INTRODUCTION
Agricultural workers, particularly swine confinement operators, are routinely exposed to organic dust environments and display a high prevalence of lung disease including chronic bronchitis, exacerbation of asthma, and obstructive lung disease.1 Exposure of naïve individuals to organic dust results in an intense systemic and pulmonary inflammatory response that attenuates over time, suggestive of adaptation.2, 3 However, despite adaptation, exposed workers are at an increased risk of lung function decline, persistent airway inflammation, and progressive respiratory impairment.1, 3, 4 While several studies have defined the acute dust-induced inflammatory response, less is known about the underlying cellular and immunologic mechanisms resulting in the chronic inflammatory adaptation-like response to repetitive organic dust exposures.
T cells are potential candidates in mediating the chronic inflammatory response. Lymphocytes are elevated in bronchoalveolar lavage fluid (BALF) sampling of animal farm operators as compared to healthy volunteers.5, 6 Moreover, in repetitive swine confinement organic dust extract (DE) animal exposure models, there is an influx of lymphocytes (primarily CD3+) within the lung.7 However, the phenotype and importance of the recruited T cells in humans and animals following repetitive organic dust exposures has not been defined. Numerous studies demonstrate that farm exposure is protective of childhood allergy because, in part, farming exposures have been associated with lower T-helper type 2 (Th2) cytokine profiles as determined by various sampling strategies.8–10 Moreover, circulating Th1 and Treg cells have been found in studies focused on farm mothers and/or farm children.11 However, it has been reported that pig farmers demonstrate increased circulating interleukin (IL)-13- and IL-4- producing Th2 cells when compared to healthy volunteers.12 Interestingly, in the lung, Th17 skewing following acute organic dust exposure has been proposed because soluble IL-17 and IL-17A-expressing lymphocytes were increased in BALF from healthy volunteers exposed once to a swine confinement facility.6 This later observation may be important because lung neutrophil accumulation is a hallmark of organic dust-induced lung disease in humans and animals,1 and IL-17 promotes neutrophil recruitment.13, 14
To understand the role of T cells in the lung in the context of repeated organic dust exposures, mice were treated daily with DE for three weeks per an established animal model7 and lung-associated T cell populations and cytokine expression profiles determined. We also evaluated responses to repetitive treatment with peptidoglycan (PGN) as recent studies demonstrate that PGN is a major component of large animal farming organic dusts.15, 16 Moreover, prior evidence suggests that PGN shares similar biologic and physiologic responses as observed with DE.17, 18 Finally, we assessed the functional role of T cells by utilizing T cell receptor (TCR) αβ knock-out (KO) mice. Our studies demonstrate that both DE and PGN exposure elicited both a Th1 and Th17-polarized lung response, and that DE-induced cellular aggregates and bronchiolar inflammation were reduced in TCR αβ KO mice, but neutrophil influx and alveolar inflammation were not altered. Collectively, these studies demonstrate that repetitive exposure to organic dust and PGN results in a αβ T cell-dependent and -independent lung inflammatory response in a mixed Th1 and Th17 environment.
METHODS
Dust extract (DE)
Dust extract (DE) was prepared as previously described7, 15 from settled surface dust samples collected 3 feet above floor level from swine confinement animal feeding operation facilities (~750 animals). Prior to extraction and filter sterilization, the dust was analyzed by gas chromatography-tandem mass spectrometry for muramic acid (PGN component), 3-hydroxy fatty acid (endotoxin component) and ergosterol (fungi component) according to previously published methods.15 Results revealed high muramic acid (424.0 pmol/mg ± 17.7 pmol/mg) and 3-hydroxy fatty acid (3109.8 ng/mg ± 152.6 ng/mg), but low ergosterol (9.3 pmol/mg ± 0.4 pmol/mg), which is consistent with previous dust sampling findings.15, 18 Dust was placed into solution, centrifuged and supernatant was filter-sterilized (0.22 μm; a process that also removes coarse particles). The aqueous DE was diluted to a final concentration of 12.5% (vol/vol) in sterile saline, which contained 2.91–3.88 mg/ml of total protein as measured by nanodrop spectrophotometry (NanoDrop Technologies, Wilmington, DE). DE (12.5%) has been previously shown to elicit optimal lung inflammation and is well-tolerated.7 The endotoxin levels in the 12.5% DE ranged from 22.1 to 91.1 EU/ml as assayed using the limulus amebocyte lysate assay according to manufacturer’s instruction (Sigma, St. Louis, MO). Peptidoglycan levels were not quantitated because commercially available measurement assays do not exist, to the best of our knowledge.
