal mass and liver weights of young and aged rats.
| Body Weight (g) | Weight of Total Renal Mass (g) | Weight of Liver (g) | |
|---|---|---|---|
| Young – 0.5 μmol | 248.24 ± 7.37 | 2.11 ± 0.09 | 8.44 ± 0.59 |
| Aged – 0.5 μmol | 615.46 ± 57.38a | 3.78 ± 0.55a | 11.41 ± 0.78a |
| Young – 2.5 μmol | 208.88 ± 11.07 | 2.33 ± 0.15 | 9.02 ± 1.08 |
| Aged – 2.5 μmol | 662.65 ± 44.79a | 4.95 ± 0.24a | 13.87 ± 0.77a |
2.2 Intravenous Injections
Rats were injected intravenously (i.v.) with either a non-nephrotoxic (0.5 μmol • kg−1 • 2 mL−1 normal saline) or a nephrotoxic (2.5 μmol • kg−1 • 2 mL−1 normal saline) dose of HgCl2 according to our previously published protocol (Bridges et al. 2008a; Bridges et al. 2008b). The injection solution contained radioactive mercury ([203Hg2+]) and was designed to deliver 1 μCi [203Hg2+] to each animal. [203Hg2+] was generated by neutron activation of mercuric oxide for four weeks at the University of Missouri Research Reactor (MURR) (Belanger et al. 2001; Bridges et al. 2008a). At the time of injection, each animal was anesthetized with isoflurane and a small incision was made in the skin in the mid-ventral region of the thigh to expose the femoral vein and artery. A 0.5 μmol or 2.5 μmol • kg−1 dose of HgCl2 was administered into the vein. The wound was closed using two 9-mm stainless steel wound clips. Animals were then housed individually in metabolic cages. Forty-eight hours after injection with HgCl2, animals were sacrificed and organs/tissues were harvested.
2.3 Collection of Organs
At the time of euthanasia, animals were anesthetized with ketamine (70 mg • kg−1) and xylazine (30 mg • kg−1). A 1-mL sample of blood was obtained from the inferior vena cava and set aside for determination of [203Hg2+] content. A separate sample of blood was placed in a Microtainer plasma separation tube in order to estimate content of [203Hg2+] in plasma and cellular fractions. The total volume of blood was estimated to be 6% of body weight (Lee and Blaufox 1985).
The liver and kidneys were also removed from each rat. The mean total renal mass and liver weights for each group of animals are shown in Table 1. Each kidney was trimmed of fat and fascia and weighed and cut in half along the mid-traverse plane. One-half of the right kidney was placed in fixative (40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH) as preparation for histological analyses. The remaining half was frozen in liquid nitrogen for future RNA analyses. One-half of the left kidney was utilized for estimation of [203Hg2+] content. A 3-mm traverse slice was obtained from the remaining half and was used for dissection of renal zones (cortex, outer stripe of the outer medulla, inner stripe of the outer medulla, and inner medulla). Each sample was weighed and placed in a separate tube for estimation of [203Hg2+]. The liver was weighed and a 1-g sample was removed for determination of [203Hg2+] content.
Urine and feces were collected in 24-h periods throughout the duration of the experiment. Urine volumes are listed in Table 2. At the end of each 24-h collection period, a 1-mL sample of urine was weighed and placed in a tube for estimation of [203Hg2+] content. All of the feces excreted during each 24-h collection period were counted for estimation of [203Hg2+] content. The content of [203Hg2+] in each sample was determined by counting in a Wallac Wizard 3 automatic gamma counter (Perkin Elmer, Boston, MA) and the content of Hg2+ in each sample was estimated using standard computational methods.
Table 2.
Absolute amounts of mercury detected in tissues, urine and feces of young and aged rats.
| Total Renal Mass (nmol/g) | Cortex (nmol/g) | OSOM (nmol/g) | Liver (nmol/g) | Blood (nmol/g) | Urine (nmol/g/ml) | Feces (nmol) | |
|---|---|---|---|---|---|---|---|
| Young – 0.5 μmol | 25.5 ± 1.97 | 25.68 ± 3.39 | 37.51 ± 3.18 | 0.92 ± 0.02 | 0.09 ± 0.01 | 0.69 ± 0.18 | 14.37 ± 0.88 |
| Aged – 0.5 μmol | 46.07 ± 6.54 | 56.67 ± 6.79 | 47.07 ± 6.65 | 4.44 ± 0.67 | 2.16 ± 0.68 | 2.31 ± 0.61 | 81.55 ± 4.6 |
| Young – 2.5 μmol | 46.46 ± 2.2 | 54.97 ± 4.47 | 70.18 ± 14.17 | 1.62 ± 1.09 | 0.14 ± 0.01 | 1.49 ± 0.55 | 18.19 ± 6.16 |
| Aged – 2.5 μmol | 76.0 ± 8.0 | 92.87 ± 10.03 | 103.02 ± 15.06 | 13.88 ± 1.34 | 1.16 ± 0.41 | 2.59 ± 0.53 | 37.77 ± 8.73 |
2.4 Real-time PCR
Kidneys from young and old Wistar rats injected with 0.5 μmol or 2.5 μmol HgCl2 • kg−1 were isolated, cut into sections, and frozen at the time of animal sacrifice. At the time of RNA isolation, frozen kidney sections were pulverized with a mortar and pestle. TRIzol Reagent (Life Technologies, Grand Island, NY) was added to the ground kidney and RNA was extracted according to the manufacturer’s protocol.
Reverse transcription of 1 μg of RNA was carried out using reverse transcriptase and random hexamers (Life Technologies). For real-time PCR analyses, 2 μL of the reverse transcriptase reaction were utilized. Analysis of kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin (Ngal) was performed with an ABI Prism 7000 detection system using a Gene Expression Assay (Rn00597701_m1 and Rn00590612_m1, respectively, Life Technologies). Glyceraldehyde 3-phosphate dehydrogenase (Gapdh; Rn01775763_g1) was used as a reference gene.
2.5 Histology
Kidneys were fixed in 40% formaldehyde, 50% glutaraldehyde in 96.7 mM NaH2PO4 and 67.5 mM NaOH for 48 hours at 4°C. Following fixation, kidneys were washed twice with normal saline and placed in 70% ethanol. Tissues were processed in a Tissue-Tek VIP processor using the following sequence: 95% ethanol for 30 min (twice); 100% ethanol for 30 min (twice); 100% xylene (twice). Tissue was subsequently embedded in POLY/Fin paraffin (Fisher) and 5 μm sections were cut using a Leitz 1512 microtome and mounted on glass slides. Sections were stained with hematoxylin and eosin (H & E) and were viewed using an Olympus IX70 microscope. Images were captured with a Jenoptix Progress C12 digital camera.
