Abstract
Background
Increased nicotinic receptor mediated relaxation in the gastroesophageal antireflux barrier may be involved in the pathophysiology of reflux. This study is designed to determine whether the defects we previously identified in GERD patients in-vivo are due to abnormalities of the gastric sling, gastric clasp or lower esophageal circular (LEC) muscle fibers.
Methods
Muscle strips from whole stomachs and esophagi were obtained from 16 normal donors and 15 donors with histologically proven Barrett's esophagus. Contractile and relaxant responses of gastric sling, gastric clasp or LEC fibers were determined to increasing concentrations of carbachol and to nicotine after inducing maximal contraction to bethanechol. Muscarinic receptor density was measured using subtype selective immunoprecipitation.
Key Results
Barrett's esophagus gastric sling and LEC fibers have decreased carbachol induced contractions. Barrett's esophagus sling fibers have decreased M2 muscarinic receptors and LEC fibers have decreased M3 receptors. Relaxations of all 3 fiber types are greater in Barrett's esophagus specimens to both high carbachol concentrations and to nicotine following bethanechol pre-contraction. The maximal response to bethanechol is greater in Barrett esophagus sling and LEC fibers.
Conclusions & Inferences
The increased contractile response to bethanechol in Barrett's specimens indicates that the defect is likely not due to the smooth muscle itself. The enhanced nicotinic receptor mediated response may be involved in greater relaxation of the muscles within the high pressure zone of the gastroesophageal junction during transient lower esophageal sphincter relaxations and during deglutitive inhibition and may be involved in the pathophysiology of gastro esophageal reflux disease.
Introduction
Prior in-vivo studies by our group demonstrated abnormal pressure profiles from the gastric sling and clasp muscle fiber complex and from the lower esophageal circular (LEC) fibers in patients with gastroesophageal reflux disease (GERD). A simultaneous endoluminal ultrasound and manometry catheter was pulled through the esophago-gastric segment before and after atropine administration which demonstrated that in GERD patients, the muscarinic receptor mediated tone was reduced in the proximal LEC fibers and absent in the distal gastric clasp and sling fiber complex (1). In an attempt to explain these abnormal pressure profiles, we evaluated the in-vitro contractile responses of these muscle groups in patients with chronic GERD compared to non-GERD subjects. Since a large volume of tissue is required to perform these experiments, it was decided to obtain viable tissue from organ transplant donors. We used normal transplant donors without a history of GERD or use of proton pump inhibitory drugs (PPIs) or H2 receptor blocking drugs as normal controls. We used donors with Barrett's esophagus as a surrogate marker for chronic reflux, because these patients are known to have chronic reflux and because we were able to definitively make a diagnosis of Barrett's esophagus based on histology (presence of goblet cells). The current study compares muscle preparations using in-vitro techniques to evaluate the area of the gastric sling and clasp muscle fibers, and the LEC fibers, by measuring the force generated in response to the mixed muscarinic and nicotinic cholinergic receptor agonist carbachol and the relaxation response to nicotine after inducing a maximal contraction with the specific muscarinic receptor agonist bethanechol (30 μM).
Purpose
To determine whether there are differences in the contractile response to muscarinic stimulation and the relaxation response to nicotinic stimulation in smooth muscle strips from muscle fibers involved in the gastroesophageal junction high pressure zone between organ donors with Barrett's esophagus and non-GERD donors.
Materials and Methods
Forty two stomach and esophagi were procured over a 52 month period by third party organ procurement agencies (the National Disease Research Interchange and the International Institute for the Advancement of Medicine) under approval from the Temple University Institutional Review Board. These organs were from brain dead donors maintained on life support who had consented to organ transplant donation. Their next of kin consented to donation of non-transplantable organs for research. The only medical records available relate to the events occurring at the time of brain death because the donors' identity was de-identified by the procurement agencies. Thus limited medical history is available and no direct medical record information is accessible to determine whether the subject had GERD diagnosed by a physician. Indirect medical history was obtained by the procurement agencies by interviewing the next of kin and determining whether the donor had heartburn, reflux, regurgitation or use of antacids or acid suppressive medication (proton pump inhibitors or histamine H2 receptor blockers) on a regular basis. As described below, 15 of these specimens were definitively identified as Barrett's esophagus specimens based on the presence of epithelial goblet cells observed with Alcian blue histochemistry. In 11 of the 42 specimens, no goblet cells were observed but the next of kin interview indicated possible reflux. Because no absolutely definitive clinical diagnosis of GERD could be made or ruled out in these donors, these specimens were excluded from this study. No next of kin reports of reflux and no epithelial goblet cells were observed in 16 of the 42 specimens which were considered non-GERD specimens.
All drugs and chemicals were obtained from Sigma Chemical Company (St. Louis, MO) with the exception of digitonin (Wako Pure Chemical Company, Osaka) and pansorbin (Calbiochem, La Jolla, CA).
In-vitro muscle strip studies
For the muscle bath studies, muscle strip samples from 15 of the 16 non-GERD donors and all 15 of the Barrett's donors were used. Contraction and relaxation studies were performed prior to histochemical identification of the presence or absence of epithelial goblet cells. The stomach and esophagi were harvested from the transplant donors within 30 minutes after cross clamping the aorta. The stomach contents were gently rinsed out with saline. The esophageal and pyloric openings were ligated and the entire specimen was transported to our laboratory on ice by overnight courier immersed in either University of Wisconsin (UW, Beltzer's Viaspan) organ transport media or Custodial HTK solution.