Animal model
C57BL/6 wild type (WT) and T cell receptor (TCR) αβ knock-out (KO) mice (mice homozygous for the Tcrbtm1Mom targeted mutation; stock number 002118) on a C57BL/6 background (6–8 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). TCR αβ KO mice were selected to study the potential functional role of T cells because the vast majority (>90%) of blood T cells are αβ+.19 Utilizing an established intranasal inhalation murine model of ODE- and PGN -induced lung inflammation, mice were treated daily with 12.5% DE, PGN (10 or 100 μg) or sterile PBS (diluent) for 3 weeks.7, 17 Mice were sacrificed 24 h after the last treatment. Staphylococcus aureus PGN was purchased from Sigma (St. Louis, MO). PGN (100 μg), which is approximately half the protein concentration in 12.5% DE, is known to elicit airway inflammatory responses similar to DE and is well tolerated.17 In this current study, two concentrations of PGN were investigated to determine a dose-dependent response. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center according to NIH guidelines for the use of rodents.
Lung-associated cell collection
Cells were isolated from whole lungs as previously described.20 Briefly, after opening the chest cavity, the right ventricle was infused with 10 ml of sterile PBS with heparin to remove blood from the pulmonary vasculature. Whole lung tissues were harvested and subjected to an automated dissociation procedure using a gentleMAC Dissociator instrument according to the manufacturer’s instructions (Miltenyi Biotech, Auburn, CA) in a solution containing collagenase type I (324 U/mL; Fisher, Pittsburgh, PA), bovine DNase (75 U/mL), porcine heparin (25 U/mL) and PBS with Ca2+ and Mg2+. To remove any large fragments, cell solution was passed through nylon mesh (40 μM; Fisher), and cell pellets were briefly resuspended in cold RBC lysing solution, centrifuged. Cell counts were obtained by hematocytometer and recorded. Viability was assessed and assured by trypan blue exclusion.
Cell surface phenotype and intracellular cytokine staining by flow cytometry
Dissociated lung-associated cells from each animal were incubated with anti-CD16/32 (Fc Block, BD Biosciences) for 10 min to minimize nonspecific Ab binding, and then stained with mAbs directed against CD3, CD4, CD8, and appropriate isotype controls (BD Biosciences, San Jose, CA) for 30 min at 4°C. Compensation was performed with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. Lymphocyte populations were gated by characteristic forward and side scatter properties and antibody specific staining fluorescence intensity using a FACSAria (BD Biosciences) with gating strategy depicted in Figure 1A. Lymphocyte numbers were calculated by multiplying the percentage of each cell type measured by flow cytometry by the original hemocytometer count of total cells recovered from each animal.
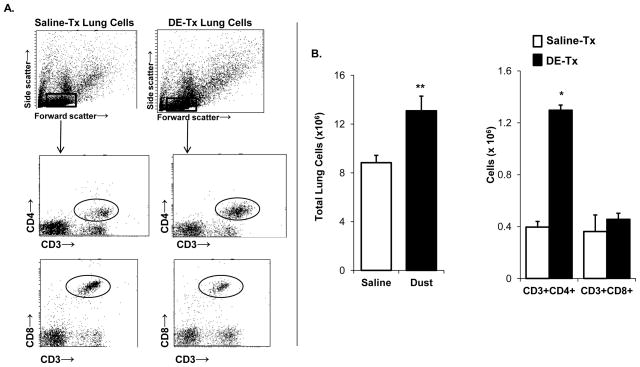
Figure 1. Lung CD4+ T cells are increased following repetitive DE exposure.
C57BL/6 mice were repetitively treated with organic DE or saline for 3 weeks whereupon cells were isolated from whole lungs. Lung-associated cells were stained for CD3, CD4, and CD8 and T cell populations were selected by characteristic forward and side scatter properties and specific antibody staining fluorescence intensity. Absolute CD4+ T cells, but not CD8+ T cells, were increased following repetitive DE exposure. A, A representative dot blot of saline-treated (tx) and dust extract (DE)-treated lung-associated cells is shown. B, Results represent mean ± SEM (N=6 mice/group) of total lung-associated cells, CD3+/CD4+ and CD3+/CD8+ T cells (percentage of cell type x total lung cell count) with statistical difference denoted by asterisks (**p<0.01).
Cytokine expression profiles of T cells that infiltrated the lungs were evaluated by intracellular cytokine staining and FACS analysis. Single cell suspensions of whole lung cells were underlaid with lymphocyte separation solution (Fisher Scientific, Pittsburgh, PA) and centrifuged at 400g for 20 min. Collected mononuclear cells were incubated in PBS containing 0.1% BSA with anti-CD16/32 (Fc Block). Next, cells were stained with mAbs directed against CD4 and CD8 and isolated by fluorescence-activated cell sorting using a FACSAria (BD Biosciences). Post-sort, flow cytometry confirmed > 95% purity for each T cell population. Subsequently, T cells were cultured at 5 × 104 cells/ml in complete L-glutamine-RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), 2-mercaptoethanol (5 × 10−5 M), and 50 μg/mL of penicillin/streptomycin (Invitrogen) in the presence of the protein transport inhibitor, GolgiPlug (BD Biosciences), and stimulated with a mixture of 50 ng/ml PMA (Sigma-Aldrich) and 1 μg/ml ionomycin (Sigma-Aldrich) at 37°C for 4 h. After activation, cells were washed and fixed in 4% paraformaldehyde for 15 min and stored overnight in staining buffer. Fixed cells were permeabilized for 15 min on ice in CytoPerm (BD Biosciences) and stained with mAbs directed against IL-17A, IFN-γ, IL-4, IL-10, Foxp3 and appropriate isotype controls (all from BD Biosciences) for 30 min at 4°C. Fluorescence intensity was analyzed using a BD FACSAria, and data files were analyzed using DIVA software.
