Abstract
Chronic wounds including diabetic foot ulcers, pressure ulcers, and venous leg ulcers are a worldwide health problem. As the traditional methods of treatment have proven ineffective against chronic wounds involving biofilms, there is an unmet clinical need for developing products with an antibiofilm component that inhibits and/or disrupts biofilms and thus make the biofilm-embedded bacteria more susceptible to antimicrobial therapy. We developed a DispersinB® antibiofilm enzyme-based wound spray for treating chronic wounds in conjunction with an antimicrobial. Under in vitro conditions, the DispersinB® and Acticoat™ combination performed significantly better (P < 0.05) than Acticoat™ alone, indicating the synergy between the two compounds because of DispersinB® enhancing the antimicrobial activity of Acticoat™. Furthermore, DispersinB® wound spray enhanced the antimicrobial activity of Acticoat™ in a chronic wound mouse model of methicillin-resistant Staphylococcus aureus (MRSA) infection. Thus, this novel combination of DispersinB® and Acticoat™, an antimicrobial dressing, prompts clinical evaluation for potential applications in biofilm-based chronic wound management.
Keywords: biofilm, DispersinB®, chronic wound, MRSA, silver
Introduction
An epidemic increase in obesity combined with an aging population has caused chronic wounds such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers to become an increasing clinical concern. More than 2% of the US population suffers from chronic, nonhealing wounds1 and with only transiently effective antimicrobials; the current standard of care is amputation with 24% of diabetic patients undergoing amputation surgery in their lifetime.2 Although chronic and acute wounds progress through similar stages of healing, chronic wounds appear to stall in the inflammatory stage of wound healing, likely because of persistent colonization by bacteria.3 Colonization by bacteria contributes to nonhealing of the wound; however, recent evidence suggests that development of a chronic wound is dependent on contaminating bacteria forming biofilms. In chronic wounds, the biofilm mode of growth is characterized by adherence to biotic or abiotic surfaces, slow development of overt symptoms, a lack of resolution by host defenses, and resistance to antibiotic therapy.4 Furthermore, recent studies by James et al2 have demonstrated biofilm as a potential reason why chronic wounds do not heal. Adaptation by biofilm communities has resulted in the failure of multiple antimicrobials; thus; synergistically acting combinations of antibiofilm and antimicrobials have the greatest likelihood of remaining efficacious in the clinic. Wolcott and Rhoads5 observed that the chronic wound treatments that specifically target biofilms transformed nonhealable wounds into healable wounds. When combined with antibiofilm compounds, the use of antibiotics declined approximately 25% during the four-year study period. Thus, the use of suitable topical agents that inhibit biofilm formation or disrupt preformed biofilm should be integral to the management of chronic wound infections.
We have developed a DispersinB® antibiofilm enzyme-based wound spray for treating chronic wounds that could be used in conjunction with an antimicrobial. Furthermore, we have tested the in vitro and in vivo efficacy of DispersinB® wound spray in combination with nanocrystalline silver containing Acticoat™ wound dressing. DispersinB® is a naturally occurring enzyme produced by an oral bacterium Aggregatibacter actinomycetemcomitans, which is associated with juvenile periodontitis.6 DispersinB® is active against poly- N- acetylglucosamine (PNAG), which is a major polysaccharide in biofilms of wound-associated bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, and Acinetobacter baumannii. It has been shown to inhibit biofilm formation and disperse preformed biofilm in these bacteria without exhibiting any antibacterial activity.7 However, DispersinB® in combination with an antimicrobial has been shown to make the bacteria more susceptible to antimicrobial killing either by inhibiting biofilm formation or by disrupting preformed biofilm.8,9
Silver is an antimicrobial present in many wound care products and has a broad spectrum antimicrobial activity because of its interaction with sulfhydryl (–SH) groups of proteins as well as bases of DNA leading either to the inhibition of respiratory processes or DNA unwinding.10,11 The efficacy of silver-impregnated dressings for treatment of wounds has remained inconclusive with some meta-analyses supportive and some not.12 Although silver has been demonstrated to be efficacious alone against biofilm growth, over the course of treatment, bacterial biofilms have exhibited a remarkable ability to overcome single-treatment antimicrobials. Indeed, silver resistance has been demonstrated in clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA).13 Therefore, development of a novel synergistic combination with an antibiofilm agent that enhances the efficacy of silver may have a significant therapeutic value.
