Abstract
It is well recognized that the prevalence of dementia is higher in diabetic patients than non‐diabetic subjects. The incidence of diabetes has been increasing because of dramatic changes in lifestyles, and combined with longer lifespans as a result of advances in medical technology, this has brought about an increase in the number of elderly diabetic patients. Together, aging and diabetes have contributed to dementia becoming a serious problem. Progression to dementia reduces quality of life, and imposes a burden on both patients themselves and the families supporting them. Therefore, preventing the complication of dementia will become more and more important in the future. Although many mechanisms have been considered for an association between diabetes and cognitive dysfunction, glucose metabolism abnormalities such as hyperglycemia and hypoglycemia, and insulin action abnormalities such as insulin deficiency and insulin resistance can be causes of cognitive impairment. Recent large‐scale longitudinal studies have found an association between glycemic control and cognitive decline, although it is still unclear how cognitive decline might be prevented by good glycemic control. However, at an early stage, it is necessary to detect moderate cognitive dysfunction and try to reduce the risk factors for it, which should result in prevention of dementia, as well as vascular events. In the present review, in addition to outlining an association between diabetes and cognitive function, we discuss how glycemic control and cognitive decline are related.
Keywords: Cognitive function, Diabetes mellitus, Glycemic control
Introduction
Thanks to the tremendous advances in medicine that have been made in recent years, the average life expectancy of diabetic patients has been increasing year by year1. Although prevention of diabetes is being promoted worldwide, patients with diabetes or glucose intolerance continue to increase. Coupled with the aging of society, an increase in the number of elderly diabetic patients is therefore inevitable.
Generally, problems for the elderly are impaired activities of daily living (ADL) and cognitive dysfunction. In this regard, dementia necessitates huge expenditure for caregiving worldwide and places an immeasurable burden on caregivers2. Previously, large‐scale epidemiological studies have reported that the incidence of dementia in diabetic patients is two‐ to threefold higher than in non‐diabetic persons3, and recent investigations carried out in Japan have found the same to be the case (Table 1)7. For this reason, prevention of dementia in elderly diabetic patients will be one of the most important issues in diabetes treatment in the future.
Table 1. Risk of dementia in diabetic patients.
| Study | Relative risk (95% confidence interval) | ||
|---|---|---|---|
| Alzheimer's disease | Vascular dementia | ||
| Yoshitake et al.3 | Hisayama Study | 2.18 (0.97–4.90) | 2.77 (2.59–2.97) |
| Leibson et al.4 | Rochester Study |
Men: 2.27 (1.55–3.31) Women: 1.37 (0.94–2.01) |
– |
| Ott et al.5 | Rotterdam Study | 1.9 (1.2–3.1) | 2.0 (0.7–5.6) |
| Peila et al.6 | Honolulu–Asia Aging Study | 1.8 (1.1–2.9) | 2.3 (1.1–5.0) |
| Ohara et al.7 | Hisayama Study | 2.05 (1.18–3.57) | 1.82 (0.89–3.71) |
Many mechanisms have been considered for an association between diabetes and cognitive dysfunction. In their review, Biessels et al.8 mentioned that: (i) atherosclerosis, such as brain infarcts; (ii) microvascular disease as a result of insidious ischemia; (iii) advanced protein glycation and oxidative stress as a result of glucose toxicity; and (iv) insufficient insulin action were major factors, and the added involvement of aging and genetic factors leads to dementia. As diabetes is a heterogeneous disease that is easily compounded by hypertension, dyslipidemia and so on, it is considered to be a clinical condition that is modified by many factors. However, for cognitive dysfunction, the underlying conditions are blood glucose disorders, such as hyperglycemia and hypoglycemia, and insulin disorders, such as insulin resistance and insulin insufficiency, have been shown to lead to cognitive dysfunction (Figure 1).
Figure 1.
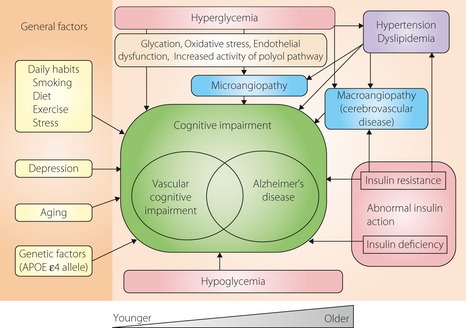
Possible mechanistic contribution to cognitive impairment seen in diabetes mellitus. Hyperglycemia, hypoglycemia and abnormal insulin action have been implicated as major causes of cognitive impairment in diabetic patients, but many other factors, such as those shown in the figure, are also involved. APOE, apolipoprotein E.
Recently, interesting findings have been reported for longitudinal research. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study, a long‐term study that followed up type 1 diabetes patients for approximately 18 years, found that a decline in cognitive function, such as motor speed and psychomotor efficiency, was associated with glycemic control level9. In contrast, the Action to Control Cardiovascular Risk in Diabetes‐Memory in Diabetes (ACCORD‐MIND) trial, whose aim was intensive control in type 2 diabetes patients, observed a decline in cognitive function over time, and effects of intensive glycemic control were not shown11. In the Atherosclerosis Risk in Communities (ARIC) study, while an analysis on all subjects including non‐diabetics found that glycosylated hemoglobin A1c (HbA1c) level was associated with progression of cognitive impairment in motor speed, an analysis on the diabetic subjects only did not find such an association12.