The specimens were dissected in a cold room (0-5°C). The greater and lesser omentum was removed. The outermost longitudinal fibers descending from the esophagus across the stomach were individually removed by sharp dissection which exposed the inner muscle layers of the esophagus and the stomach. The LEC fibers are the circular muscle fibers running circumferentially at the lower esophagus 2-3 cm above the cardiac notch. The sling muscle fibers are seen as a U shaped group of fibers approximately 8 mm wide enveloping the esophagus around the greater curvature of the stomach and the semicircular clasp muscle fibers along the lesser curvature opposite to the cardiac notch. The clasp muscle fibers are oriented perpendicular to the sling muscle fibers (Figure 1). The LEC was carefully dissected from the underlying mucosa as a complete ring starting from 4 cm above the cardiac notch to 2 cm above the cardiac notch. The ring of the circular muscles were then separated from the esophagus and muscle strips of approximately 3 × 3 × 8 mm with the long axis parallel to the direction of the muscle fibers were prepared. Beginning at the cardiac notch, the sling muscle fibers were separated from the underlying submucosa by sharp dissection and this tissue plane was followed completely around the lesser curvature thus separating the clasp muscle fibers from underlying submucosa. The clasp muscle fiber complex was removed from the sling fiber complex by sharp dissection and cut into 10-12 strips as described above. Similar muscle strips were cut from the middle of the sling muscle fibers such that these strips were derived from the sling muscle fibers in the cardiac notch as well as sling fibers extending along both sides of the esophageal opening of the stomach. These smooth muscle strips were suspended in 10 ml muscle baths in Tyrode's solution continuously bubbled with 95%O2 / 5%CO2 and maintained at 37 °C.
Figure 1.

Photograph and sketches of the dissections. Center photograph shows a dissection immediately before separation of the mucosa from the muscle as sketched at the upper right after removing the longitudinal fibers sketched at the upper left and opening the esophagus sketched at the lower left.
The muscle strips were stretched to approximately 150% of their slack length, which produced approximately 1 gram of basal tension and were then allowed to accommodate to the muscle bath for at least 60 minutes prior to investigation of contractile response and or relaxation response. Carbachol was added to the muscle baths to obtain carbachol concentration response curves. Bethanechol was added at a concentration of 30 μM to induce a maximal contraction after which 1 mM nicotine was added to induce relaxation. Because the relaxation response is dependent on the contractile response, the relaxation response to carbachol is expressed as a % of maximum contraction response to carbachol. The relaxation response (% of contraction) to nicotine was determined after inducing a maximal contraction with 30 μM bethanechol.
Histological determination of Barrett's versus non-GERD
After all muscle strips were collected from the specimens, the stomach and esophagus of each specimen was opened by a longitudinal incision along the lesser curvature and extending up the esophagus, exposing the transition zone separating the mucosa of the stomach from the esophageal mucosa (Z-line). This transition zone was removed for histology by transecting the esophagus 1.5 cm proximal to the Z-line and transecting the stomach 0.5 cm distal to the Z-line. After fixation in 4% phosphate buffered paraformaldehyde for 2 days, followed by cryopreservation in 30% sucrose for 3 days, the specimen was cut into 4 equal longitudinal sections that were mounted side-by-side so that all 4 stomach and esophageal mucosa sections of each specimen (representing all 4 quadrants of the gastroesophageal junction) could be embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek USA Inc., Torrance, CA) and sectioned together on a cryostat at 12 μm before mounting on charged slides (Fisher Plus). Sections were dried onto slides overnight at room temperature, and were stained with hematoxylin and eosin (H&E), or with Alcian blue for discrimination of goblet cells, using a method described by Edgett et al, 2004 (2).
Three independent investigators that were blinded to the subjects' donor information examined the sections for presence or absence of goblet cells. Presence of goblet cell metaplasia in the esophageal mucosa (Alcian Blue stained) was used to define Barrett's esophagus in a donor specimen. Specimens were then coded as either having Barrett's esophagus or as non-GERD. Data obtained from specimens from donors without goblet cells but a positive history of acid suppressive medication or of heartburn or symptoms suggesting heartburn, as elicited from family members was not used for this study.