2.6 Measurement of Glucose, Protein, Creatinine and Blood Urea Nitrogen
Urine samples were frozen for determination of urinary glucose and protein. For analyses of urinary glucose, 5 μL of urine were used and glucose was quantitated using the QuantiChrome glucose assay kit (BioAssay, Hayward, CA). For analyses of urinary protein, 20 μL of urine were added to 1 mL Bradford’s Reagent (Sigma, St. Louis, MO) and absorbance was read at 460 nm. Protein concentrations were calculated from a standard curve.
Plasma creatinine and blood urea nitrogen (BUN) levels were assessed in order to estimate alterations in renal function. Following separation of plasma from cellular components of blood, samples were stored at −20°C. For determination of plasma creatinine, 30 μL of plasma was utilized and the concentration of creatinine was assessed using the QuantiChrome creatinine assay (BioAssay). Similarly, using a 5 μL sample of plasma, the concentration of BUN was determined using the QuantiChrome urea assay (BioAssay).
2.7 Statistical Analyses
Data for each experiment were analyzed first with the Kolmogorov-Smirnov test for normality and then with Leven’s test for homogeneity of variances. Data were then analyzed using a two-way analysis of variance (ANOVA) to assess differences among the means. When statistically significant F-values were obtained with ANOVA, the data were analyzed using Tukey’s post hoc multiple comparison test. A p-value of ≤ 0.05 was considered statistically significant. Each group of animals contained four rats.
3. Results
3.1 Disposition of Mercuric Ions in Total Renal Mass
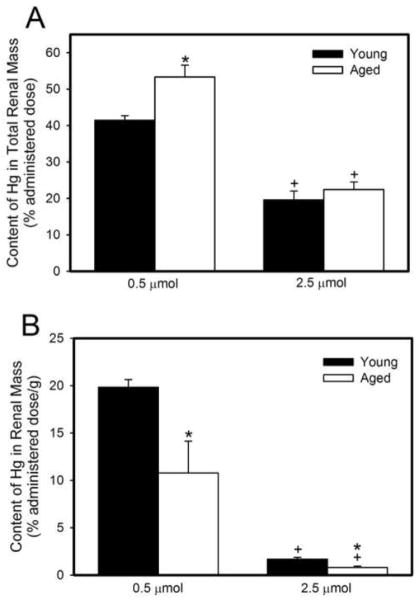
Figure 1A shows the renal burden of Hg2+ (% administered dose) in young and old rats following a 48-h exposure to either 0.5 μmol or 2.5 μmol HgCl2 • kg−1. In rats exposed to 0.5 μmol HgCl2, the renal burden of Hg2+ (% administered dose) was significantly greater in older rats than in younger rats. Following exposure to 2.5 μmol HgCl2 • kg−1, the renal burden of mercury, in both young and aged rats, was significantly lower than that in rats of corresponding age exposed to 0.5 μmol HgCl2 • kg−1. The absolute amount of Hg2+ (nmol/g tissue) in the total renal mass is shown in Table 2.
Figure 1.
Content of mercury in total renal mass (% administered dose) (A) or concentration of mercury in renal mass (% administered dose/g tissue) (B) of young and aged rats 48 hours after intravenous exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Each value represents a mean ± SE for four rats. * Significantly different (p<0.05) from the mean of young rats exposed to the same dose. + Significantly different (p<0.05) from the mean of corresponding rats exposed to 0.5 μmol HgCl2.
Figure 1B shows the amount of mercury (% administered dose/g tissue) in kidneys from young and old rats following a 48-h exposure to either 0.5 μmol or 2.5 μmol HgCl2 • kg−1. The amount of Hg2+ in kidneys of young rats exposed to 0.5 μmol HgCl2 • kg−1 was significantly greater than that of aged rats exposed to the same dose of HgCl2. Similarly, the amount of Hg2+ in kidneys of young rats exposed to the 2.5 μmol • kg−1 dose of HgCl2 was greater than that of kidneys of aged rats exposed to the same dose of HgCl2. The amount of Hg2+ in kidneys of both young and aged rats exposed to 2.5 μmol HgCl2 • kg−1 was significantly less than that of corresponding rats exposed to 0.5 μmol HgCl2 • kg−1.
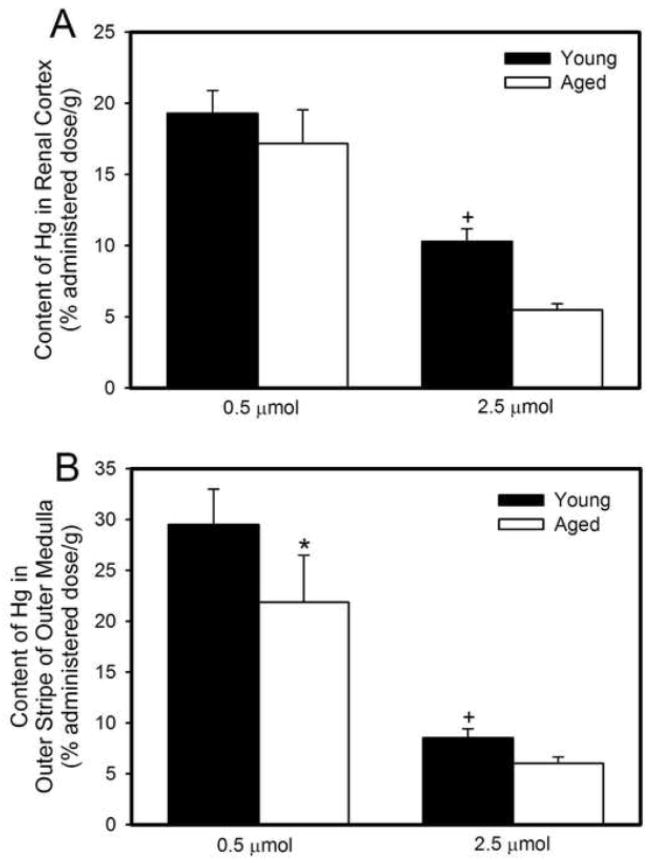
The disposition of Hg2+ in the renal cortex and the outer stripe of the outer medulla (OSOM) was examined because segments of the proximal tubule, which is the primary site of Hg2+ accumulation and intoxication, are located within these two zones of the kidney. Figure 2A shows the amount of Hg2+ in the renal cortex (% administered dose/g tissue) 48 hours after exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. When rats were treated with 2.5 μmol HgCl2 • kg−1, the cortical burden of Hg2+ in aged rats was significantly lower than that in younger rats. The amount of Hg2+ in the cortex of both young and old rats, exposed to 2.5 μmol HgCl2 • kg−1 was significantly less than that in the cortex of corresponding rats exposed to 0.5 μmol HgCl2 • kg−1.