The findings of these studies are at odds with those of many studies carried out in the past, as they targeted relatively young middle‐aged and elderly persons, and provide new insights into an association between progression of cognitive impairment and blood glucose levels. However, the cognitive impairment observed in the respective studies was mainly mild impairment of frontal lobe function, and it is unclear as to whether it would have lead to future dementia or not. We will therefore have to wait and see what future research reveals. Also, the recent Hisayama study showed a high prevalence of Alzheimer's disease and vascular dementia in subjects in whom glucose intolerance had been observed 15 years previously7.
The findings of the aforementioned studies raise the following questions. In patients with diabetes and glucose intolerance: What will cause decline in cognitive function? What needs to be done to prevent future dementia in such patients? What is the impact of glycemic control on cognitive function, and can good control suppress cognitive impairment and also prevent its progression to dementia? Furthermore, what methods could be used for the early discovery of cognitive impairment or to predict its progression?
In the present review, in addition to outlining an association between diabetes and cognitive function, we discuss how glycemic control and cognitive decline are related.
Impairment of Cognitive Function in Diabetes
Dementia is mainly classified into Alzheimer's disease, vascular dementia, dementia with Lewy bodies and frontotemporal dementia. Although Alzheimer's disease, the most common type, is considered to be a neurodegenerative disease, it is frequently accompanied by cerebrovascular lesions. Thus, the current thinking is that a cerebrovascular component is also greatly involved in Alzheimer's disease8.
It has been reported that Alzheimer's disease and vascular dementia are more frequent in diabetic patients than in non‐diabetic persons3, and that aspects of frontal lobe function, such as psychomotor efficiency, processing speed and executive function, are generally more impaired in diabetics12. As causes, abnormal glucose metabolism and cerebrovascular lesions in the frontal lobe have been suggested, as described later, but the details of this remain unclear.
Damage to many cognitive function domains has been reported in diabetic patients, but there are some differences in the aspects of function that are damaged between type 1 and type 2 diabetes. Many studies have found that such aspects as information processing, psychomotor efficiency, attention, visuoconstruction and mental flexibility are greatly impaired in type 1 diabetes. In type 2 diabetes, however, psychomotor speed and executive function, as well as memory, are greatly affected15. As a result, walking speed is reduced, balance is impaired, risk of falls is increased and fractures are more frequent in elderly diabetic patients, reducing quality of life. Although it has still to be adequately explained how it is associated with changes in the brain, executive dysfunction has been reported to be associated with inability to carry out lower‐extremity tasks16.
It is generally considered that vascular damage is closely associated with impairment of aspects of frontal lobe function, such as psychomotor speed, executive function and attention, and that atrophy of the hippocampus, which is related to memory, and reduced blood flow in the parietal lobe, the posterior portion of cingulated gyrus and precuneus are associated with Alzheimer's disease. Differing from type 1 diabetes, compounded by age and various risk factors associated with it in a complicated fashion, in type 2 diabetes, a very wide range of functional damage is observed.
Influence of Hyperglycemia and Glycemic Control on Cognitive Function
Findings from Cross‐Sectional Studies
Based on the findings of many cross‐sectional studies, there seems to be broad agreement that the hyperglycemic state causes cognitive function impairment. The ACCORD‐MIND study observed a 0.14‐point drop in Mini‐Mental State Examination (MMSE) score for each 1% increase in HbA1c, and that its elevation impaired such aspects of cognitive function as psychomotor speed (Digital symbol substitution test [DSST]), memory (Rey Auditory Verbal Learning Test) and executive function (Stroop test), suggesting a significant negative association between HbA1c level and cognitive function17. In our own research on elderly diabetic patients, baseline HbA1c level was an independent factor for functional impairment as indicated by DSST, Stroop test and word recall performance18.
Neuronal changes as a result of advanced glycosylated end‐product production and oxidative stress have been cited as directly‐related factors in the process by which hyperglycemia causes functional damage. Among other mechanisms under consideration is one in which damage to neurons and vascular endothelium as a result of high osmotic stress induced by hyperglycemia disrupts the blood–brain barrier causing local leakage of vascular substances, which leads to further neuronal damage8.