Materials and Methods
Reagents, microorganisms, and culture conditions
All the chemicals (including media ingredients) were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO, USA) or BD Diagnostic Systems (Sparks, MD, USA). DispersinB® enzyme was purified from a recombinant Escherichia coli fermentation as previously described.14,15 The enzyme had a specific activity of ~103 units/mg of protein.14,15 DispersinB® wound spray comprising 200 μg mL−1 clinical grade DispersinB® in 50 mM sodium phosphate buffer containing 100 mM NaCl (pH 5.9) was formulated in a current good manufacturing practice (cGMP) facility, Therapure Biopharma Inc. (Mississauga, ON, Canada). The DispersinB® enzyme concentration in the wound spray was determined empirically. The commercial nanocrystalline silver containing wound dressing Acticoat™ was obtained from Smith and Nephew. The clinical wound isolates of S. epidermidis 1457, K. pneumoniae P30, and A. baumannii 63270 were provided by Dr. George G. Zhanel (Department of Medical Microbiology, University of Manitoba, Winnipeg, MB, Canada). The clinical wound isolates of MRSA Gav 16a and coagulase-negative staphylococci (CoNS) 42 were obtained from Dr. Randolph D. Wolcott (Southwest Regional Wound Care Center, Lubbock, TX, USA). All the strains were maintained at −80°C in 15% (v/v) glycerol stocks and recovered onto tryptic soy agar (TSA) and tryptic soy broth (TSB).
Biofilm assay
To evaluate the effect of DispersinB® wound spray on biofilm inhibition and dispersal, a 96-well biofilm assay was performed.8 For biofilm inhibition assay, the overnight grown cultures were diluted 100 times in TSB medium and grown in a 96-well microtiter plate in the presence or absence of DispersinB® wound spray for 18 hours at 37°C. For biofilm dispersal assay, cultures were grown in 96-well microtiter plates for 18 hours at 37°C and treated with or without DispersinB wound spray for 3 hours at 37°C. Biofilm was washed and stained with crystal violet, and the absorbance was measured at 630 nm.
Antibiofilm–antimicrobial activity
The MRSA biofilm was developed in a 12-well microtiter plate for 18 hours at 37°C and treated with Acticoat™ alone and in combination with DispersinB® wound spray for three hours at 37°C.16 The biofilms were washed and suspended in 2 mL phosphate buffered saline (PBS). The plate was sonicated for 30 seconds, and the suspension was transferred into 15 mL tubes. After vortexing the tubes for one minute, serial dilutions were plated onto TSA.
In vivo efficacy
The efficacy of the combination of Acticoat™ and DispersinB® wound spray was studied using a chronic wound mouse model of MRSA infection.17 A 1.5 cm2 surgical excision wound was created on the back of 27 mice and an OPSITE dressing was applied over the wound. The wounds were infected with 104 CFU of S. aureus. After 24 hours of post-infection, mice were divided into three groups of nine mice each and treated with either placebo (50 mM phosphate buffer + 100 mM NaCl, pH 5.9) or 1.5 cm2 Acticoat™ alone or a combination of DispersinB® wound spray and Acticoat™. At day 2 of post-wound infection, mice were euthanized, wound sections were harvested, and serial dilutions were plated onto TSA to determine the CFU per gram of tissue. The bacterial load was compared between different groups by using one-way analysis of variance (ANOVA).