Cytokine Assays
Cytokine profiles were also characterized in total lung homogenates. Briefly, total lung homogenates were prepared by homogenizing whole-lung samples in 500 μl of sterile PBS, and 100 μl of cell-free homogenate was analyzed by a custom SearchLight protein multiplex immunoassay array at Aushon Biosystems, Inc (Billerica, MA) for IL-1β, IL-6, TNF-α, IL-17A, IL-23, IFN-γ, IL-12p40, IL-4. IL-22 and IL-2 levels were determined according to manufacturer’s instruction using a quantikine ELISA kit (R&D Systems, Minneapolis, MN).
BALF
BALF was collected as previously described.7 Briefly, the total cell number recovered from pooled lavages (3 × 1 ml lavages) was enumerated and differential cell counts determined using cytospin-prepared slides (Cytopro Cytocentrifuge, Wescor Inc, Logan, UT) stained with DiffQuick (Dade Behring, Newark, DE). Cell counts determined the differential ratio of cell types in 400 cells per mouse.
Lung histology and semiquantitative evaluation of inflammation
For histology studies, whole lungs were excised after lavage and inflated to 10 cm H2O pressure with 10% formalin (Sigma) to preserve pulmonary architecture. Next, lungs were processed, embedded in paraffin and sections (4–5 μm) were cut and stained with hematoxylin and eosin (H&E). Slides were microscopically reviewed and semi-quantitatively assessed by a pathologist who was blinded to the treatment conditions utilizing a previously published scoring system7 for the distribution of inflammatory changes for each parameter that included alveolar compartment inflammation, bronchiolar compartment inflammation, and intrapulmonary cellular aggregates. Each parameter was independently assigned a value from 0 to 3 with the greater the score, the greater the inflammatory changes in the lung.
Statistical Methods
Data are presented as the mean ± standard error of mean (SEM). Statistics were performed using a two-tailed, non-paired or paired t-test where appropriate to determine significant changes between two treatment groups. One-way analysis of variance (ANOVA) with Tukey multi-comparison post-test was employed to compare differences among three or more treatment groups using GraphPad (version 5.01) software. In all analyses, p values less than 0.05 were considered statistically significant.
RESULTS
DE exposure elicits elevated CD4+ infiltrates that are predominately Th17-polarized
The total number of lung-associated cells was increased following repetitive DE exposure as compared to saline control concomitant with significant elevations in the absolute number of lung CD4+ T cells, but not CD8+ T cells (Figure 1B).
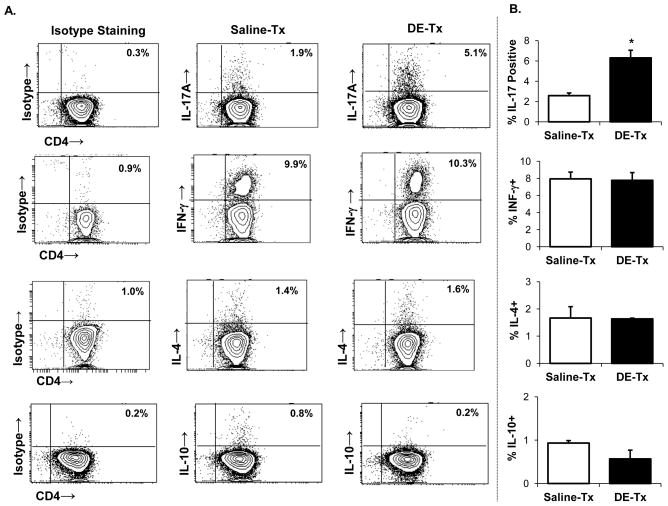
Next, CD4+ and CD8+ T cells were isolated by FACS and stimulated with PMA and ionomycin to determine cytokine expression profile. We found across four independent experiments, a consistent two- to three-fold increase in the number of IL-17 producing CD4+ cells with no major changes in IFN-γ-, IL-10-, or IL-4-producing CD4+ T cells following DE exposure as compared to saline (Figure 2A–B). There were also no significant differences in Foxp3+ Treg infiltrates following DE or saline treatment (Supplemental Figure 1). Likewise, there were no significant differences in the frequency of IFN-γ- or IL-17-producing CD8+ T cells in the lungs of DE- and saline-treated mice (Supplemental Figure 2).
Figure 2. IL-17-producing lung CD4+ T cells are increased following repetitive DE exposure.