Muscarinic receptor subtype immunoprecipitation
Tissues from 20 (10 from non-GERD, 10 from Barrett's esophagus) of the 31 donors were used to quantify muscarinic receptor density. Rabbit polyclonal antibodies were used for the muscarinic receptor subtype immunoprecipitation assays. The M2 antibody was raised to a fusion protein of glutathione-S-transferase and a sequence corresponding to the third intracellular loop of the M2 receptor. The M3 antibody was raised to a synthetic peptide corresponding to the C terminus of the M3 receptor coupled to thyroglobulin. The production and specificity of these antibodies as determined by immunoprecipitation of clonal cell lines expressing individual receptor subtypes has been previously described.(3-6) This immunoprecipitation assay makes use of tandem specificity: the specificity of [3H]QNB binding to only muscarinic receptors and the specificity of the individual antibody binding to only the given subtype. If the antibody binds to other proteins that do not bind [3H]QNB then those proteins would not be detected in the assay. Likewise if [3H]QNB binds to other proteins that do not bind to the antibody then those proteins would not be detected by the assay. Briefly, the tissues were homogenized at 100 mg/ml in cold Tris EDTA buffer (TE) with 10μg/mL of the following protease inhibitors: soybean and lima bean trypsin inhibitors, aprotinin, leupeptin, pepstatin, and α2-macroglobulin. 20μL of the non-subtype selective muscarinic receptor antagonist [3H] QNB (49 Curies/mM, approximately 4,000 cpm /μL) per mL assay homogenate was added and incubated at room temperature for 30 minutes with inversion every 5 minutes. Samples were pelleted via centrifugation at 20,000 g for 10 minutes at 4°C and the pellet was solubilized in TE buffer containing 1% digitonin and 0.2% cholic acid (1% TEDC) with the above protease inhibitors at 100 mg wet weight per ml. Samples were incubated for 50 minutes at 4°C, with inversion every 5 minutes then centrifuged at 30,000 g for 45 minutes at 4°C. The supernatant containing the solubilized receptors was incubated overnight after addition of the M2 antibody, the M3 antibody or vehicle at 4°C. To determine total receptor density, samples were desalted over Sephadex G-50 minicolumns with 0.1% TEDC. M2 and M3 receptors were precipitated by adding 200μL pansorbin, and incubated at 4°C for 50 minutes, with inversion every 5 minutes. The precipitated receptors were pelleted via centrifugation at 15,000 g for one minute at 4°C and the pellet was surface washed with 500μL of 0.1% TEDC. 50μL of 72.5mM deoxycholate/ 750 mM NaOH was added and incubated for 30 minutes at room temperature. The pellet was resuspended in 1 mL of TE buffer and neutralized with 50μL of 1M HCl. Radioactive counts were determined by liquid scintillation spectrometry. Protein content was determined by a Coomassie blue dye binding protein assay using bovine serum albumin as a standard. Receptor density (mean ± SEM) is reported as femtomoles (fmoles) receptor per mg solubilized protein
Statistical analysis
Data is shown as mean ± SEM for the number of muscle strips (n) from the number of donor organs (N). Tension developed by the muscle fibers is not normalized to cross sectional area because analysis of over 300 clasp, 300 sling and 300 LEC muscle strips demonstrated that there is no statistically significant correlation between the maximal response to carbachol or bethanechol and the cross sectional area of the muscle strips. This may be due to the manner in which the muscle strips are suspended in the tissue baths. The strips are attached via tissue clips (Radnoti LLC, Monrovia, CA, cat # 158801), which meet at a point and do not encompass the entire cross sectional area of the strip. Hence the contraction measured may be due to only a linear portion of the muscle strip located between the clips. Statistical analyses are performed using analysis of variance. Because the variance is significantly different between groups, a nonparametric Mann-Whitney U-test is used to compare groups. Probability values less than 0.05 are considered statistically significant.
Results
Subject Demographics
Of the 31 specimens included in the study, 16 had no history of GERD and 15 had Barrett's esophagus, the latter confirmed by observation of epithelial goblet cells after Alcian blue histochemical staining. Non-Barrett's (Non-GERD) subjects were selected based on a lack of epithelial goblet cells on Alcian blue histochemistry, and no history of PPI use, or history of heartburn or symptoms suggesting heartburn, as elicited from family members. For the non-GERD donors, the average age was 47.3 ± 4.4 years, the average height was 1.7 ± 0.03 meters, the average weight was 77.7 ± 5.2 kg and the average BMI was 26.8 ± 1.6. These donors consisted of 7 females and 9 males, 2 were Asian, 13 were Caucasian and 1 was Hispanic. For the donors with Barrett's esophagus, the average age was 44.8 ± 4.2 years, the average height was 1.7 ± 0.02 meters, the average weight was 87.9 ± 5.0 kg and the average BMI was 31.0 ± 1.8. These donors consisted of 6 females and 9 males, 2 were African American, 1 was Asian, 10 were Caucasian and 2 were Hispanic. There were no significant differences between non-GERD donors and Barrett's esophagus donors in age, height, weight or BMI.
Response to carbachol
Carbachol induces a contractile response at lower concentrations and then abrupt relaxations at concentrations of 100 μM and higher (Figure 2). There is a marked and profound difference between the Barrett's donors and the non-GERD subjects in both the contraction and relaxation of the gastric sling muscle fibers. The Barrett's donors' sling fibers contract less than the non-GERD donors and relax more at all subsequent concentrations of carbachol (p<0.01, Figure 3, middle graph). The relaxation response is significantly greater in the sling muscle fibers from the Barrett's esophagus donors, when compared to non-GERD donors expressed in grams of tension (p<0.01, Figure 4, middle graph) or expressed as a percentage of the maximal carbachol induced contraction (p<0.01, Figure 4, bottom graph).
Figure 2.

Representative trace of gastric clasp, gastric sling and LEC muscle fiber contractile response to the mixed muscarinic and nicotinic receptor agonist carbachol. Low carbachol concentrations induce contraction while carbachol concentrations above 10 μM induce an abrupt relaxation response. This relaxation response is not induced by the muscarinic receptor agonist bethanechol, but is induced by nicotine following bethanechol induced contraction.