A study utilizing proton magnetic resonance spectroscopy reported that glucose and glutamate levels in the prefrontal regions of type 1 diabetics were elevated as compared with non‐diabetics20. It is thought that elevated glucose concentrations lead to enhanced oxidative phosphorylation and increased levels of glutamate, an excitatory neurotransmitter, whose enhanced levels bring about cognitive dysfunction by causing neuronal damage. It has also been suggested that changes in brain metabolites, such as myo‐inositol and N‐acetylaspartate, occur as glucose, and glutamate levels in the white matter and cortex of the frontal lobe and the thalamus rise20. It is likely that neuronal damage is induced by higher osmotic pressure as a result of elevated myo‐inositol levels, and that reduced levels of the non‐specific amino acid N‐acetylaspartate are associated with neuronal impairment. In this regard, it has been reported that elevated glutamate levels in type 1 diabetic patients were correlated with aspects of cognitive function, such as executive function and memory. Also, elevated glutamate concentrations were seen in subjects with poor glycemic control in whom HbA1c exceeded 7%21. Although almost all of this research was on type 1 diabetes patients, it is likely that the frontal lobe dysfunction often seen in type 2 diabetic patients is a result of abnormal glucose metabolism induced by hyperglycemia.
Findings from Longitudinal Studies
In contrast to cross‐sectional research, longitudinal research has not really produced a consensus view regarding blood glucose levels and cognitive impairment. In a 7‐year follow up of elderly diabetic patients aged 70 years and older, Bruce et al.22 reported that the number of years of diabetes duration and atherosclerosis were important risk factors for dementia, but factors such as HbA1c, insulin levels and hypertension, were not independent factors. As shown in Figure 2, in the ARIC study during 6 years of follow up, although there was a statistically significant relationship between reduction in motor speed and HbA1c levels, for diabetic patients alone, such a relationship disappeared12. Although Yaffe et al.23 reported a fourfold increase in cognitive impairment in subjects with HbA1c ≥ 7%, as compared with those with HbA1c < 7%, the majority of the subjects were not diabetic. Also, the DCCT/EDIC study, which was carried out with type 1 diabetes patients, reported that motor speed and psychomotor efficiency were reduced in a group with HbA1c ≥ 8.8%, as compared with a group with HbA1c ≤ 7.4%9. Our 3‐year follow up of elderly diabetic patients showed that baseline HbA1c level was an independent risk factor for reduced perceptual speed (DSST), attention and executive function (Stroop test)24; although with MMSE scores of 24 or above, these subjects were evaluated as not demented. However, HbA1c level was not a predictive factor for cognitive impairment in the same study population when patients considered to have mild cognitive impairment were included25. This suggests that the involvement of blood glucose in cognitive impairment varies with the subjects selected.
Figure 2.
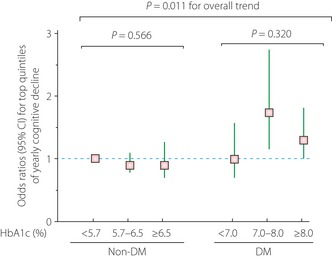
Association between Digit Symbol Substitution Test (DSST) decline and glycosylated hemoglobin A1c levels. A total of 8958 subjects with an average age of 56 years were followed up for 6 years, and the results were adjusted by age, sex, race, income, education, drinking, smoking, body mass index, systolic blood pressure, diastolic blood pressure, hypertension medication use and total cholesterol level. The odds ratio with 95% confidence interval (CI) for yearly DSST decline was 1.42 (1.14–1.75) in diabetic patients as compared with non‐diabetic subjects. This figure is based on a table published in Diabetologia 201112. DM, diabetes mellitus.
In the recently published ACCORD‐MIND study, after 40 months, no difference in the degree of cognitive impairment was observed between an intensive treatment group with a target of <6% for HbA1c and a standard treatment group for which the HbA1c target was 7–7.9%. However, in a magnetic resonance imaging (MRI) examination, overall brain volume was significantly greater in the intensive treatment group than in the standard treatment group. Thus, there could already have been systemic changes in the brain before the cognitive impairment and therefore, there might be an interval between the protection of organic changes as a result of intensive glycemic control and suppression of cognitive decline11. Furthermore, it has been reported that persisting hyperglycemia with HbA1c > 9.0% and severe hypoglycemia episodes caused minute structural changes in the brain in a 2‐year observation of type 1 diabetic patients26. As many previous studies also show that brain atrophy is associated with cognitive impairment27, brain structural changes as a result of slight variations in glycemic control might lead to future cognitive impairment.
As aforementioned, discrepancies in the findings of individual studies are not just because of differences between type 1 and type 2 diabetes; they are also considered to arise from the complex interaction of factors that affect cognitive function, such as bias due to the subjects selected, age group and duration of diabetes, presence of complications affecting large and small vessels, and hypertension. It is therefore difficult to study the effect of glycemic level by itself. In the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) and the Rotterdam study, although a decline in cognitive function was observed in diabetic patients as compared with non‐diabetic subjects, no significant association was noted between fasting blood glucose levels and cognitive impairment in non‐diabetics. Owing to this, the authors argue that there is a certain threshold above which abnormal blood glucose levels cause cognitive impairment or the involvement of factors other than hyperglycemia is greater in diabetic patients28. Furthermore, with intensive treatment, hypoglycemia readily occurs, which leads to an increase in events and mortality29, and there is a definite possibility that it has an influence on cognitive impairment. However, a conclusion has still to be reached on optimal glycemic control indexes that will not bring about cognitive impairment.