Figure 2.
Concentration of mercury (% administered dose/g) in renal cortex (A) or outer stripe of outer medulla (B) of young and aged rats 48 hours after intravenous exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Each value represents a mean ± SE for four rats. * Significantly different (p<0.05) from the mean of young rats exposed to the same dose. + Significantly different (p<0.05) from the mean of corresponding rats exposed to 0.5 μmol HgCl2.
Figure 2B shows the burden of Hg2+ (% administered dose/g tissue) in the OSOM. In rats exposed to 0.5 μmol HgCl2 • kg−1, the amount of Hg2+ per gram of OSOM was greater in young rats than in aged rats. The amount of Hg2+ in the OSOM of young and aged rats exposed to 2.5 μmol HgCl2 • kg−1 was significantly lower than that of corresponding rats exposed to 0.5 μmol HgCl2 • kg−1. The amount of Hg2+ in either the inner stripe of the outer medulla or the inner medulla was very low and was significantly less than that in either the cortex or OSOM (data not shown). This finding was observed in all groups of rats. The absolute amount of Hg2+ (nmol/g) in the cortex and the OSOM is shown in Table 2.
3.2 Disposition of Mercuric Ions in Liver and Blood
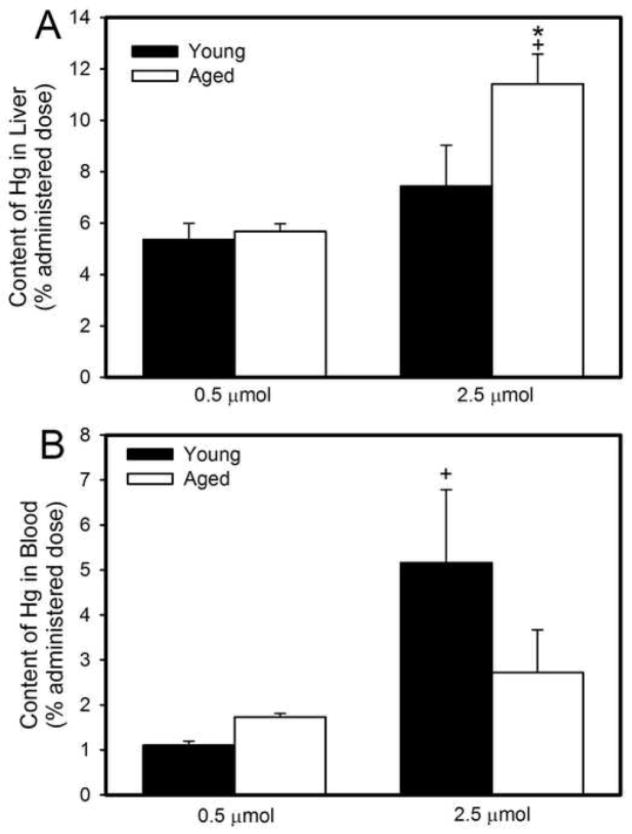
Figure 3A shows that approximately 5% of the administered dose of Hg2+ was present in the liver of young and aged rats 48h after exposure to the 0.5 μmol • kg−1 dose of HgCl2. Following exposure to 2.5 μmol HgCl2 • kg−1, the hepatic burden of Hg2+ was significantly greater in aged rats than that in younger rats (7% administered dose).
Figure 3.
Amount of mercury (% administered dose) in liver (A) and total blood volume (B) of young and aged rats 48 hours after intravenous exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Each value represents a mean ± SE for four rats. * Significantly different (p<0.05) from the mean of young rats exposed to the same dose. + Significantly different (p<0.05) from the mean of corresponding rats exposed to 0.5 μmol HgCl2.
Figure 3B shows the hematologic burden of Hg2+ in young and aged rats 48h following exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. When rats were exposed to 2.5 μmol HgCl2 • kg−1, the hematologic burden of Hg2+ was significantly greater in young rats than in corresponding aged rats. The hematologic burden of Hg2+ in young rats exposed to 2.5 μmol HgCl2 • kg−1 was significantly greater than that in corresponding young rats exposed to 0.5 μmol HgCl2 • kg−1. The absolute amount of Hg2+ (nmol/g) in the liver and blood is shown in Table 2.
3.3 Elimination of Mercuric Ions in Urine and Feces
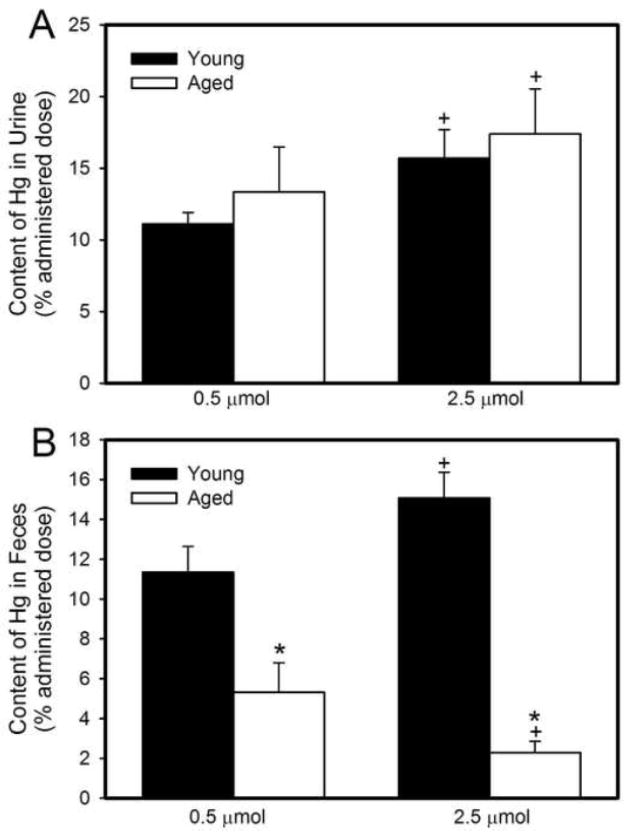
Figure 4A shows the total amount of Hg2+ excreted in the urine of young and old rats during a 48-h exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. There were no significant differences in urinary excretion of Hg2+ between young and aged rats at either dose of HgCl2. However, the urinary elimination of Hg2+ in young and aged animals was significantly greater following exposure to 2.5 μmol HgCl2 • kg−1 than that after exposure to 0.5 μmol HgCl2 • kg−1. The absolute amount of Hg2+ (nmol/g tissue/ml urine) excreted in urine is shown in Table 2.
Figure 4.