Figure 3.
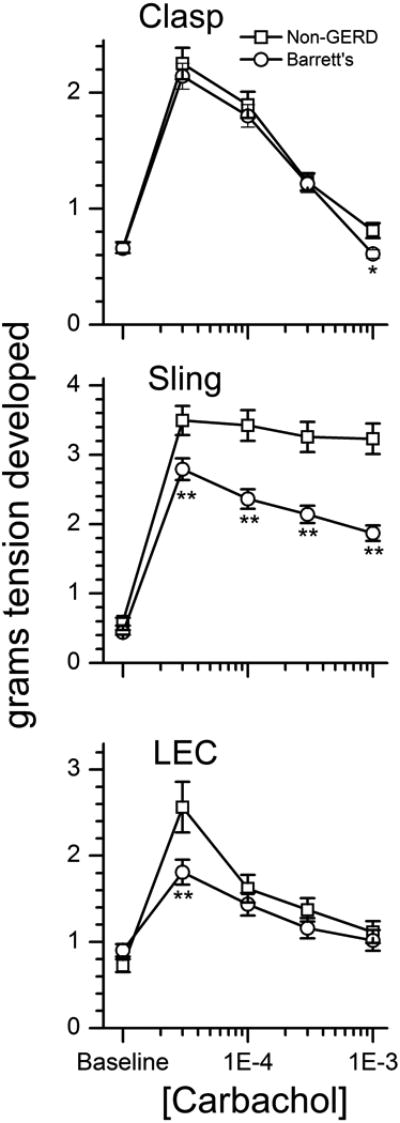
Carbachol concentration response curves for gastric clasp, gastric sling and LEC muscle fibers from non-GERD, and Barrett's esophagus donors. * p<0.05 or ** p<0.01 comparing either probable GERD or Barrett's to non-GERD. # p<0.05 or ## p<0.01 comparing Barrett's to probable-GERD
Figure 4.

Carbachol induced maximal contraction, relaxation and relaxation as a percentage of contraction for gastric clasp, gastric sling and LEC muscle fibers from non-GERD and Barrett's esophagus donors. There was no significant difference in the contractile or relaxation response of gastric clasp muscle fibers between Barrett's and non-GERD subjects when evaluated as the absolute tension in grams. However, the relaxation response as normalized to percent contraction of the gastric clasp muscle fibers was significantly greater from Barrett's esophagus donors than from non-GERD donors (p<0.01). The contractile response of the gastric sling muscle fibers when evaluated as the absolute tension in grams was significantly less from donors with Barrett's esophagus when compared to non-GERD donors (p<0.05). The relaxation response was significantly greater in the gastric sling fibers from Barrett's esophagus when compared to non-GERD donors (p<0.01). The relaxation response as normalized as a percent of contraction of the gastric sling muscle fibers was significantly greater from Barrett's donors when compared with the non-GERD donors (p<0.01) The contractile response of LEC fibers was significantly less from donors with Barrett's esophagus when compared to non-GERD donors (p<0.01), while the relaxation response was significantly greater in LEC fibers from donors with Barrett's esophagus compared to non-GERD donors. For figures 3-5 N refers to the number of different donors and n refers to the number of muscle strips. * p<0.05 or ** p<0.01 compared to non-GERD.
The contractile response to carbachol of the LEC fibers is significantly less from donors with Barrett's esophagus compared to non-GERD donors in grams of tension (p<0.01, Figure 3, bottom graph). The relaxation response, expressed as a percentage of the maximal contraction, is significantly greater in the LEC fibers from Barrett's esophagus donors (p<0.01, Figure 4, bottom graph). There is no significant difference in the contractile or relaxation response of the gastric clasp muscle fibers between Barrett's esophagus donors and non-GERD donors when expressed as the absolute grams of tension, except at the very highest concentration of carbachol in which the Barrett's esophagus clasp fibers relax more than the non-GERD fibers (p<0.05, Figure 3, top graph). However, the maximal relaxation response, expressed as a percentage of the maximal contraction of the gastric clasp muscle fibers is significantly greater in muscle strips from the Barrett's esophagus donors than from the non-GERD donors (p<0.01, Figure 4, bottom graph).
Bethanechol (30 μM) induced contraction and nicotine (1 mM) induced relaxation
There is no difference in the contractile or relaxation response between the gastric clasp muscle fibers from non-GERD donors and donors with Barrett's esophagus when evaluated as the absolute grams of tension produced (Figure 5, top and middle graphs). However, the sling muscle fibers from the Barrett's esophagus donors demonstrate a greater contractile response (p<0.01) and more of a relaxation response (p<0.01) to bethanechol and nicotine respectively, than the non-GERD donors, when evaluated as the absolute grams of tension (figure 5, top and middle graphs). The relaxation response, normalized as a percentage of the maximal contraction is significantly greater in the Barrett's esophagus donors when compared to the non-GERD donors, in both the sling muscle fibers (p<0.01) and the clasp fibers (p<0.05, Figure 5, bottom graph). LEC fibers from Barrett's esophagus donors contract significantly more to 30 μM bethanechol than LEC fibers from non-GERD donors (p<0.05, Figure 5, top graph) and relax more to the subsequent addition of nicotine, when expressed in terms of grams of tension (p<0.01, Figure 5, middle graph). There is no difference in the relaxation response of LEC fibers between the groups, when normalized as a percentage of the maximal contraction (Figure 5, bottom graph).