Results
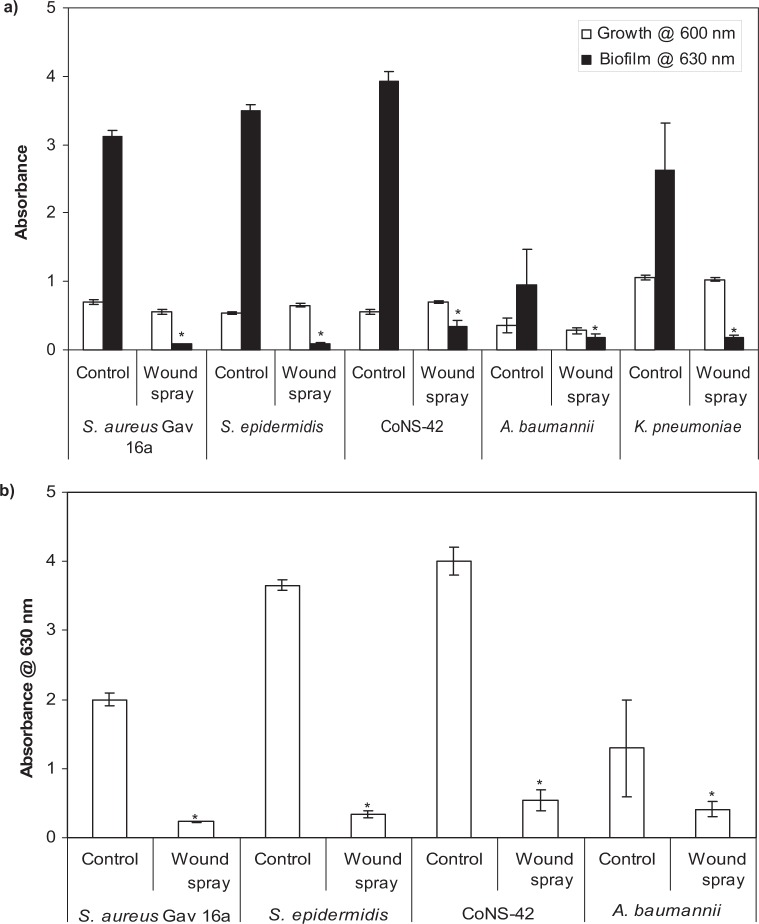
DispersinB® wound spray was effective in inhibiting and dispersing biofilms without affecting the growth of wound- associated pathogens such as S. aureus, S. epidermidis, CoNS-42, A. baumannii, and K. pneumoniae (Figs. 1a and b).
Figure 1.
Effect of DispersinB® wound spray on (a) growth and biofilm formation and (b) biofilm dispersal of wound-associated bacteria. The biofilm was grown in 96-well microtiter plates at 37°C for 18 hours. The biofilm was stained with crystal violet and washed with water, and absorbance was measured at 630 nm. Error bars represent the standard deviation. The values are means ± standard deviations. *P < 0.05, compared with untreated control.
In addition, MRSA biofilm was treated with Acticoat™ alone and in combination with DispersinB® wound spray using a 12-well biofilm assay.
Although biofilm treated with Acticoat™ alone showed 0.4log10 more bacteria compared to control, the difference was not significant (P > 0.05) (Fig. 2). The combination showed 3log10 reduction in biofilm-embedded S. aureus compared to both control and Acticoat™ alone.
Figure 2.
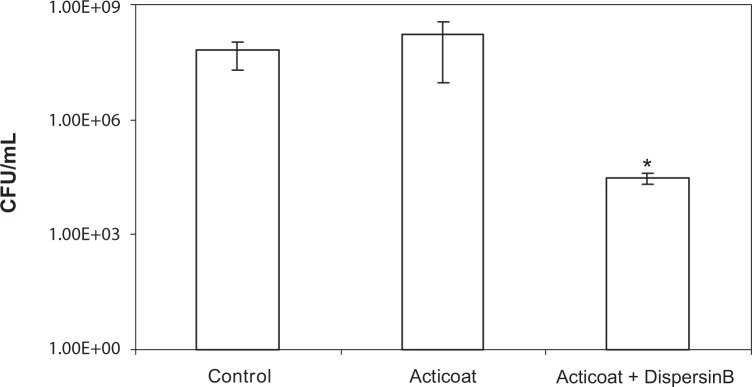
Increased sensitivity of MRSA biofilm to Acticoat™ in the presence of DispersinB® wound spray. The biofilm was developed in 12-well plates for 18 hours at 37°C. After treating biofilm with water control, Acticoat™ alone, and a combination of DispersinB® wound spray and Acticoat™ for three hours at 37°C, total viable counts were determined. Error bars represent the standard deviation. The values are means ± standard deviations. *P < 0.05, compared with untreated control and Acticoat™ alone treated.