Amount of mercury (% administered dose) excreted in urine (A) or feces (B) of young and aged rats during the 48-hour period after an intravenous injection of 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Each value represents a mean ± SE for four rats. * Significantly different (p<0.05) from the mean of young rats exposed to the same dose. + Significantly different (p<0.05) from the mean of corresponding rats exposed to 0.5 μmol HgCl2.
Figure 4B shows the cumulative fecal excretion of Hg2+ in young and aged rats during the initial 48 hours following exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. In rats exposed to 0.5 μmol HgCl2, the fecal excretion of Hg2+ was significantly greater in young rats than in older rats. Similarly, in rats exposed to 2.5 μmol HgCl2 • kg−1, the fecal excretion of Hg2+ was also significantly greater in young rats than in aged rats. The absolute amount of Hg2+ (nmol) eliminated in feces is shown in Table 2.
3.4 Biochemical Analyses of Renal Function
There were significant differences in the total amount of glucose excreted in urine (per 48h) of young and aged rats (Table 3). The total amount of glucose (mg/48h) excreted in urine of young rats exposed to 0.5 μmol HgCl2 • kg−1 was significantly greater than that excreted in urine of corresponding aged rats exposed to the same dose of HgCl2. Similarly, the total amount of glucose excreted in urine of young rats exposed to a 2.5 μmol • kg−1 dose of HgCl2 was significantly greater than that excreted in urine of aged rats exposed to the same dose of HgCl2. When the urinary excretion of glucose by rats exposed to a non-nephrotoxic dose of HgCl2 (0.5 μmol • kg−1) was compared to that of rats exposed to a nephrotoxic dose of HgCl2 (2.5 μmol • kg−1), we found that the total urinary excretion of glucose increased significantly in both young and aged rats. The amount of glucose excreted in urine of young rats exposed to 2.5 μmol HgCl2 • kg−1 was approximately six-fold greater and significantly different than that of young rats exposed to the non-nephrotoxic dose of HgCl2 (0.5 μmol • kg−1). Similarly, in aged rats exposed to 2.5 μmol HgCl2 • kg−1, the total amount of glucose excreted in urine was approximately three-fold greater and significantly different than that excreted in urine of aged rats exposed to 0.5 μmol HgCl2 • kg−1.
Table 3.
Urinary glucose, protein and volume of urine collected in each 24h period.
| Glucose (mg/total urine volume) | Protein (mg/total urine volume) | Urine volume First 24h | Urine volume Second 24h | |
|---|---|---|---|---|
| Young – 0.5 μmol | 12.6 ± 2.9 | 4.0 ± 0.74 | 16.5 ± 5.13 | 11.67 ± 2.69 |
| Aged – 0.5 μmol | 5.74 ± 1.4a | 33.4 ± 8.5a | 14.67 ± 5.81 | 9.0 ± 2.65 |
| Young – 2.5 μmol | 75.15 ± 19.6b | 11.3 ± 4.9b | 12.5 ± 2.33 | 23.75 ± 7.58bc |
| Aged – 2.5 μmol | 18.5 ± 1.2ab | 44.7 ± 6.4a | 33.75 ± 5.54ab | 16.25 ± 1.44c |
When the total amount of protein excreted in urine following a 48-h exposure to HgCl2 was measured, we found significant differences between young and aged rats (Table 3). The total urinary excretion of protein (per 48h) in aged rats exposed to 0.5 μmol HgCl2 • kg−1 was significantly greater than that excreted by young rats exposed to the same dose of HgCl2. Similarly, in aged rats exposed to 2.5 μmol HgCl2 • kg−1, the total amount of protein excreted in urine of aged rats was significantly greater than that excreted in urine of corresponding young rats exposed to the same dose of HgCl2. Furthermore, exposure to a nephrotoxic dose of HgCl2 (2.5 μmol • kg−1) led to significant increases in the total amount of protein excreted in urine of young rats exposed to a non-nephrotoxic dose of HgCl2.
Plasma creatinine (mg/dL) was assessed in order to estimate renal clearance and net renal function (Table 4). Plasma creatinine of control rats (not exposed to HgCl2) was similar to that of corresponding rats exposed 0.5 μmol HgCl2 • kg−1. In aged rats exposed to HgCl2 (0.5 or 2.5 μmol • kg−1), plasma creatinine was significantly greater than that in young rats exposed to the same dose of HgCl2. Exposure of rats (young and aged) to a nephrotoxic dose of HgCl2 resulted in a significant increase in plasma creatinine, compared with that following exposure to a non-nephrotoxic dose of HgCl2.
Table 4.
Plasma creatinine and blood urea nitrogen (BUN) of young and aged rats.
| Creatinine (mg/dL) | BUN (mg/dL) | |
|---|---|---|
| Young – control | 0.32 ± 0.01 | 5.33 ± 0.1 |
| Aged – control | 0.37 ± 0.01 | 8.09 ± 0.25 |
| Young – 0.5 μmol | 0.34 ± 0.05 | 7.42 ± 0.97 |
| Aged – 0.5 μmol | 0.69 ± 0.18a | 17.63 ± 5.99a |
| Young – 2.5 μmol | 2.53 ± 0.38b | 52.22 ± 4.60b |
| Aged – 2.5 μmol | 3.42 ± 0.60a | 40.11 ± 5.30b |
significantly different (p<0.05) from mean of group of young rats exposed to same dose of HgCl2
significantly different (p<0.05) from mean of group of rats of the same age exposed to 0.5 μmol HgCl2 • kg−1
significantly different (p<0.05) from mean of 24 h volume
BUN was measured as a means to estimate retention of nitrogen byproducts of intermediary metabolism and consequently, renal function (Table 4). The levels of BUN in control rats were similar to those of rats exposed to 0.5 μmol HgCl2 • kg−1. In rats exposed to 0.5 μmol HgCl2 • kg−1, BUN was significantly greater in aged rats than in corresponding young rats. In contrast, following exposure to 2.5 μmol HgCl2 • kg−1, no significant differences were noted between young and aged rats. As expected, BUN was significantly greater in rats (young and aged) exposed to 2.5 μmol HgCl2 • kg−1 than in rats exposed to 0.5 μmol HgCl2 • kg−1.