C57BL/6 mice were repetitively exposed to DE and saline for 3 wk whereupon CD4+ T cells pooled from three animals and isolated by FACS were immediately stimulated ex vivo with PMA + ionomycin for 4 h, and stained for IL-17 (Th17), IFN-γ (Th1), IL-4 (Th2), and IL-10 (Treg) to demonstrate cytokine profiles. A, A representative contour plot depicting cytokine staining of one of four independent experiments is shown. Left column depicts background or isotype staining for each respective cytokine. Middle and right column depict saline- and DE-treated mice, respectively. B, Results represent mean ± SEM of the percentage positive cytokine staining of CD4+ T cells with statistical difference denoted by asterisk (*p<0.05).
DE exposure induces a cytokine milieu that favors Th1/17-polarization in whole lung
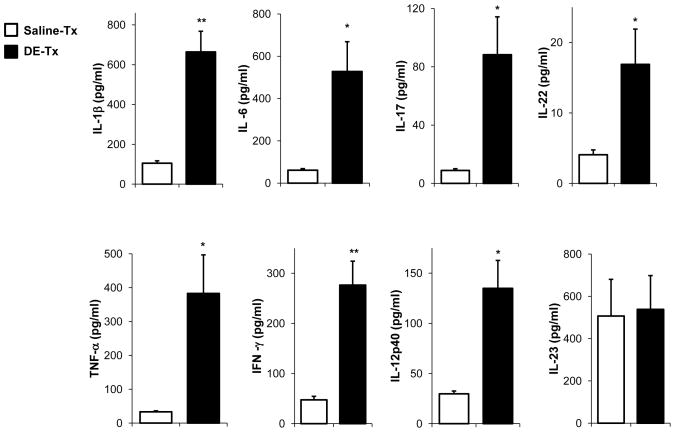
In the lung homogenates from DE-treated mice there was evidence of a cytokine milieu that would promote both Th17 and Th1 responses. First, there was significant increase in the cytokines that impact Th17 development and function. Namely, IL-6, IL-1β, IL-17, and IL-22 levels were significantly increased in DE-treated mice (Figure 3). However, there were also significant increases in IFN-γ, TNF-α, and IL-12p40, consistent with a Th1 phenotype. There was no difference in IL-23 levels in saline- and DE-treated lungs. IL-4, a prototypical Th2 cytokine, was at the lower limit of detection and not significantly different between groups (data not shown).
Figure 3. DE exposure induces a Th1/17-polarized cytokine response in the whole lung.
C57BL/6 mice were repetitively exposed to DE or saline for 3 wk and whole lung samples were homogenized and cytokine profiles associated with T cell subsets were analyzed from cell-free supernatants of total lung homogenates by protein multiplex immunoassay. Results represent the mean ± SEM of six mice from two independent experiments with statistical difference denoted by asterisks (*p<0.05, **p<0.01).
Repetitive intranasal challenge with PGN induces a Th1/Th17 polarized lung cell phenotype
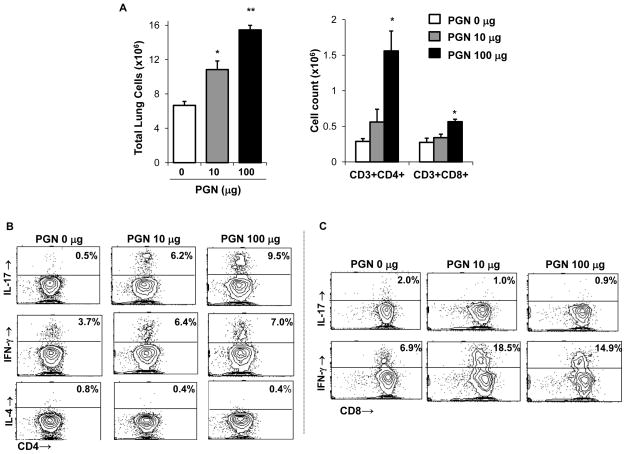
Because recent studies suggest that bacterial PGN in industrial animal farming environments are highly prevalent and may be responsible for mediating DE-induced airway disease,15, 21 we investigated the effects of repetitive PGN challenges in separate experiments to determine if PGN shared similar effects with DE on T cell development and accumulation. Similar to what was observed with DE-treated mice, PGN exposure resulted in a dose-dependent increase in total lung cell infiltrates and CD4+ T cells as compared to saline (Figure 4A). Interestingly, the highest dose of PGN tested caused an increase in CD8+ T cells infiltrating the lung (Figure 4A).
Figure 4. Repetitive intranasal challenge with peptidoglycan (PGN) induces a Th1/Th17-polarized lung cell phenotype.