In a chronic wound mouse model of MRSA infection, the combination of DispersinB® wound spray and Acticoat™ showed 80% reduction (P < 0.05) in bioburden as compared to placebo treatment, and a moderate 14% reduction (P > 0.05) when wounds were treated with Acticoat™ alone (Table 1), indicating an increased antimicrobial activity of silver in combination with DispersinB®.
Table 1.
In vivo efficacy of DispersinB® wound spray and Acticoat™ combination against MRSA infection in a chronic wound mouse model.
| UNINFECTED WOUND | INFECTED WOUND (n = 9) | ACTICOAT™ TREATED (n = 9) | DISPERSINB®-ACTICOAT™ TREATED (n = 9) | |
|---|---|---|---|---|
| Bioburden MRSA (CFU/gm tissue) | 0 | 8.6 × 106 | 7.4 × 106 | 1.6 × 106 |
| P value | Vs infected > 0.05 | Vs Infected = 0.0119 Vs Acticoat™ > 0.05 |
Discussion
It is well recognized that infections involving biofilms are difficult to eradicate, as sessile bacteria employ mechanisms that raise survival and resistance to antimicrobials up to 1000 times compared to their planktonic counterparts.4 Wound treatment is primarily managed through mechanical manipulation such as debridement and choice of wound dressing.5 Rationally designing wound dressings with effective antibiofilm control has the potential to significantly improve chronic wound therapy. The inhibition of biofilm formation and/or dispersal of preformed biofilms may make biofilm-embedded bacteria more susceptible to antimicrobial therapy. DispersinB® wound spray showed broad spectrum biofilm inhibition and dispersal activity against wound-associated bacteria. Furthermore, we evaluated the effect of combining DispersinB® wound spray with Acticoat™ on wound-associated MRSA. DispersinB® wound spray in combination with Acticoat™ showed significantly (P < 0.05) more reduction in biofilm-embedded MRSA compared to that by Acticoat™ alone, indicating the synergistic antibiofilm–antimicrobial activity of the combination. These findings are in agreement with previous reports describing the synergistic activity of the combination of antibiofilm enzyme DispersinB and antibiotics (cefamandole nafate or ampicillin) and non-antibiotic compounds such as triclosan.7–9 The synergy between these two compounds could be attributed to DispersinB® making the bacteria embedded in biofilms more susceptible to antimicrobial Acticoat™ killing either by inhibiting biofilm formation or by disrupting preformed biofilms.
Furthermore, the efficacy of Acticoat™ and DispersinB® wound spray combination was studied in vivo using a chronic wound mouse model of MRSA infection.17 DispersinB® wound spray enhanced the antimicrobial activity of Acticoat™ wound dressing. As two cysteines containing thiol groups present in the active site of C-terminal of DispersinB® were substituted with alanine, Acticoat™ did not affect the enzyme activity.
MRSA poses a major threat to hospital patients and accounts for 93.5% of venous leg ulcers- and 40% of diabetic foot ulcers-associated infections.18 In addition, our previous study showed that silver containing wound gel (Silver-Sept™) did not inhibit the growth of S. aureus, including methicillin-resistant as well as sensitive strains.19 However, the DispersinB® wound spray and Acticoat™ combination showed a synergistic inhibitory effect on biofilm-embedded MRSA under both the in vitro and in vivo conditions. The other advantage of using antibiofilm and antimicrobial compounds in combinations with different modes of action is to minimize the probability of bacteria developing resistance. Treatment with a naturally occurring biocompatible DispersinB® enzyme9 combined with a clinically safe Acticoat™ wound dressing could meet the unmet clinical need for biofilm-based chronic wound management. Thus, it would be prudent to evaluate the potential clinical application of this novel antibiofilm–antimicrobial combination for prevention and treatment of chronic wound-associated infections.