3.5 Real-time PCR Analyses of Renal Biomarkers
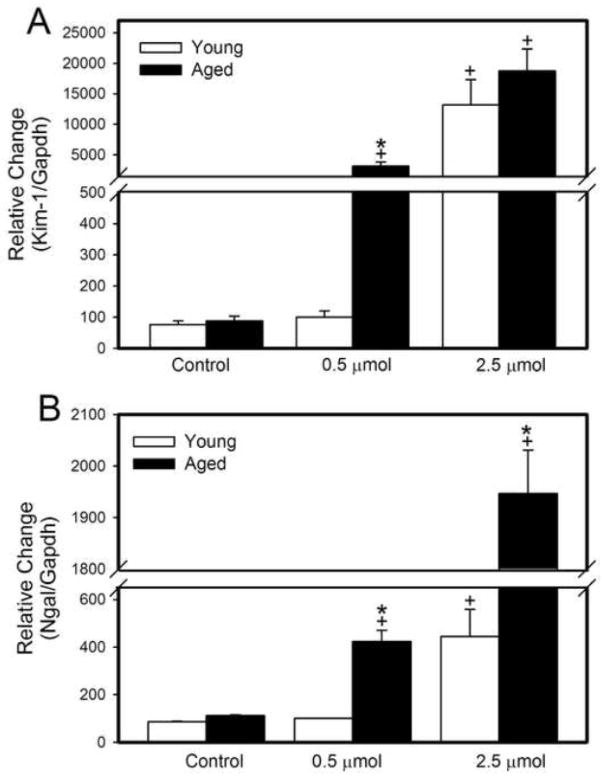
Real-time PCR was utilized to analyze renal expression of kidney injury molecule-1 (Kim-1) and neutrophil gelatinase associated lipocalin (Ngal) in the kidneys of young and aged rats exposed to saline (control), 0.5 μmol HgCl2 • kg−1 or 2.5 μmol HgCl2 • kg−1 (Figure 5). Kim-1, which is also known as hepatitis A virus cellular receptor 1 (Havcr 1) is a transmembrane protein that is expressed at high levels in proximal tubular epithelial cells following ischemic or toxic injury (Ichimura et al. 2004). Expression of Kim-1 mRNA in control rats was similar between young and aged rats (Figure 5A). In contrast, Kim-1 expression was significantly greater in aged rats than in corresponding young rats exposed to 0.5 μmol • kg−1. Exposure of rats to a nephrotoxic dose of HgCl2 (2.5 μmol • kg−1) led to a dramatic increase in the mRNA expression of Kim-1 in both young and aged rats, compared to corresponding control rats or rats exposed to 0.5 μmol HgCl2 • kg−1.
Figure 5.
Real-time PCR analyses of kidney injury molecule-1 (Kim-1) and neutrophil gelatinase associated lipocalin (Ngal). RNA was extracted from kidneys removed from young and aged rats 48 hours after intravenous exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Each value represents a mean ± SE for twelve samples from four rats. *, Significantly different (p<0.05) from the mean of young rats exposed to the same dose. +, Significantly different (p<0.05) from the mean of corresponding rats exposed to 0.5 μmol HgCl2.
Ngal is a small polypeptide that is secreted by injured proximal tubular cells and has been shown to be a marker for renal injury (Mishra et al. 2003; Supavekin et al. 2003). The expression of Ngal mRNA was significantly greater in aged rats exposed to either dose of HgCl2 than in corresponding young rats or control rats (Figure 5B). Moreover, mRNA expression of Ngal in rats (young and aged) exposed to 2.5 μmol HgCl2 • kg−1 was significantly greater than that in corresponding groups of rats exposed to the 0.5 μmol • kg−1 dose of HgCl2.
3.6 Histological Analyses of Renal Structure
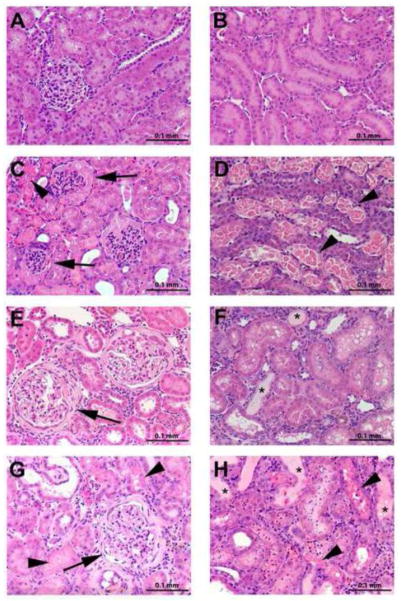
Figure 6 shows photomicrographic images of hematoxylin and eosin (H & E) stained sections of kidneys removed from young and aged Wistar rats exposed to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Panels A and B shown representative photomicrographs of renal sections from young rats exposed to 0.5 μmol HgCl2 • kg−1. Panel A demonstrates normal cortical tissue while Panel B shows normal tissue from the outer stripe of the outer medulla (OSOM). The inner stripe of the outer medulla and the inner medulla were also examined and no evidence of pathological alterations was observed (data not shown). Since the 0.5 μmol • kg−1 dose of HgCl2 has been shown previously to be non-toxic (Zalups 1993b), it is assumed that the histological features observed in Panels A and B are similar to that which would be observed in sections of kidneys from rats that have not been exposed to HgCl2. Indeed, no pathological alterations were observed in any of these sections. Panels C and D are representative photomicrographs of kidneys from young rats exposed to a nephrotoxic dose of HgCl2 (2.5 μmol • kg−1). The major histopathological finding was significant necrosis along the pars recta of proximal tubules, particularly in the inner cortex and OSOM. Panel C shows representative glomeruli in the cortex. The capillary tuft appears shrunken and compressed with proteinaceous material surrounding it in Bowman’s space. Areas of cellular necrosis (arrowheads) were also observed in the cortex. Panel D shows a section of the OSOM, where extensive areas of cellular necrosis (arrowheads) were observed along the pars recta of proximal tubules. Panels E and F show representative photomicrographs from kidneys obtained from aged rats exposed to a 0.5 μmol • kg−1 dose of HgCl2. Panel E shows affected glomeruli with significant thickening of glomerular basement membranes and Bowman’s capsule. Glomerular changes were consistent with glomerulosclerosis and were characterized by thickening of the glomerular basement membranes and Bowman’s capsule. Interestingly, some glomeruli were surrounded by a tubular-like, columnar epithelium. Tubular alterations included dilation of tubular lumina, presence of proteinaceous casts within lumina, tubular atrophy and the formation of vacuoles within tubular epithelial cells. In some areas, interstitial infiltration of lymphocytes (interstitial nephritis) was observed. Panel F shows a section of the OSOM with atrophied tubules and proteinaceous casts (asterisks). Panels G and H show representative photomicrographs from sections of kidney removed from aged rats exposed to 2.5 μmol HgCl2 • kg−1. Panel G shows sclerotic glomeruli (arrows) and necrotic tissue (arrowheads). Panel H shows a section of the OSOM following exposure to a toxic dose of HgCl2. Tubular necrosis (arrowheads) was induced along the pars recta of proximal tubules in the inner cortex as well as throughout the OSOM. Proteinaceous casts (asterisks) were also identified within lumina of tubules. Although Hg-induced necrosis was observed in kidneys of aged rats exposed to 2.5 μmol HgCl2 • kg−1, the severity was less than that in corresponding young rats exposed to the same dose.