Taking the findings of previous research together, keeping HbA1c below 7% should maintain a good state of cognitive function. Furthermore, in glycemic control it is also necessary to pay attention to postprandial hyperglycemia and hyperinsulinemia, as well as hypoglycemia, as described in more detail later. However, less intensive control, for example with keeping approximately 8% for HbA1c, would be desirable for elderly patients, especially those with functional disability and other conditions30.
Relationship Between Fluctuation in Blood Glucose Levels and Cognitive Function
In a study utilizing a continuous glucose monitoring system, it was reported that diurnal variation in blood glucose was more closely associated with MMSE scores and other aspects of cognitive function than HbA1c, fasting blood glucose and postprandial blood glucose31. Also, the Hisayama study found that an increase in postprandial blood glucose was more closely associated with cognitive impairment than an increase in fasting blood glucose7. The ACCORD‐MIND baseline analysis did not observe a relationship between fasting blood sugar and cognitive impairment17. Furthermore, in research in which subjects were randomized to either repaglinide or glibenclamide as oral hypoglycemic agents, after 1 year, cognitive function testing showed that there was a significantly greater decline in cognitive function in the glibenclamide group. However, this disappeared after adjusting for postprandial blood glucose, indicating the involvement of postprandial hyperglycemia in cognitive decline32. It is therefore likely that oxidative stress arising from a glucose spike as a result of fluctuation in blood glucose or postprandial hyperglycemia is associated with deterioration in cognitive function, through a similar mechanism to one in which oxidative stress causes cardiovascular events.
There have also been studies on a relationship between metabolic syndrome and cognitive decline33. In this case, although blood glucose abnormalities will of course be involved, the contribution of hyperinsulinemia and hypertension would also be great. This will be discussed later.
Relationship Between Drug Therapy and Cognitive Function
Many previous studies have reported that hypoglycemic agents achieved a short‐term improvement in cognitive function19. Although a large‐scale cohort study on elderly females observed that diabetes enhanced cognitive decline over 2 years of follow up, there was no difference between a group of subjects treated with oral hypoglycemic agents and non‐diabetic subjects36. Also, in a 24‐week study in which rosiglitazone, a thiazolidinedione, or glyburide, a sulfonylurea, was combined with metformin, it was observed that both agents improved fasting blood glucose and also working memory37. Another study found that oral hypoglycemic agents were more effective when the duration of disease was longer, and that multiple drug therapy was better at improving cognitive function than monotherapy38. These previous studies show that glucose control leads to better short‐term improvement in cognitive function than individual oral hypoglycemic agents. However, most of the research up until now has focused on cognitive improvement using thiazolidinediones, as described later, and very little research has been carried out on other types of oral hypoglycemic agents. There are now hopes that agents enhancing incretin effects will have beneficial effects on neurons39 and disruption of brain insulin signaling in mice40, and they could be effective in preventing dementia in the future.
For some time, cognitive decline has been reported in patients receiving insulin therapy5. In our own research, we noted significant cognitive decline in patients receiving insulin therapy as compared with those being treated by diet alone or with oral hypoglycemic agents25. Although it is possible that insulin causes such decline by acting as a neurogenerator, in patients receiving insulin therapy, the involvement of various other factors, such as long duration of disease, poor glycemic control, hypoglycemia and presence of multiple cardiovascular complications, would be great.
Insulin Action and Cognitive Function
Hyperglycemia in diabetes is due to the insufficient action of insulin, and cognitive impairment is not only closely associated with hyperglycemia, but also with the action of insulin. Insulin enters the brain through the blood–brain barrier where it binds with insulin receptors. In the brain, insulin is involved in various cognitive functions. There is a particularly large number of insulin receptors in the hippocampus and cerebral cortex, which play a central role in memory. Insulin induces the release of β‐amyloid peptide (Aβ) in cells to the cell exterior, and also promotes the expression of insulin degrading enzyme (IDE). As IDE also degrades Aβ, if there is lack of insulin, Aβ will accumulate8.
In the case of hyperinsulinemia or insulin‐resistance, due to downregulation, there is a decrease in insulin receptors and less insulin comes into the brain. Also, as insulin is degraded by IDE, in the high insulin state, IDE is consumed and its amount decreases, resulting in an increase in Aβ causing cognitive impairment to progress. In this regard, a cohort study on middle‐aged adults reported an association between hyperinsulinemia and cognitive decline44. Also, in the Hisayama study, autopsy findings showed that hyperinsulinemia and hyperglycemia as a result of insulin resistance enhanced neuritic plaque formation45. Furthermore, Rönnemaa et al.46 reported that a reduction in insulin secretion, not in insulin sensitivity, was associated with the onset of Alzheimer's disease. Thus, insulin seems to be definitely connected with the Alzheimer's disease pathology and insulin resistance to be associated with vascular dementia through atherosclerosis. Also, obesity accompanying the high insulin state is a future risk factor for dementia, especially in middle‐age48; whereas in the elderly, a decrease in weight was a possible early symptom of Alzheimer's disease, and no association between obesity and Alzheimer's was observed49. Regarding the effects of weight reduction, a meta‐analysis showed that it was somewhat useful in preventing cognitive decline, at least in obese persons, although there were many controversial aspects50. Regarding therapeutic agents, it has been reported that insulin‐sensitizing compounds, such as the thiazolidinediones rosiglitazone and pioglitazone, were effective in improving cognitive function51, though this has still to be firmly established. In addition, the intranasal administration of insulin has been shown to be effective when its action in the brain is insufficient, and there are expectations that this could become a new type of therapy53.