Figure 5.

Bethanechol (30 μM) induced contraction, nicotine (1 mM) induced relaxation and nicotine induced relaxation as a percentage of the bethanechol induced contraction for gastric clasp, gastric sling and LEC muscle fibers from non-GERD, and Barrett's esophagus donors. There was no difference in the contractile or relaxation response between clasp fibers from non-GERD donors and donors with Barrett's esophagus when evaluated as the absolute tension in grams. However the gastric clasp muscle fibers relaxed more in the Barrett's donors than in the non-GERD donors when normalized as a percent of contraction (p<0.05). The gastric sling muscle fibers from the Barrett's subjects had a greater contractile response (p<0.01) and relaxation response (p<0.01) to bethanechol and nicotine respectively, than the non-GERD subjects when evaluated as the absolute tension in grams. Gastric sling muscle fibers from Barrett's esophagus donors relaxed significantly greater than gastric sling muscle fibers from non-GERD donors when normalized as a percent of contraction. LEC fibers from Barrett's esophagus donors contracted significantly more to bethanechol and relaxed more to nicotine than LEC fibers from non-GERD donors (p<0.05) when evaluated as the absolute tension in grams. There was no difference in the relaxation response of LEC fibers between the groups when evaluated as the percent of the contractile response. All fibers relaxed significantly more with bethanechol and nicotine than with Carbachol alone except for the clasp fibers from the Barrett's donors.
All muscle fibers relax significantly more with bethanechol and nicotine than with cumulative addition of carbachol alone (p<0.05), except for the clasp fibers from the Barrett's subjects in which no statistically significant differences in relaxation response between the two protocols is found.
Muscarinic receptor density
Tissues from 20 (10 from non-GERD, 10 from Barrett's esophagus) of the 31 donors were used to quantify muscarinic receptor density. For the non-GERD donors, the average age was 50.6 ± 5.6 years, the average height was 1.7 ± 0.04 meters, the average weight was 73.9 ± 6.3 kg and the average BMI was 25.6 ± 1.7. These donors consisted of 5 females and 5 males, 1 was Asian and 9 were Caucasian. For the donors with Barrett's esophagus, the average age was 42.1 ± 5.6 years, the average height was 1.7 ± 0.03 meters, the average weight was 86.8 ± 5.9 kg and the average BMI was 28.7 ± 2.2. These donors consisted of 3 females and 7 males, 2 were African American, 1 was Asian, 5 were Caucasian and 2 were Hispanic. There were no significant differences between non-GERD donors and Barrett's esophagus donors in age, height, weight or BMI.
The density of total, M2 and M3 muscarinic receptors were determined in each specimen using either duplicate or triplicate determinations depending on the availability of sufficient tissue. There are no differences in the clasp fiber total, M2 or M3 receptor densities between Barrett's esophagus specimens and non-GERD specimens. The density of M2 receptors are significantly lower in sling fibers from Barrett's esophagus specimens than in non-GERD specimens (p<0.05). Total and M3 receptor density is significantly lower in the LEC fibers of Barrett's esophagus specimens than in the non-GERD specimens (p<0.01 for total, p<0.05 for M3, Figure 6).
Figure 6.
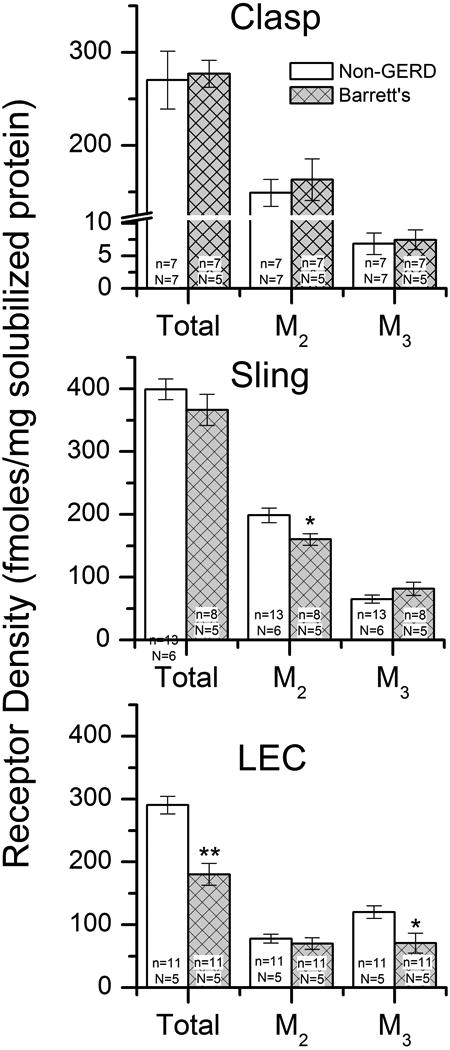
Total, M2 and M3 muscarinic receptor density in clasp, sling and LEC muscle from non-GERD donors and Barrett's esophagus donors as determined by immunoprecipitation. There are no differences in total, M2 or M3 receptor density in clasp fibers from non-GERD donors and Barrett's esophagus donors. Sling fibers from Barrett's esophagus donors have a lower density of M2 receptors than sling fibers from non-GERD donors. There is a lower density of total and M3 receptors in LEC fibers from Barrett's esophagus donors than from non-GERD donors.