Figure 6.
Photomicrographs of histological sections of kidneys removed from young and aged rats 48 hours after intravenous exposure to 0.5 μmol or 2.5 μmol HgCl2 • kg−1. Sections were stained with H & E. Arrows indicate glomeruli, arrowheads indicate cellular necrosis, and asterisks denote proteinaceous casts. A, cortex from young rats exposed to 0.5 μmol HgCl2 • kg−1; B, outer stripe of outer medulla from young rats exposed to 0.5 μmol HgCl2 • kg−1; C, cortex from young rats exposed to 2.5 μmol HgCl2 • kg−1; D, outer stripe of outer medulla from young rats exposed to 2.5 μmol HgCl2 • kg−1; E, cortex from aged rats exposed to 0.5 μmol HgCl2 • kg−1; F, outer stripe of outer medulla from aged rats exposed to 0.5 μmol HgCl2 • kg−1; G, cortex from aged rats exposed to 2.5 μmol HgCl2 • kg−1; H, outer stripe of outer medulla from aged rats exposed to 2.5 μmol HgCl2 • kg−1. Sections are representative samples tissue obtained from each rat (n = 4/group).
4. Discussion
Renal function declines significantly with age and has been shown to be related directly to pathological changes in the kidney (Choudhury 2004; Macias-Nunez 2008; Shock 1984). Since humans and other mammals possess significant renal functional reserve, the capacity to maintain proper fluid and solute (including electrolytes) balance is generally not compromised in an aging individual even though overall glomerular filtration rate (GFR) may be lower than normal (Bohler et al. 1993; Esposito et al. 2007; Fliser et al. 1993). However, when challenged, such as following exposure to a nephrotoxicant like inorganic mercury (Hg2+), the renal functional reserve of an individual may become depleted and lead to additional reductions in filtration capacity and/or substantial alterations in fluid and electrolyte homeostasis (Barai et al. 2010; Uriu et al. 2000). Indeed, it has been suggested that long-term exposure to nephrotoxic heavy metals such as cadmium may exacerbate the effects of age-related decline in GFR (Gonick 2008; Lauwerys et al. 1992).
In the current study, we examined the disposition and toxicity of Hg2+ in young and aged rats exposed to a non-toxic (0.5 μmol • kg−1) or a nephrotoxic (2.5 μmol • kg−1) dose of HgCl2. The present study is novel in that is the first to characterize the disposition and toxic effects of Hg2+ in aged animals. We found that mercuric ions accumulate primarily in kidneys of both young and aged rats. When considering the total burden of mercury in each kidney (% administered dose), we found that more mercury was present in kidneys of aged animals. This was not surprising since the kidneys of these older rats were larger than those of the younger animals. When the renal burden of mercury was factored by the weight of the kidney (% administered dose/g), we found that the concentration of Hg2+ was significantly lower in aged rats than in young rats. This was the case following treatment with either dose of HgCl2. This finding may relate to the presence of glomerulosclerosis, interstitial fibrosis and impaired renal plasma flow that are characteristic of aged kidneys (Baylis and Schmidt 1996; Choudhury 2004; Zhou et al. 2008). It has been suggested that kidneys of aged individuals may be more susceptible to toxicants (Mueller et al. 1998; Uriu et al. 2000). This theory may be true of kidneys that are undergoing the initial steps of age-induced pathological change but it may not be applicable to kidneys that already have significant pathological alterations. We propose that there is a period of increased renal susceptibility in which aged kidneys are more susceptible to intoxication because of compensatory changes that occur within the remaining functioning nephrons. The initial point at which pathological alterations begin to develop in nephrons may represent the beginning of the period where remaining functional nephrons may be more susceptible to injury by tubular nephrotoxicants. We postulate that this increase in susceptibility may be related to compensatory changes such as glomerular hyperfiltration and increased peritubular blood flow in functional nephrons. These changes could lead to increased exposure of these nephrons to metabolic wastes and nephrotoxicants. As the pathological changes progress and the majority of nephrons become sclerotic, the overall renal plasma flow is decreased and nephrons may become less susceptible to nephrotoxicants simply because of the inability of these compounds to gain access to renal tissue. In the current study, it appears that glomerulosclerosis in the aged kidneys has progressed to a point where renal plasma flow is decreased and consequently, exposure to toxicants is reduced. We were unable to assess changes in susceptibility at the single nephron level; however, it is possible that at the level of the individual nephron, the effects of mercury may have been more severe in aged rats than in younger rats.
Biochemical analyses of renal function were carried out in order to assess differences in renal function among groups of rats. In young rats exposed to a non-nephrotoxic dose of HgCl2, plasma creatinine and BUN levels were similar to normal values reported in the current study (Table 4) and by others (Amini et al. 2012; Moeini et al. 2013; Palm and Lundblad 2005). Similarly, total urinary excretion of glucose and protein reflected previous reports from normal rats not treated with HgCl2 (Dai et al. 2012; Gray 1977; McDermott et al. 1996), which indicates that young rats exposed to a non-nephrotoxic dose of HgCl2 can serve as controls for other groups. In contrast, plasma creatinine and BUN levels of aged rats exposed to a non-toxic dose of HgCl2 were found to be elevated. These data support previous reports indicating that glomerular filtration is compromised in aged animals and humans (Corman 1993; Shock 1984). Interestingly, the total urinary excretion of glucose was lower in aged animals than in young animals, suggesting that filtration of glucose at the site of the glomerulus may be reduced in aged animals. In contrast, urinary excretion of protein was greater in older rats than in younger rats. This protein may originate from the lumina of atrophied tubules or be due to hyperfiltration of protein and diminished absorption.
We also assessed biochemical parameters of renal function in young and aged rats exposed to a nephrotoxic dose of HgCl2. In young rats, plasma creatinine, BUN, and urinary excretion of glucose and protein increased significantly following exposure to a nephrotoxic dose of HgCl2. These changes are related directly to Hg2+-induced renal necrosis and tubular dysfunction, which leads to increased urinary excretion of filtered solutes such as glucose (Barbier et al. 2005). Exposure of aged rats to a nephrotoxic dose of HgCl2 led to similar increases in the aforementioned biochemical parameters. The magnitude of these changes was less in older animals than in young animals, suggesting that a toxic dose of HgCl2 had a greater effect on renal tissue of younger animals than that of older animals. To our knowledge, these data represent the first characterization of these biochemical parameters in aged rats exposed to a non-nephrotoxic or nephrotoxic dose of HgCl2.