Hypoglycemia and Cognitive Function
The matter of an association between hypoglycemia and cognitive function has been continually discussed for some time. Although it was reported that hypoglycemia and cognitive function were not associated in the elderly55, a large‐scale, longitudinal cohort study found that severe hypoglycemia was a risk factor for dementia in elderly subjects56. Also, a 16‐year follow up of type 1 diabetes patients showed that severe hypoglycemia at a young age affected cognitive function57. In contrast, the DCCT/EDIC study did not observe an association between hypoglycemia and cognitive impairment9. The study group in this research was well managed, indicating that cognitive decline as a result of hypoglycemia might be kept to a minimum through such careful management.
However, in the case that hypoglycemia in the elderly is combined with pre‐existing atherosclerosis, organic brain disease will readily develop and any nerve damage that occurs will probably be more difficult to repair than in type 1 diabetics or young diabetic patients. In addition, as impaired cognitive function is likely to increase the risk of severe hypoglycemia, measures against hypoglycemia should be taken in the treatment of elderly patients42.
Microvascular Disease and Cognitive Function
Diabetic microvascular disease (in particular, retinopathy and nephropathy) has been reported to be associated with cognitive impairment in type 1 and type 2 diabetic patients59. Also, an association between cognitive impairment and retinal microvascular abnormalities or chronic kidney disease has been reported in non‐diabetic patients63, and it was explained that this type of relationship was mainly with cerebral small vessel disease (SVD). More specifically, retinal and cerebral small vessels are considered to have a similar embryological origin and structures, and share common physiological characteristics, and it is thought that damage to retinal and cerebral small vessels as a result of ischemia and so on leads to decline in a cognitive function. Furthermore, the vascular bed of the kidney and cerebral small vessels are structurally similar, and disorders of blood flow or shear stress as a result of hypertension and so on cause damage to both the brain and kidney, showing ‘The brain and the kidney connection’67. Therefore, in cognitive impairment occurring when there are retinal abnormalities and kidney disease, there is more likely to be impairment of executive function and processing speed, which stem from cerebrovascular disorders, than memory. Regarding neuropathy, it is thought that nerve sheaths in the brain are damaged by a similar mechanism to the one for peripheral neuropathy, and that this leads to a decline in cognitive function. In this connection, it has been suggested that increased activity of the polyol pathway and abnormal myo‐inositol metabolism, which are closely associated with the development of diabetic neuropathy, could alter glucose metabolism in the frontal lobe, as mentioned previously, resulting in cognitive impairment20. However, it has also been reported that decline in cognitive function and diabetic microvascular disease are not associated69.
Although it cannot be said that an association between microvessel damage in the retina and kidney is necessarily confined to diabetics, it can be considered that a mechanism involving abnormal metabolism as a result of diabetes gives rise to cerebral microvessel lesions or nerve damage, which in turn leads to a decline in cognitive function. It is therefore no exaggeration to call cognitive impairment the fourth diabetic microvascular disease70.
Regarding decline in cognitive function, it is important to consider the association with SVD. Types of SVD usually seen on brain MRI are silent brain infarction (SBI), white matter lesions and microbleeds, and all of them are reportedly related to cognitive dysfunction71. Among them, SBI has been found to be more prevalent in diabetics than in non‐diabetics74. In our own research, we found that the presence, severity and progression of SVD were associated with cognitive impairment in elderly diabetic patients25. An association was observed between SVD and frontal lobe impairment, the latter assumed to be a result of damage to the prefrontal loop caused by SBI in the thalamus, and deep and periventricular white matter lesions. Diabetic patients have a high incidence of SBI in the thalamus and basal ganglia75.
Hypertension plays a major role in the onset of SVD, but hyperglycemia is also considered to cause it. Hyperglycemia damages the endothelium of brain microvessels, resulting in disruption of the blood–brain barrier, which in turn leads to SVD76. In this regard, we previously reported that a rise in serum levels of intercellular adhesion molecule‐1 (ICAM‐1), which indicates vascular endothelial damage, was associated with the presence of SBI77 and its progression78, and more recently that it was associated with progression of white matter lesions79. An association between vascular endothelial function and SVD has also been reported80. We further reported that there was an association between a rise in ICAM‐1 levels and a decline in DSST79.