Footnotes
Author Contributions
PVG and APC performed experiments. KL and NY produced DispersinB® enzyme. PVG, KPR, and SM planned the experiments. PVG wrote and edited the manuscript. All authors contributed to discussion about the results and read the manuscript. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Raul Rivas, Editor in Chief
FUNDING: The in vivo efficacy study using a mouse chronic wound model was partly funded by Kane Biotech. Kane Biotech had no influence on the study or the content of this article. The authors disclose no other funding sources.
COMPETING INTERESTS: PVG, KL, NY and SM are employees of Kane Biotech Inc, manufacturer of DispersinB®, and own company stocks and have options. APC and KPR are employees of Texas Tech University Health Science Center and have nothing to declare.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
REFERENCES
- 1.Gottrup F. A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg. 2004;187:38S–43S. doi: 10.1016/S0002-9610(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 2.James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 3.Konturek PC, Brzozowski T, Kontuerk SJ, Kwiecien S, Dembinski A, Hahn EG. Influence of bacterial lipopolysaccharide on healing of chronic experimental ulcer in rat. Scand J Gastroenterol. 2001;36:1239–1247. doi: 10.1080/003655201317097065. [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care. 2008;17:145–155. doi: 10.12968/jowc.2008.17.4.28835. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J Bacteriol. 2003;185:4693–4698. doi: 10.1128/JB.185.16.4693-4698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int J Artif Organs. 2009;32:545–554. doi: 10.1177/039139880903200903. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche RO, Mansuri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB® combination. J Antimicrob Chemother. 2009;51:2733–2740. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 9.Donelli G, Francolini I, Romoli D, et al. Synergistic activity of dispersin B and cefamandole nafate in inhibition of Staphylococcal biofilm growth on polyurethanes. Antimicrob Agents Chemother. 2007;51:2733–2740. doi: 10.1128/AAC.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragg PD, Rannie DJ. The effect of silver ions on the respiratory chain of E. coli. Can J Microbiol. 1974;20:883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 11.Batarseh KI. Anomaly and correlation of killing in the therapeutic properties of silver (I) chelation with glutamic and tartaric acids. J Antimicrob Chemother. 2004;54:546–548. doi: 10.1093/jac/dkh349. [DOI] [PubMed] [Google Scholar]
- 12.Chambers H, Dumville JC, Cullum N. Silver treatments for leg ulcers: a systematic review. Wound Repair Regen. 2007;15:165–173. doi: 10.1111/j.1524-475X.2007.00201.x. [DOI] [PubMed] [Google Scholar]
- 13.Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J. 2009;6:32–38. doi: 10.1111/j.1742-481X.2008.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta- hexosaminidase activity. J Bacteriol. 2003;185:4693–4698. doi: 10.1128/JB.185.16.4693-4698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakandawala N, Gawande PV, LoVetri K, Romeo T, Kaplan JB, Madhyastha S. Enhanced expression of engineered ACA-less b-1,6-N-acetylglucosaminidase (dispersin B) in Escherichia coli. J Ind Microbiol Biotechnol. 2009;36:1297–1305. doi: 10.1007/s10295-009-0613-0. [DOI] [PubMed] [Google Scholar]
- 16.Darouiche RO, Mansuri MD, Gawande PV, Madhyastha S. Efficacy of combination of chlorhexidine and protamine sulfate against device-associated pathogens. J Antimicrob Chemother. 2008;61:651–657. doi: 10.1093/jac/dkn006. [DOI] [PubMed] [Google Scholar]
- 17.Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 18.Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J. 2006;3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawande PV, Yakandawala N, LoVetri K, Madhyastha S. In vitro antimicrobial and antibiofilm activity of DispersinB®-Triclosan wound gel against chronic wound-associated bacteria. Open Antimicrob Agents J. 2011;3:12–16. [Google Scholar]