Discussion
Barrett's esophagus is a condition in which chronic gastroesophageal reflux causes a change in the lining of the esophagus from a normal squamous mucosa to an abnormal specialized columnar mucosa (intestinal metaplasia) that can become dysplastic and predispose to adenocarcinoma of the distal esophagus. The underlying pathophysiology of the chronic reflux in patients with Barrett's esophagus has never been fully explained. In the current study, we used Barrett's esophagus as a surrogate marker for chronic reflux in order to study the physiology and pathophysiology of the muscles that make up the antireflux barrier (gastric clasp, gastric sling and esophageal LEC). Detailed pharmacologic studies of in-vitro contractile responses are not feasible using the minute muscle tissue obtained from an endoscopic biopsy but are feasible using whole organs obtained from organ transplant donors. Our goal was to determine the underlying pathophysiology of GERD and Barrett's esophagus.
We are confident that all of the Barrett's esophagus donors in this study had chronic reflux based on the intestinal metaplasia diagnosed by the presence of goblet cells on histology using Alcian Blue staining. However, it is more difficult to exclude reflux in the non-GERD, normal control donors, since a direct interview could not be obtained from the subjects and since pH testing was not possible as these were tissues obtained from organ transplant donors. GERD was excluded to the best of our ability by obtaining a medical history from the subject's family and by reviewing all available medical records. None of the non-GERD, “normal control subjects” had a history of reflux, a history or regular use of histamine H2 receptor blockers or proton pump inhibitors, presence of goblet cells after Alcian blue staining, presence of inflammatory cells (including eosinophils, neutrophils, and macrophages), noticeable necrosis or erosions, basal cell hyperplasia, elongated vascular papillae, or dilated intracellular spaces within the squamous mucosa of the distal esophagus consistent with acute or chronic reflux after hematoxylin and eosin staining.
In our previous studies (7), we employed simultaneous ultrasound and manometry, as well as pharmacologic attenuation of the intrinsic smooth muscle components of the high pressure zone (HPZ) with the non-subtype selective muscarinic receptor antagonist atropine, to evaluate the HPZ anti-reflux barrier in-vivo. In normal control subjects, we demonstrated two atropine attenuated intrinsic smooth muscle components (one proximal and one distal) and an atropine resistant skeletal muscle component (the external crural diaphragm). The proximal smooth muscle component is consistent with the physiologic LEC muscle fiber sphincter while the distal component is consistent with the gastric clasp/sling muscle fibers (8, 9). We used the same technique to compare the HPZ of normal subjects to patients with GERD (1). We demonstrated that GERD patients lack the muscarinic receptor mediated pressure contribution from the gastric sling/clasp muscle fiber complex and demonstrated a weakened muscarinic pressure profile within the region of the LEC fibers. This implies that these defects in both the gastric sling/clasp muscle fiber complex and LEC muscle fibers may be a contributing factor to the pathophysiology of GERD. Because we found that the in-vitro contractile response to bethanechol is greater in the sling and LEC fibers of Barrett's specimens, this is not likely a muscle defect but proximal to the smooth muscle.
The purpose of the current study is to determine if a defect exists in-vitro that is comparable to the defect previously recognized in-vivo, in patients with GERD (1), and to determine if this defect is due to an abnormality of the gastric sling muscle fibers, the gastric clasp muscle fibers, the LEC fibers, or some combination of these individual muscle groups. We anticipated an attenuated contractile response to muscarinic stimulation in the Barrett's donors, which might account for the attenuated pressure profile previously demonstrated in GERD patients in-vivo. By performing concentration response curves with carbachol we found a significant decrease in the contractility of both the gastric sling and LEC muscle fibers in the Barrett's donors compared to the non-GERD donors. There was no difference in the contractile response of the gastric clasp muscle fibers. These studies confirm the prior muscle contraction studies, in which patients with reflux associated with Barrett's esophagus exhibited a reduction in cholinergic muscle contraction while retaining similar features of basal tone and responses to tachykinins (10). These findings may account for the attenuated pressure profiles that were observed in the in-vivo studies, in that decreased contractility of the sling muscle fibers will have the effect of decreasing the pressure from the entire clasp and sling muscle fiber complex, even when the clasp fibers have no decrease in contractility. Likewise, decreased contractility of the LEC muscle fibers will decrease the in-vivo pressure profile from the LEC muscle fiber sphincteric component as demonstrated in the prior in-vivo studies (1).