Other Factors Influencing Cognitive Function in Diabetic Patients
A review by Barnes and Yaffe showed that up to half of all cases of Alzheimer's disease are attributable to seven potentially modifiable risk factors – diabetes, midlife hypertension, midlife obesity, smoking, depression, low education level and physical inactivity – and a 25% reduction in all of these risk factors could reduce the number of dementia cases by up to 3 million81. Most of these risk factors also contribute to the incidence or progression of diabetes mellitus. Also, besides the risk associated with the apolipoprotein E (APOE) ε4 allele, multiple potentially modifiable risk factors, such as intelligence, fruit and vegetable consumption, depression and diabetes, were identified in a prospective 7‐year cohort study in populations aged over 65 years, suggesting that improvement of modifiable risk factors might have a great impact in reducing the incidence of dementia82. It has also been reported that lifestyle‐related diseases in middle age, such as diabetes, hypertension and dyslipidemia, increase the future incidence of dementia83, and risk factors for cardiovascular events readily accumulate in diabetic patients. Thus, diabetes mellitus plays a central role in cognitive decline.
There have also been a large number of studies on an association between hypertension and cognitive function84, and antihypertensive therapy is thus also important for the prevention of dementia. A recent meta‐analysis found that renin–angiotensin system blockers, especially angiotensin II receptor blockers, were effective in preventing cognitive decline85. Although an association between hypercholesterolemia and cognitive impairment is still the subject of much debate, statins are though to be useful in preventing dementia. However, they are considered to achieve this through a range of actions other than cholesterol lowering, including anti‐inflammatory and vascular endothelial stabilizing actions86. Needless to say, symptomatic cerebrovascular disease caused by cardiovascular risk factors will lead to cognitive impairment87.
A bidirectional association between depression and diabetes has been reported88, and the depressive state is closely associated with dementia89. This is considered to arise from damage to the hypothalamic–pituitary–adrenal axis, but it will not be discussed in detail in the present review90.
Regarding genetics, an association between the APOE gene and dementia is well known, and it has been reported that APOE ε4 allele carriers raise the incidence of Alzheimer's disease several fold91. In cross‐sectional6 as well as longitudinal research93, it has been reported that the presence of the APOE ε4 allele in diabetic patients synergistically increased the incidence of Alzheimer's disease and other types of dementia as compared with non‐diabetic patients, despite the fact that people with diabetes were reported to have a low incidence of the APOE ε4 allele95. Although possession of this genotype is generally associated with the development of Alzheimer's disease, associations with vascular dementia and mixed dementia have also been reported93. Also, the Hisayama study reported that in the presence of the APOE ε4 allele, hyperinsulinemia and hyperglycemia as a result of insulin resistance enhanced the formation of neuritic plaque45. Furthermore, it was found that the development of dementia was enhanced in APOE ε4 allele carriers in a middle‐aged cohort as a result of diabetes and other vascular risk factors, so early action needs to taken regarding lifestyle improvement96.
Future Outlook for Therapy to Arrest Cognitive Impairment
Recent large‐scale studies have clearly shown that comprehensive management of risks encompassing blood pressure and lipid control, as well as glycemic control, will prevent or stop the progress of vascular diseases in diabetic patients97. Future advances in diabetic therapy are expected to increase the life expectancy of diabetic patients by reducing the incidence of cardiovascular events. However, as it has been reported that not only dementia, but also cancer, frequently occur in diabetic patients98, it will be important to take measures against both diseases in elderly diabetics (Figure 3)99. Major problems of cognitive impairment are not only that it affects life expectancy, the deterioration in ADL due to cognitive impairment greatly increases the burden on caregivers, as well as the related medical costs. Mild cognitive impairment is considered to be the stage before dementia, and it has been said that when accompanied by diabetes, progression to dementia is accelerated100. In addition, modest cognitive decrements have been reported to be already present at the early stage of type 2 diabetes101. Thus, early diagnosis and countermeasures are very important. However, besides MMSE and other basic screening tools102, there are really very few ways of evaluating cognitive impairment.
Figure 3.
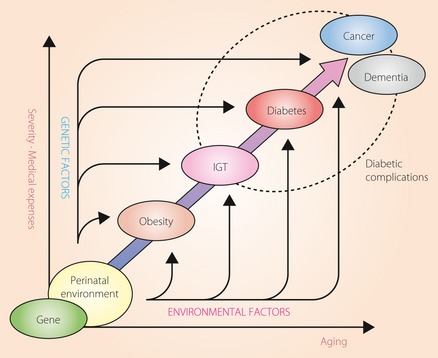
Natural history of patients with diabetes mellitus – past, present and future. The figure shows the natural history of patients with diabetes mellitus, whose status is affected by genetic and environmental factors. Thanks to recent advances in medicine, the incidence of vascular events in patients with diabetes has been decreasing; but from now on, both cancer and dementia will become serious problems in elderly diabetic patients. The results of the Framingham Heart Study showed an inverse association between cancer and Alzheimer's disease, with cancer survivors having a lower risk of Alzheimer's disease than those without cancer, and showed that patients with Alzheimer's disease had a lower risk of incident cancer99. IGT, impaired glucose tolerance.