Kim-1 and Ngal have been shown to be sensitive markers of renal injury following exposure to a number of nephrotoxicants, including heavy metals (Han et al. 2012; Prozialeck and Edwards 2010; Prozialeck et al. 2007; Singer et al. 2013; Sinha et al. 2013). In control animals (not exposed to HgCl2), the expression of Kim-1 and Ngal was similar in young and aged animals. Exposure to a non-nephrotoxic dose of HgCl2 led to an increase in Kim-1 and Ngal in aged, but not young animals. This finding is most likely due to the significant structural alterations that are associated with aging rather than Hg-related nephrotoxicity. The renal expression of Kim-1 and Ngal also increased dramatically in young and aged rats following exposure to a nephrotoxic dose of HgCl2. This finding is similar to other studies which have reported increases in Kim-1 and Ngal following exposure to nephrotoxicants (Han et al. 2012; Prozialeck and Edwards 2010; Prozialeck et al. 2007).
Histological analyses were carried out to characterize structural and pathological changes in kidneys of young and aged rats exposed to non-nephrotoxic or nephrotoxic doses of HgCl2. Kidneys of young rats exposed to a non-nephrotoxic dose of HgCl2 appeared normal. In kidneys of aged animals exposed to a non-nephrotoxic dose of HgCl2, we identified widespread glomerulosclerosis, proteinaceous casts, atrophied tubules, and interstitial fibrosis similar to that which has been reported previously (Corman 1993; Short 1993; Zhou 2008). When rats were exposed to a nephrotoxic dose of HgCl2, significant pathological changes were evident in both old and young rats. Similar to previous reports (Zalups and Diamond 1987), widespread cellular necrosis was found in pars recta segments of proximal tubules located in the OSOM and along medullary rays in the cortex. It should be noted that the proximal tubule, which is localized in the OSOM, has been shown to be the primary target of Hg2+ accumulation and intoxication (Bridges and Zalups 2005; Bridges and Zalups 2010; Zalups 2000). The necrosis was more severe in kidneys of younger rats, which was not surprising considering that the amount of Hg2+ detected in the OSOM of young rats was significantly greater than that of older rats (at both doses of HgCl2).
The current study not only provides additional documentation of functional and structural alterations in aged kidneys, but also is the first to describe the disposition of and toxicity related to HgCl2 administered acutely to aged rats. It has been postulated previously that kidneys of older animals may be more susceptible to the intoxicating effects of nephrotoxic compounds; however, in the current study, the sclerotic state of the kidneys appears to be somewhat protective against nephrotoxicants. Additional experiments carried out earlier in the aging process are necessary to fully characterize the susceptibility of aged kidneys to toxicants.
Highlights.
Humans are exposed to Hg environmentally and occupationally
Hg can cause nephrotoxic effects in normal and aging kidneys
Aging has a profound effect on the disposition of Hg
Hg accumulation is greater in young kidneys than in aged kidneys
Hg-induced proximal tubular necrosis is more severe in young kidneys
Acknowledgments
This work was supported by NIH grant (ES019991) awarded to Christy C. Bridges
Abbreviations
- Hg2+
inorganic mercury
- CH3Hg+
methylmercury
- HgCl2
mercuric chloride
- [203Hg2+]
radioactive mercury
- SNGFR
single nephron glomerular filtration rate
- GFR
glomerular filtration rate
- BUN
blood urea nitrogen
- Kim-1
kidney injury molecule-1
- Ngal
neutrophil gelatinase-associated lipocalin
- H & E
hematoxylin and eosin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. Am J Pathol. 1995;146:742–752. [PMC free article] [PubMed] [Google Scholar]
- Amini FG, Rafieian-Kopaei M, Nematbakhsh M, Baradaran A, Nasri H. Ameliorative effects of metformin on renal histologic and biochemical alterations of gentamicin-induced renal toxicity in Wistar rats. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2012;17:621–625. [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Brenner BM. Effects of aging on the renal glomerulus. The American journal of medicine. 1986;80:435–442. doi: 10.1016/0002-9343(86)90718-7. [DOI] [PubMed] [Google Scholar]
- Barai S, Gambhir S, Prasad N, Sharma RK, Ora M. Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology (Carlton) 2010;15:350–353. doi: 10.1111/j.1440-1797.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P. Effect of heavy metals on, and handling by, the kidney. Nephron Physiol. 2005;99:p105–110. doi: 10.1159/000083981. [DOI] [PubMed] [Google Scholar]
- Baylis C, Schmidt R. The aging glomerulus. Seminars in nephrology. 1996;16:265–276. [PubMed] [Google Scholar]
- Belanger M, Westin A, Barfuss DW. Some health physics aspects of working with 203Hg in university research. Health Phys. 2001;80:S28–30. [PubMed] [Google Scholar]
- Bohler J, Gloer D, Reetze-Bonorden P, Keller E, Schollmeyer PJ. Renal functional reserve in elderly patients. Clinical nephrology. 1993;39:145–150. [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008a;105:211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008b;324:383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. Journal of toxicology and environmental health Part B, Critical reviews. 2010;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury D, Raj DSC, Levi M. Effect of Aging on Renal Funtion and Disease. In: BMB, editor. The Kidney. Elsevier; Philadelphia: 2004. [Google Scholar]
- Corman BJ, Owen RA. Normal Development, Growth, and Aging of the Kidney. In: Mohr U, Dungworth DL, Capen CC, editors. Pathobiology of the Aging Rat. ILSI Press; Washington, DC: 1993. [Google Scholar]
- Dai GL, He JK, Xie Y, Han R, Qin ZH, Zhu LJ. Therapeutic potential of Naja naja atra venom in a rat model of diabetic nephropathy. Biomedical and environmental sciences: BES. 2012;25:630–638. doi: 10.3967/0895-3988.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Ding G, Franki N, Kapasi AA, Reddy K, Gibbons N, Singhal PC. Tubular cell senescence and expression of TGF-beta1 and p21(WAF1/CIP1) in tubulointerstitial fibrosis of aging rats. Exp Mol Pathol. 2001;70:43–53. doi: 10.1006/exmp.2000.2346. [DOI] [PubMed] [Google Scholar]
- Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, Mangione F, Castoldi F, Serpieri N, Cornacchia F, Dal Canton A. Renal function and functional reserve in healthy elderly individuals. Journal of nephrology. 2007;20:617–625. [PubMed] [Google Scholar]
- Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Decreased glomerulosclerosis in aging by angiotensin-converting enzyme inhibitors. J Am Soc Nephrol. 1994;5:1147–1152. doi: 10.1681/ASN.V541147. [DOI] [PubMed] [Google Scholar]