C57BL/6 mice were repetitively exposed to PGN (10 and 100 μg) or saline for 3 wk, whereupon lung-associated cells were stained for CD3+CD4+ and CD3+CD8+ and T cells and analyzed by FACS. A, Results represent mean ± SEM (N=6 mice/group) of total lung cells, CD3+/CD4+ and CD3+/CD8+ T cells (percentage of cell type x total lung cell count). Statistical difference as compared to saline control (PGN 0 μg) is denoted by asterisk (*p<0.05, **p<0.01). CD4+ and CD8+ T cells pooled from 3 animals and isolated by FACS were immediately stimulated ex vivo with PMA + ionomycin for 4 h, and stained for IL-17, IFN-γ, and IL-4 to demonstrate cytokine profiles. A representative contour plot depicting cytokine staining of isolated CD4+ T cells (B) and CD8+ T cells (C) of one of three independent experiments is shown.
There was a consistent and robust increase in IL-17-producing CD4+ T cells (i.e. six to nine-fold across three independent experiments) following repetitive PGN exposure (Figure 4B). In addition, PGN challenge augmented the percentages of IFN-γ-producing CD4+ T cells, but not IL-4 or IL-10 (Figure 4B and data not shown). Moreover, PGN treatment resulted in a consistent increase in IFN-γ-, but not IL-17-producing lung CD8+ T cells as compared to saline control (Figure 4C). The cytokine profile of lung homogenates following repetitive PGN exposure resembled what was observed following DE and confirmed the establishment of a mixed Th1 and Th17-polarized immune response (Supplemental Figure 3).
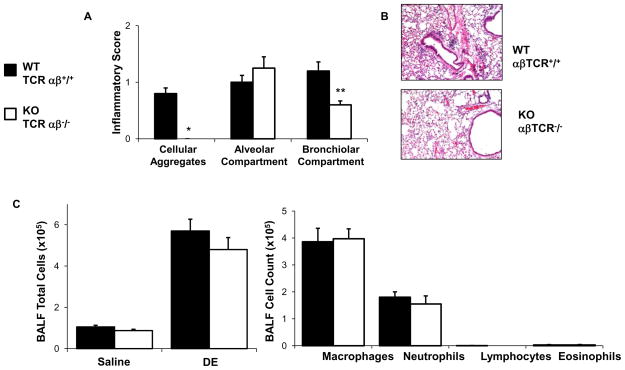
αβ-expressing T cells are essential for the formation of DE-induced peribronchiolar mononuclear aggregates, but not neutrophil infiltrates
To determine the functional importance of T cells in mediating repetitive DE-induced lung inflammation, αβ-expressing T cells were targeted through utilization of TCR αβ KO mice. There was an absence of DE-induced mononuclear aggregates and a significant reduction in peri- bronchiolar inflammation in the TCR αβ KO animals as compared to WT mice (Figure 5A–B). The number of neutrophils recovered from BALF did not differ between TCR αβ KO and WT mice following repetitive DE exposure (Figure 5C). These studies confirm that αβ-expressing T cells are critical for the genesis of the DE-induced mononuclear infiltrates, but do not appear to explain the persistent neutrophilic influx following repetitive DE exposure.
Figure 5. αβ T cells are essential for the formation of DE-induced peri-bronchiolar mononuclear aggregates, but not neutrophil infiltrates.
C57BL/6 wild type (WT, +/+) and TCR αβ knock-out (KO, −/−) mice on a C57BL/6 background were repetitively exposure to DE for 3 weeks. Whole lungs were excised and inflated to 10 cm H20 pressure with 10% formalin to preserve pulmonary architecture, processed, embedded in paraffin and sections (4–5 μm) were cut and stained with H&E. A, Results represent the mean ± SEM (n=6 mice/group) of the semiquantitative degree and distribution of lung cellular aggregates, alveolar inflammation, and bronchiolar inflammation. B, A representative section of one of six mice per treatment group is shown at 20x magnification. C, Results represent the mean ± SEM of the total cells and cell differentiation recovered from the BALF of WT and TCR αβ KO mice following repetitive DE exposure (n=6 mice per group). Statistical difference is denoted with asterisks between groups (**p<0.01).
DISCUSSION
Our studies demonstrate that repetitive exposure to swine confinement organic DE resulted in the influx of CD4+ T cells concomitant with a mixed Th1 and Th17 lung microenvironment. Exposure to PGN, a major component of DE in porcine and other large animal confinement facilities, 15, 16, 21 also resulted in the accumulation of CD4+ T cells and CD8+ T cells, with a mixed Th1 and Th17-polarized lung cytokine profile. These studies established that the development of lung parenchymal mononuclear aggregates and peribronchiolar inflammation following DE exposure is dependent on αβ-expressing T cells, but that DE-induced airway neutrophil influx is independent of these cells. Collectively, these studies establish that T cells skewed to Th1/Th17 phenotype are important in the inflammatory murine lung response to repeated organic dust exposures.