The density of total, M2 and M3 muscarinic receptors in the clasp muscle fibers of the Barrett's donors is the same when compared to non-GERD subjects. This is completely consistent with the equal contractile responses to both carbachol and bethanechol in clasp fibers from Barrett's compared to non-GERD donors. However, the M2 receptor density in the gastric sling muscle and both total and M3 receptor density of the LEC fibers of Barrett's donors is less when compared to non-GERD subjects. We previously found, using a new analysis method relating dual occupation of M2 and M3 receptors to contraction, that the M2 muscarinic receptor plays a greater role in mediating contraction of clasp and sling fibers than in LEC muscles in which the M3 receptor predominantly mediates contraction (3). This decreased M2 receptor density in sling fibers and decreased M3 receptor density in LEC fibers in Barrett's esophagus specimens is consistent with the decreased contractile responses to carbachol but not consistent with the increased contractile responses to bethanechol in sling and LEC (Figure 5, top graph). It is possible that in sling and LEC fibers from Barrett's donors, muscarinic receptors located on non-muscle cells, such as cells of the enteric nervous system, may act to augment the direct effect of bethanechol on the muscle cells thus increasing the contractile response.
We originally set out to determine whether defects in the muscular components of the lower gastro esophageal high pressure zone could explain our earlier findings that the in-vivo contractile response is absent in the gastric clasp and sling fiber complex and attenuated in the LEC fibers of patients with GERD as compared to normal control subjects (1). We found in-vitro contractile defects in both the sling and LEC muscle fibers in response to carbachol that may account for the in-vivo findings. However, in the process of performing these experiments we also found that concentrations of carbachol higher than 30 μM caused the muscle fibers to relax. The gastric clasp, gastric sling and LEC muscle fibers relaxed to a greater extent as a percent of the maximal contraction to high concentrations of carbachol in the Barrett's donors (Figure 4, bottom graph). This increase in the relaxation response is particularly pronounced in the gastric sling muscle fibers of subjects with Barrett's esophagus, when compared to non-GERD subjects at increasing doses of carbachol (Figure 3, middle graph). Subsequent experiments demonstrated that the carbachol induced relaxation could be blocked by nicotinic receptor antagonists, indicating that this relaxation is mediated by nicotinic receptors (11).
Because of the dual muscarinic mediated contractile response and nicotinic mediated relaxation response of carbachol, we sought a method to separate the contractile response from the relaxation response. During the contractile response to carbachol, the nicotinic receptors responsible for the relaxant response to subsequent higher concentrations of carbachol are also activated along with the muscarinic receptors mediating contraction and this likely affects both the contractile and subsequent relaxant response. We therefore designed a study in which we used bethanechol, a pure muscarinic agonist and nicotine, a pure nicotinic agonist to further explore the relaxation response. Preliminary concentration-response studies using bethanechol demonstrated that the maximal contractile response occurs at 30 μM in normal donor tissue. Because relaxation experiments always have to be performed in the setting of underlying baseline contraction, we chose a bethanechol concentration that induces maximal contraction to determine the relaxation effects of nicotine on these muscle groups. Interestingly, the maximal contractile response of the gastric sling and LEC muscle fibers to bethanechol was greater in the Barrett's donors than in non-GERD donors. In gastric clasp and sling muscle fibers, we found a significantly greater relaxation response to nicotine as a percent of the maximal contraction of bethanechol, in Barrett's esophagus donors when compared with non-GERD donors. Therefore, the nicotinic receptors in the sling and clasp fibers of Barrett's esophagus donors may be different than in non-GERD donors. We hypothesize that these enhanced nicotinic receptor mediated relaxations may also be involved in the pathophysiology of GERD.
The findings in this study demonstrate that the gastric clasp and gastric sling muscle fibers in Barrett's esophagus donors relax significantly more than in non-GERD donors. Transient lower esophageal sphincter relaxation (TLESR) is thought to be the underlying cause of GERD. While the frequency (12) and duration (13) of TLESR's may not be different between patients with GERD and normal subjects, GERD patients have a greater amount of reflux during transient LES relaxations compared to normal subjects (13-15). If the gastric clasp and gastric sling muscle fibers relax more in GERD patients to equivalent nicotinic receptor stimulation than in non-GERD subjects, it is tempting to speculate that this may explain why GERD subjects have a greater amount of refluxate during TLESR.
We believe that these results may explain a number of prior in-vivo findings including the fact that the yield pressure in patients with GERD is less than in non-GERD subjects (16). Abnormal compliance curves obtained in GERD subjects using the functional luminal imaging probe (FLIP), demonstrated greater compliance of the GEJ with balloon distension (17). Deglutitive inhibition during swallowing, used to measure the mechanical properties of the distal esophageal high pressure zone in normal control subjects and patients with GERD, demonstrates that the stiffness of the muscle wall surrounding the gastroesophageal junction is approximately six-fold less in the GERD patients than in the normal control subjects (18). Therefore, these findings may also explain why patients with GERD reflux more during TLESR than non-GERD subjects.
In conclusion, we found that the gastric clasp, gastric sling and LEC muscle fibers from donors with Barrett's esophagus contract less in response to increasing carbachol concentrations and relax more in response to nicotinic mediated stimulation than the same muscle fibers from non-GERD donors. This enhanced nicotinic receptor mediated response may be involved in greater relaxation of the muscles within the high pressure zone of the distal esophagus during TLESR's and during deglutitive inhibition and may be involved in the pathophysiology of GERD. The results of the current study imply that either there is a difference in the number or in the type of nicotinic receptors on the muscles or on the nerves innervating the muscles of the distal esophageal high-pressure zone in subjects with GERD. Future studies should evaluate these differences.
Key Message.