Regarding non‐invasive methods of evaluation, although new neuroimaging techniques using positron emission tomography and single photon emission computed tomography have been recently developed to predict Alzheimer's disease, it is difficult to apply them in general clinical practice. In contrast, MRI is in widespread use and it has been reported that hippocampus volume, which is associated with cognitive function, could be easily semi‐quantitated by computer‐assisted analysis using a MRI voxel‐based specific regional analysis system developed for Alzheimer's disease103. Despite such advances, the development of methods based on measurement of predictive markers in blood samples would be more beneficial. So far, inflammatory markers and cytokines, such as C‐reactive protein, interleukin‐1β, interleukin‐6 and tumor necrosis factor‐α, have been reported to be associated with cognitive function105, and a study has been carried out on the use of an algorithm combining various serum protein‐based biomarkers for the detection of Alzheimer's disease107. However, useful blood markers are yet to be found. Surprisingly, an association between elevated adiponectin levels and future dementia has recently been reported in women108, and as weight loss in the elderly is said to be an early symptom of Alzheimer's disease, that study is of great interest109. In any case, it will be necessary for future research to discover and develop specific high‐sensitivity markers.
In conclusion, hyperglycemia and hyperinsulinemia, as well as hypoglycemia, are associated with the progression of cognitive decline. Diabetic control from an early stage would be useful in preventing the onset of vascular events, as well as cognitive decline. Although the usefulness of intensive glycemic control has yet to be shown, good control that avoids hypoglycemia and hyperinsulinemia would suppress cognitive impairment. Much research is currently underway on dementia, and in the near future, epoch‐making diagnosis techniques and therapies will be developed. However, at the current stage, lifestyle improvement with regard to diet, exercise habits, smoking and stress should help prevent diabetes and vascular complications, as well as the future development of dementia.
Acknowledgements
The authors declare no conflict of interest.
(J Diabetes Invest, doi: 10.1111/j.2040-1124.2012.00234.x, 2012)
The values of glycosylated hemoglobin A1c in the present review are expressed according to the National Glycohemoglobin Standardization Program.
References
- 1.Hotta N, Nakamura J, Iwamoto Y, et al Causes of death in Japanese diabetics: a questionnaire survey of 18,385 diabetics over a 10‐year period. J Diabetes Invest 2010; 1: 66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luengo‐Fernandez R, Leal J, Gray AM. Cost of dementia in the pre‐enlargement countries of the European Union. J Alzheimers Dis 2011; 27: 187–196 [DOI] [PubMed] [Google Scholar]
- 3.Yoshitake T, Kiyohara Y, Kato I, et al Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: The Hisayama Study. Neurology 1995; 45: 1161–1168 [DOI] [PubMed] [Google Scholar]
- 4.Leibson CL, Rocca WA, Hanson VA, et al Risk of dementia among persons with diabetes mellitus: a population‐based cohort study. Am J Epidemiol 1997; 145: 301–308 [DOI] [PubMed] [Google Scholar]
- 5.Ott A, Stolk RP, van Harskamp F, et al Diabetes mellitus and the risk of dementia. The Rotterdam Study. Neurology 1999; 53: 1937–1942 [DOI] [PubMed] [Google Scholar]
- 6.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. The Honolulu‐Asia Aging Study. Diabetes 2002; 51: 1256–1262 [DOI] [PubMed] [Google Scholar]
- 7.Ohara T, Doi Y, Ninomiya T, et al Glucose tolerance status and risk of dementia in the community. The Hisayama Study. Neurology 2011; 77: 1126–1134 [DOI] [PubMed] [Google Scholar]
- 8.Biessels GJ, Staekenborg S, Brunner E, et al Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006; 5: 64–74 [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complication Trial/Epidemiology of Diabetes Intervention and Complications (DCCT/EDIC) Study Research Group . Long‐term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007; 356: 1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson AM, Ryan CM, Cleary PA, et al Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow‐up of diabetes control and complications trial (DCCT) cohort. Diabetologia 2011; 54: 233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launer LJ, Miller ME, Williamson JD, et al Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomized open‐label substudy. Lancet Neurol 2011; 10: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christman AL, Matsushita K, Gottesman RF, et al Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 2011; 54: 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelick PB, Scuteri A, Black SE, et al Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 2011; 42: 2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes‐systematic overview of prospective observational studies. Diabetologia 2005; 48: 2460–2469 [DOI] [PubMed] [Google Scholar]