Farming exposures have gained interest over the past decade as early life exposures to farming related environments have been associated with a protection against the development of atopy or IgE-mediated diseases.8, 9 While this association is strong in European cohort studies, there is also evidence that farming exposures are protective against IgE-mediated disease development in the United States.22 However, farming practices differ in the United States with the rise in large scale, concentrated, closed animal feeding operations.1 Moreover, it has been demonstrated that despite a lower risk of atopy, children living on farms that raise swine in Iowa had a self-reported asthma prevalence of 46 to 56%.22 In adults, repetitive or chronic farm exposure is well-recognized to result in a high prevalence of airway inflammatory diseases including workplace exacerbated asthma, chronic bronchitis, and obstructive lung disease.23 Differing lineage types of effector CD4+ T cells induced by these farm organic dust exposures within the lung are likely responsible for skewing the adaptive immune response and regulating tissue inflammation and disease development.
We found that repetitive organic dust exposures induced both a Th1 and Th17 lung response, but not a Th2 or Treg response. Th1 and Th17 cell lineages are associated with promoting neutrophil, as opposed to eosinophil, accumulation in the lungs. Th17 influx was augmented in the lungs of mice following repetitive DE exposure, which was evident by enhanced CD4+ IL-17-producing cells. However, cytokine array analysis of whole lung homogenates demonstrated a mixed Th1/Th17 response. Namely, IL-6, IL-1β, IL-17, and IL-22 were increased, which is consistent with driving a Th17 response; however, IL-12 and IFN-γ were also significantly increased, suggestive of a Th1-promoting environment. IL-23 was not different between groups, which may be due to the time interval investigated or represents a distinction from other lung inflammatory models such as asthma whereby IL-23 and IL-17A are important in the enhancement and maintenance of eosinophilic and neutrophilic inflammatory responses.24–27 A similar Th1/Th17 response was demonstrated following repetitive PGN exposures, supporting a role for microbial components like PGN in driving organic dust-induced airway inflammatory responses. Our findings of a mixed Th1/Th17 response would be consistent with observations in smoking subjects with chronic obstructive lung disease (COPD).28 However, these results differ from the murine model of hypersensitivity pneumonitis and lung fibrosis whereby Th17, but not Th1 or Th2, lung responses predominate.29 It might be warranted to investigate the lung pathogenic response following organic dust exposure in mice deficient in IL-17R signaling as well as investigate IL-17 receptor variant expression levels. However, our findings suggest to us that strategies to target a specific cytokine to reduce organic dust-induced airway disease might be difficult due to the myriad of cytokines involved. Another potential future approach could be to examine the alteration of Th1/Th17 transcription factors such as Tbet, GATA3, and Retinoid-Related Orphan Receptor (ROR)γt. However, our findings suggest to us that strategies to target a specific cytokine to reduce organic dust-induced airway disease might be difficult due to the multiple cytokines involved.
The numbers of CD4+ T cells were increased in the lungs of mice repetitively exposed to organic dusts. Our studies also confirmed that αβ-expressing T cells are the critical T cell infiltrate following repetitive DE exposure as cellular aggregates failed to form in TCR αβ KO mice and bronchiolar inflammation was significantly reduced following DE exposures. Interestingly, alveolar inflammation and neutrophil influx was found to be αβ T cell-independent. This later observation implies that alternative cell types, such as macrophages and/or airway epithelia, are also likely important contributors for sustaining neutrophil influx. A potential future approach to further understand the role of cellular players would be the generation of chimera mice or adoptive transfer models of TCR-deficient and healthy naïve mice. Alternatively, CD8+, γδ T cells, NK cells, and NKT cells are also important sources of a number of these lung-associated cytokines that promote airway inflammation.24 However, repetitive DE exposure did not increase the number of CD8+ T cells or IFN-γ- or IL-17-producing CD8+ T cells, which suggests that they may not be as important in this model. In addition, the frequencies of γδ T cells, NK cells, and NKT cells were quite low and did not differ among groups (data not shown). Although we did not quantitate γδ T cells in the TCR αβ KO mice, an earlier publication from our laboratory7 demonstrated that T cell influx following organic dust treatment was clustered in cellular aggregates throughout the lung parenchyma. In the current study, these cellular aggregates were absent in the αβ TCR deficient mice following organic dust exposure, confirming that the T lymphocytes within the aggregates express αβ TCR. It also suggests that a compensatory increase in other lymphocyte types, such as γδT cells, did not occur because the aggregates were absent, although admittedly, a minor population may still be discernible by more sensitive detection methods such as FACS. The potential involvement of γδT cells in this model can be investigated in future studies. Overall, because studies demonstrated an important role for T cells, future studies could investigate whether lymphocyte-targeted interventions with immunosuppressive drugs including cyclosporine or mycophenolate mofetil might be beneficial in reducing DE-induced airway disease. This may be a novel future direction because pharmacologic interventions that include inhaled sodium cromoglycate, corticosteroids, and long-acting beta agonists have shown modest and sometimes conflicting effects in the prevention and/or treatment of exposed agriculture workers.30–32