- Fine LG, NJ, Kujubu DA, Knecht A. Renal Hypertrophy. In: Seldin DWGG, editor. The Kidney: Physiology and Pathophysiology. Raven Press; New York: 1992. [Google Scholar]
- Fine LG, Norman J. Cellular events in renal hypertrophy. Annual review of physiology. 1989;51:19–32. doi: 10.1146/annurev.ph.51.030189.000315. [DOI] [PubMed] [Google Scholar]
- Fliser D, Zeier M, Nowack R, Ritz E. Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol. 1993;3:1371–1377. doi: 10.1681/ASN.V371371. [DOI] [PubMed] [Google Scholar]
- Floege J, Johnson RJ, Couser WG. Mesangial cells in the pathogenesis of progressive glomerular disease in animal models. The Clinical investigator. 1992;70:857–864. doi: 10.1007/BF00180756. [DOI] [PubMed] [Google Scholar]
- Fogo AB. Glomerular hypertension, abnormal glomerular growth, and progression of renal diseases. Kidney international Supplement. 2000;75:S15–21. [PubMed] [Google Scholar]
- Gonick HC. Nephrotoxicity of cadmium & lead. The Indian journal of medical research. 2008;128:335–352. [PubMed] [Google Scholar]
- Gray JE. Chronic progressive nephrosis in the albino rat. CRC critical reviews in toxicology. 1977;5:115–144. doi: 10.3109/10408447709003377. [DOI] [PubMed] [Google Scholar]
- Han M, Li Y, Liu M, Cong B. Renal neutrophil gelatinase associated lipocalin expression in lipopolysaccharide-induced acute kidney injury in the rat. BMC nephrology. 2012;13:25. doi: 10.1186/1471-2369-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett JP, Kashgarian M, Epstein FH. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968;47:774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- Lauwerys R, Bernard A, Cardenas A. Monitoring of early nephrotoxic effects of industrial chemicals. Toxicol Lett. 1992;64–65(Spec No):33–42. doi: 10.1016/0378-4274(92)90170-o. [DOI] [PubMed] [Google Scholar]
- Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- Lopez-Novoa JM. The Mechanisma of Age-Associated Glomerular Sclerosis. In: Macias Nunez JF, Cameron JS, Oreopoulos DG, editors. The Aging Kidney in Health and Disease. Springer; New York, NY: 2008. [Google Scholar]
- Macias-Nunez JF, Lopez-Novoa JM. Physiology of the Healthy Aging Kidney. In: Macias Nunez JF, Cameron JS, Oreopoulos DG, editors. The Aging Kidney in Health and Disease. Springer; New York, NY: 2008. [Google Scholar]
- McDermott GF, Ingram A, Scholey J, Kirkland JL, Whiteside CI. Glomerular dysfunction in the aging Fischer 344 rat is associated with excessive growth and normal mesangial cell function. The journals of gerontology Series A, Biological sciences and medical sciences. 1996;51:M80–85. doi: 10.1093/gerona/51a.2.m80. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Moeini M, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Azarkish F, Eshraghi-Jazi F, Pezeshki Z. Protective role of recombinant human erythropoietin in kidney and lung injury following renal bilateral ischemia-reperfusion in rat model. International journal of preventive medicine. 2013;4:648–655. [PMC free article] [PubMed] [Google Scholar]
- Mueller PW, Price RG, Finn WF. New approaches for detecting thresholds of human nephrotoxicity using cadmium as an example. Environ Health Perspect. 1998;106:227–230. doi: 10.1289/ehp.98106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm M, Lundblad A. Creatinine concentration in plasma from dog, rat, and mouse: a comparison of 3 different methods. Veterinary clinical pathology/American Society for Veterinary Clinical Pathology. 2005;34:232–236. doi: 10.1111/j.1939-165x.2005.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR. Early biomarkers of cadmium exposure and nephrotoxicity. Biometals. 2010;23:793–809. doi: 10.1007/s10534-010-9288-2. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock NW, Greulick RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. U.S. Department of Health and Human Services P.H.S, editor. Normal Human Aging: the Baltimore Longitudinal Study of Aging. 1984. [Google Scholar]
- Short BGGRS. Nonneoplastic Lesions in the Kidney. In: Mohr U, Dungworth DL, Capen CC, editors. Pathobiology of the Aging Rat. ILSI Press; Washington, D.C: 1993. [Google Scholar]
- Singer E, Marko L, Paragas N, Barasch J, Dragun D, Muller DN, Budde K, Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf) 2013;207:663–672. doi: 10.1111/apha.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha V, Vence LM, Salahudeen AK. Urinary tubular protein-based biomarkers in the rodent model of cisplatin nephrotoxicity: a comparative analysis of serum creatinine, renal histology, and urinary KIM-1, NGAL, and NAG in the initiation, maintenance, and recovery phases of acute kidney injury. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2013;61:564–568. doi: 10.2310/JIM.0b013e31828233a8. [DOI] [PubMed] [Google Scholar]
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ. Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol. 1998;9:231–242. doi: 10.1681/ASN.V92231. [DOI] [PubMed] [Google Scholar]
- Uriu K, Kaizu K, Qie YL, Ito A, Takagi I, Suzuka K, Inada Y, Hashimoto O, Eto S. Long-term oral intake of low-dose cadmium exacerbates age-related impairment of renal functional reserve in rats. Toxicol Appl Pharmacol. 2000;169:151–158. doi: 10.1006/taap.2000.9063. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Anderson S. The aging kidney: physiological changes. Advances in chronic kidney disease. 2010;17:302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology. 1993a;79:215–228. doi: 10.1016/0300-483x(93)90213-c. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J Pharmacol Exp Ther. 1993b;267:791–800. [PubMed] [Google Scholar]
- Zalups RK. Enhanced renal outer medullary uptake of mercury associated with uninephrectomy: implication of a luminal mechanism. J Toxicol Environ Health. 1997;50:173–194. doi: 10.1080/009841097160564. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- Zalups RK, Diamond GL. Mercuric chloride-induced nephrotoxicity in the rat following unilateral nephrectomy and compensatory renal growth. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53:336–346. doi: 10.1007/BF02890261. [DOI] [PubMed] [Google Scholar]
- Zatz R, Fujihara CK. Glomerular hypertrophy and progressive glomerulopathy. Is there a definite pathogenetic correlation? Kidney international Supplement. 1994;45:S27–29. discussion S30-21. [PubMed] [Google Scholar]
- Zhou XJ, Laszik ZG, Silva FG. Anatomical Changes in the Aging Kidney. In: Nunez JFM, Cameron JS, Oreopoulos DG, editors. The Aging Kidney in Health and Disease. Springer; New York, NY: 2008. [Google Scholar]
- Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG. The aging kidney. Kidney Int. 2008;74:710–720. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]