An increased nicotinic receptor mediated relaxation in the muscle fibers that comprise the gastroesophageal antireflux barrier may be involved in the pathophysiology of gastroesophageal reflux disease (GERD). This study is designed to determine whether the defects that we previously identified in patients with GERD in-vivo are due to abnormalities of the gastric sling, clasp or lower esophageal circular (LEC) muscle fibers. Muscle bath contraction studies on strips from whole stomachs and esophagi were obtained from 16 normal organ transplant donors and 15 donors with histologically proven Barrett's esophagus. In specimens from Barrett's esophagus donors, nicotinic receptor stimulated relaxations by either high concentrations of carbachol or nicotine following bethanechol pre-contraction are greater in gastric clasp, gastric sling and LEC muscle fibers.
Acknowledgments
Grant support: Award Number R01DK079954 from the NIDDK (to LSM and MRR) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Author contributions: study concept and design: LSM, AKV, ASB and MRR; generation, collection, assembly, analysis and/or interpretation of data: AKV, ASB, MFB and MRR; drafting of the manuscript: LSM and MRR; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: ASB and MRR; obtained funding: LSM and MRR; technical, or material support: AKV, ASB; study supervision: LSM and MRR.
Disclosures: No competing interests declared.
References
- 1.Miller L, Dai Q, Vegesna A, Korimilli A, Ulerich R, Schiffner B, et al. A missing sphincteric component of the gastro-oesophageal junction in patients with GORD. Neurogastroenterol Motil. 2009;21(8):813–e52. doi: 10.1111/j.1365-2982.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgett W. Alcian Blue-H&E-Metanil Yellow Stain for Diagnosing Barrett's Esophagus. HistoLogic. 2004;37(2):35–8. [Google Scholar]
- 3.Braverman AS, Miller LS, Vegesna AK, Tiwana MI, Tallarida RJ, Ruggieri MR., Sr Quantitation of the contractile response mediated by two receptors: M2 and M3 muscarinic receptor-mediated contractions of human gastroesophageal smooth muscle. J Pharmacol Exp Ther. 2009;329(1):218–24. doi: 10.1124/jpet.108.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luthin GR, Harkness J, Artymyshyn RP, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Molecular Pharmacology. 1988;34(3):327–33. [PubMed] [Google Scholar]
- 5.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273(2):959–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, et al. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Molecular Pharmacology. 1993;43(2):149–57. [PubMed] [Google Scholar]
- 7.Brasseur JG, Ulerich R, Dai Q, Patel DK, Soliman AM, Miller LS, et al. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J Physiol (Lond) 2007;580(Pt.3):961–75. doi: 10.1113/jphysiol.2006.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebermann-Meffert D, Allgower M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76(1):31–8. [PubMed] [Google Scholar]
- 9.Stein HJ, Liebermann-Meffert D, DeMeester TR, Siewert JR. Three-dimensional pressure image and muscular structure of the human lower esophageal sphincter. Surgery. 1995;117(6):692–8. doi: 10.1016/s0039-6060(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 10.Smid SD, Blackshaw LA. Neuromuscular function of the human lower oesophageal sphincter in reflux disease and Barrett's oesophagus. Gut. 2000;46(6):756–61. doi: 10.1136/gut.46.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braverman AS, Vegesna AK, Miller LS, Barbe MF, Tiwana M, Hussain K, et al. Pharmacologic specificity of nicotinic receptor-mediated relaxation of muscarinic receptor precontracted human gastric clasp and sling muscle fibers within the gastroesophageal junction. J Pharmacol Exp Ther. 2011;338(1):37–46. doi: 10.1124/jpet.110.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988;95(3):593–9. doi: 10.1016/s0016-5085(88)80003-9. [DOI] [PubMed] [Google Scholar]
- 13.Schoeman MN, Tippett MD, Akkermans LM, Dent J, Holloway RH. Mechanisms of gastroesophageal reflux in ambulant healthy human subjects. Gastroenterology. 1995;108(1):83–91. doi: 10.1016/0016-5085(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 14.Dent J, Dodds WJ, Hogan WJ, Toouli J. Factors that influence induction of gastroesophageal reflux in normal human subjects. Dig Dis Sci. 1988;33(3):270–5. doi: 10.1007/BF01535748. [DOI] [PubMed] [Google Scholar]
- 15.Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307(25):1547–52. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- 16.Vegesna A, Besetty R, Kalra A, Farooq U, Korimilli A, Chuang KY, et al. Induced opening of the gastroesophageal junction occurs at a lower gastric pressure in gerd patients and in hiatal hernia subjects than in normal control subjects. Gastroenterology research & practice. 2010:857654. doi: 10.1155/2010/857654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon BP, Bligh S, Vegesna AK, Miller LS. Sa1442 FLIP Used to Evaluate the Gastroesophageal Junction High-Pressure Zone (GEJ HPZ) in Normal Control Subjects Before and After Muscarinic Blockade With Atropine and in Patients With GERD. Gastroenterology. 2012;142(5, Suppl 1):S307–S. [Google Scholar]
- 18.Miller L, Dai Q, Korimilli A, Levitt B, Ramzan Z, Brasseur J. Use of endoluminal ultrasound to evaluate gastrointestinal motility. Dig Dis. 2006;24(3-4):319–41. doi: 10.1159/000092886. [DOI] [PubMed] [Google Scholar]