Research into respiratory disease exposure in various agricultural settings has primarily focused Gram-negative bacterial endotoxins. Endotoxins have also been recognized to induce Th1 and Th17 responses in the lung.33, 34 Although endotoxins are clearly important, they may explain only a relative small proportion of respiratory disease seen in agricultural workers.35 Bioaerosols in current swine production facilities contain up to 80% Gram-positive bacteria and high levels of PGN/muramic acid.15, 17, 21, 36 Moreover, our previous work has implicated non-endotoxin components, such as PGN, as a driver of swine facility DE-induced inflammatory consequences.17, 18 In this current study, similar to that observed with DE, PGN alone induced Th17/Th1-polarized CD4+ T cells. However, one difference was the observation of increased in CD8+ T cells skewed toward a Th1 state. Whereas this could be related to biological potency, it also highlights that environmental organic dust exposures are inherently complex and immune responses cannot simply be ascribed to one single agent. The observations with PGN alone might be broadly applicable to other environments whereby Gram-positive bacteria and/or PGNs are detected, and this includes school rooms, domestic homes, and other animal farming environments.15, 37, 38
In conclusion, organic DE from porcine animal confinement facilities promotes a mixed Th1 and Th17 lung microenvironment with an important role for αβ-expressing T cells in mediating DE-induced lung pathology. Moreover, PGN, a component of organic dust elicited a similar lung response, suggesting that this microbial motif is a major driver of pathogenic lung inflammation. Overall, this information could be important when investigating potential cellular-directed therapies to reduce organic dust-induced airway inflammatory consequences, particularly those related to chronic bronchitis and obstructive lung disease in exposed agricultural workers.
Supplementary Material
Acknowledgments
The authors wish to thank Victoria Smith and Charles Kuszynski, Ph.D., of the UNMC Cell Analysis Facility for assistance with flow cytometric measurements, Greg Dooley, Ph.D., of the Colorado State University Center for Environmental Medicine, for assistance with mass spectrometry analysis of dust samples, UNMC Tissue Science Facility for assistance with digital microscopy images prepared for the manuscript, and Elizabeth Klein at UNMC for technical assistance.
Footnotes
- Jill A. Poole: (1) conception and design of the study; (2) data generation (when applicable); (3) analysis and interpretation of the data and (4) preparation and critical revision of the manuscript.
- Angela Gleason: (1) conception and design of the study; (2) data generation (when applicable); (3) analysis and interpretation of the data and (4) preparation and critical revision of the manuscript.
- Chris Bauer: (1) data generation (when applicable); (2) analysis and interpretation of the data and (3) critical revision of the manuscript.
- William West: (1) data generation (when applicable); (2) analysis and interpretation of the data and (3) critical revision of the manuscript.
- Neil Alexis: (1) conception and design of the study; (2) analysis and interpretation of the data and (3) critical revision of the manuscript.
- Debra Romberger: (1) conception and design of the study; (2) analysis and interpretation of the data and (3) critical revision of the manuscript.
- Tammy Kielian: (1) conception and design of the study; (2) analysis and interpretation of the data and (3) preparation or critical revision of the manuscript.
Disclosure: Study supported by grants from the National Institute of Environmental Health Sciences (R01: ES019325; K08: ES015522-01 and [ARRA] ES015522-03S1 to JAP), National Institute of Occupational Safety Health (1R01OH008539-01 to DJR and 5U50 OH008085 to SJR), and the National Institute of Neurological Disorders and Stroke (3R01NS040730 to TK)
References
- 1.Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol. 2012;12:126–132. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health. 2003;9:185–96. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 3.Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J. 2009;34:80–8. doi: 10.1183/09031936.00105308. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DA, Landas SK, Lassise DL, Burmeister LF, Hunninghake GW, Merchant JA. Airway injury in swine confinement workers. Ann Intern Med. 1992;116:630–5. doi: 10.7326/0003-4819-116-8-630. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen B, Iversen M, Bundgaard Larsen B, Dahl R. Pig farmers have signs of bronchial inflammation and increased numbers of lymphocytes and neutrophils in BAL fluid. Eur Respir J. 1996;9:524–530. [PubMed] [Google Scholar]
- 6.Ivanov S, Palmberg L, Venge P, Larsson K, Linden A. Interleukin-17A mRNA and protein expression within cells from the human bronchoalveolar space after exposure to organic dust. Respir Res. 2005;6:44. doi: 10.1186/1465-9921-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole JA, Wyatt TA, Oldenburg PJ, et al. Intranasal Organic Dust Exposure-Induced Airway Adaptation Response Marked By Persistent Lung Inflammation and Pathology in Mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1085–L1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Mutius E, Radon K. Living on a farm: impact on asthma induction and clinical course. Immunol Allergy Clin North Am. 2008;28:631–47. ix–x. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 10.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 11.Lluis A, Schaub B. Lesson from the farm environment. Curr Opin Allergy Clin Immunol. 2012;12:158–163. doi: 10.1097/ACI.0b013e32835109a8. [DOI] [PubMed] [Google Scholar]
- 12.Sahlander K, Larsson K, Palmberg L. Altered innate immune response in farmers and smokers. Innate Immun. 2010;16:27–38. doi: 10.1177/1753425909106317. [DOI] [PubMed] [Google Scholar]
- 13.Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells. Clin Sci (Lond) 2010;119:75–86. doi: 10.1042/CS20100033. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 15.Poole JA, Dooley GP, Saito R, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A. 2010;73:684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szponar B, Larsson L. Use of mass spectrometry for characterising microbial communities in bioaerosols. Ann Agric Environ Med. 2001;8:111–7. [PubMed] [Google Scholar]
- 17.Poole JA, Wyatt TA, Kielian T, et al. Toll-like receptor 2 (TLR2) Regulates Organic Dust-Induced Airway Inflammation. Am J Respir Cell Mol Biol. 2011:719. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole JA, Alexis NE, Parks C, et al. Repetitive organic dust exposure in vitro impairs macrophage differentiation and function. J Allergy Clin Immunol. 2008;122:375–82. 382 e1–4. doi: 10.1016/j.jaci.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam R, Gorska M. 3. Lymphocytes. J Allergy Clin Immunol. 2003;111:S476–85. doi: 10.1067/mai.2003.121. [DOI] [PubMed] [Google Scholar]
- 20.Poole JA, Kielian T, Wyatt TA, et al. Organic Dust Augments Nucleotide-Binding Oligomerization Domain (NOD2) Expression via an NF-{kappa}B Pathway to Negatively Regulate Inflammatory Responses. Am J Physiol Lung Cell Mol Physiol. 2011:L296–L306. doi: 10.1152/ajplung.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol. 2008;10:665–675. doi: 10.1111/j.1462-2920.2007.01489.x. [DOI] [PubMed] [Google Scholar]
- 22.Merchant JA, Naleway AL, Svendsen ER, et al. Asthma and farm exposures in a cohort of rural Iowa children. Environ Health Perspect. 2005;113:350–356. doi: 10.1289/ehp.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136:716–25. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 24.Vanaudenaerde BM, Verleden SE, Vos R, et al. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med. 2011;183:977–986. doi: 10.1164/rccm.201007-1196PP. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima H, Hirose K. Role of IL-23 and Th17 Cells in Airway Inflammation in Asthma. Immune Netw. 2010;10:1–4. doi: 10.4110/in.2010.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011 doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Sun M, Cheng H, et al. Silencing IL-23 expression by a small hairpin RNA protects against asthma in mice. Exp Mol Med. 2011;43:197–204. doi: 10.3858/emm.2011.43.4.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 29.Simonian PL, Roark CL, Wehrmann F, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson K, Larsson BM, Sandstrom T, Sundblad BM, Palmberg L. Sodium cromoglycate attenuates pulmonary inflammation without influencing bronchial responsiveness in healthy subjects exposed to organic dust. Clin Exp Allergy. 2001;31:1356–1368. doi: 10.1046/j.1365-2222.2001.01193.x. [DOI] [PubMed] [Google Scholar]
- 31.Ek A, Palmberg L, Larsson K. The effect of fluticasone on the airway inflammatory response to organic dust. Eur Respir J. 2004;24:587–593. doi: 10.1183/09031936.04.00018304. [DOI] [PubMed] [Google Scholar]
- 32.Strandberg K, Ek A, Palmberg L, Larsson K. Fluticasone and ibuprofen do not add to the effect of salmeterol on organic dust-induced airway inflammation and bronchial hyper-responsiveness. J Intern Med. 2008;264:83–94. doi: 10.1111/j.1365-2796.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 33.Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doreswamy V, Peden DB. Modulation of asthma by endotoxin. Clin Exp Allergy. 2011;41:9–19. doi: 10.1111/j.1365-2222.2010.03628.x. [DOI] [PubMed] [Google Scholar]
- 35.Burch J, Svendsen E, Siegel P, et al. Endotoxin exposure and inflammation markers among agricultural workers in Colorado and Nebraska. J Toxicol and Environ Health. 2010;73:5–22. doi: 10.1080/15287390903248604. [DOI] [PubMed] [Google Scholar]
- 36.Szponar B, Larsson L. Determination of microbial colonisation in water-damaged buildings using chemical marker analysis by gas chromatography-mass spectrometry. Indoor Air. 2000;10:13–8. doi: 10.1034/j.1600-0668.2000.010001013.x. [DOI] [PubMed] [Google Scholar]
- 37.Fox A, Harley W, Feigley C, et al. Large particles are responsible for elevated bacterial marker levels in school air upon occupation. J Environ Monit. 2005;7:450–456. doi: 10.1039/b418038k. [DOI] [PubMed] [Google Scholar]
- 38.Sebastian A, Larsson L. Characterization of the microbial community in indoor environments: a chemical-analytical approach. Appl Environ Microbiol. 2003;69:3103–9. doi: 10.1128/AEM.69.6.3103-3109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.