- 15.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008; 29: 494–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munshi M, Grande L, Hayes M, et al Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care 2006; 29: 1794–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cukierman‐Yaffe T, Gerstein HC, Williamson JD, et al Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors. The Action to Control Cardiovascular Risk in Diabetes‐Memory in Diabetes (ACCORD‐MIND) trial. Diabetes Care 2009; 32: 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umegaki H, Kawamura T, Mogi N, et al Glucose control levels, ischaemic brain lesions, and hyperinsulinaemia were associated with cognitive dysfunction in diabetic elderly. Age Ageing 2008; 37: 458–461 [DOI] [PubMed] [Google Scholar]
- 19.Strachan MWJ. The brain as a target organ in Type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med 2011; 28: 141–147 [DOI] [PubMed] [Google Scholar]
- 20.Heikkiä O, Lundbom N, Timonen M, et al Hyperglycaemia is associated with changes in the regional concentrations of glucose myo‐inositol within the brain. Diabetologia 2009; 52: 534–540 [DOI] [PubMed] [Google Scholar]
- 21.Lyoo IK, Yoon SJ, Musen G, et al Altered prefrontal glutamate‐glutamine‐γ‐aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in Type 1 diabetes mellitus. Arch Gen Psychiatry 2009; 66: 878–887 [DOI] [PubMed] [Google Scholar]
- 22.Bruce DG, Davis WA, Casey GP, et al Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia 2008; 51: 241–248 [DOI] [PubMed] [Google Scholar]
- 23.Yaffe K, Blackwell T, Whitmer RA, et al Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging 2006; 10: 293–295 [PubMed] [Google Scholar]
- 24.Umegaki H, Kawamura T, Kawano N, et al Factors associated with cognitive decline in elderly diabetics. Dement Geriatr Cogn Disord Extra 2011; 1: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imamine R, Kawamura T, Umemura T, et al Does cerebral small vessel disease predict future decline of cognitive function in elderly patients with type 2 diabetes? Diabetes Res Clin Pract 2011; 94: 91–99 [DOI] [PubMed] [Google Scholar]
- 26.Perantie DC, Koller JM, Weaver PM, et al Prospectively determined impact of Type 1 diabetes on brain volume during development. Diabetes 2011; 60: 3006–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EE, Egorova S, Blacker D, et al Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008; 65: 94–100 [DOI] [PubMed] [Google Scholar]
- 28.Euser SM, Sattar N, Wittman CM, et al A prospective analysis of elevated fasting glucose levels and cognitive function in older people. Results from PROSPER and the Rotterdam study. Diabetes 2010; 59: 1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwok C‐F, Ho L‐T. Severe hypoglycemia predicts major adverse outcomes in diabetic patients undergoing glycemic control regimes. J Diabetes Invest 2012; 3: 34–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araki A, Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int 2009; 9: 105–114 [DOI] [PubMed] [Google Scholar]
- 31.Rizzo MR, Marfella R, Barbieri M, et al Relationships between daily acute glucose fluctuations and cognitive performance among aged Type 2 diabetic patients. Diabetes Care 2010; 33: 2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbatecola AM, Rizzo MR, Barbieri M, et al Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 2006; 67: 235–240 [DOI] [PubMed] [Google Scholar]
- 33.Schuur M, Henneman P, van Swieten JC, et al Insulin‐resistance and metabolic syndrome are related to executive function in women in a large family‐based study. Eur J Epidemiol 2010; 25: 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raffaitin CR, Féart C, Le Goff M, et al Metabolic syndrome and cognitive decline in French elders. The Three‐City Study. Neurology 2011; 76: 518–525 [DOI] [PubMed] [Google Scholar]
- 35.Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep 2007; 7: 373–380 [DOI] [PubMed] [Google Scholar]
- 36.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. BMJ 2004; 328: 5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan CM, Fried MI, Rood JA, et al Improving metabolic control leads to better working memory in adults with Type 2 diabetes. Diabetes Care 2006; 29: 345–351 [DOI] [PubMed] [Google Scholar]
- 38.Wu JH, Haan MN, Liang J, et al Impact of antidiabetic medications on physical and cognitive functioning of older Mexican Americans with diabetes mellitus: a population‐based cohort study. Ann Epidemol 2003; 13: 369–376 [DOI] [PubMed] [Google Scholar]
- 39.Himeno T, Kamiya H, Naruse K, et al Benefical effects of Exendin‐4 on experimental polyneuropathy in diabetic mice. Diabetes 2011; 60: 2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bomfim TR, Forny‐Germano L, Sathler LB, et al An anti‐diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease‐ associated Aβ oligomers. J Clin Invest 2012; 122: 1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mogi N, Umegaki H, Hattori A, et al Cognitive function in Japanese elderly with type 2 diabetes mellitus. J Diabetes Complications 2004; 18: 42–46 [DOI] [PubMed] [Google Scholar]
- 42.Feil DG, Rajan M, Soroka O, et al Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc 2011; 59: 2263–2272 [DOI] [PubMed] [Google Scholar]
- 43.Craft S. Insulin resistance syndrome and Alzheimer's disease: age‐ and obesity‐related on memory, amyloid, and inflammation. Neurobiol Aging 2005; 26S: S65–S69 [DOI] [PubMed] [Google Scholar]
- 44.Young SE, Mainous AG, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle‐aged cohort. Diabetes Care 2006; 29: 2688–2693 [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki T, Sasaki K, Tanizaki Y, et al Insulin resistance is associated with the pathology of Alzheimer disease. The Hisayama Study. Neurology 2010; 75: 764–770 [DOI] [PubMed] [Google Scholar]
- 46.Rönnenmaa E, Zethelius B, Sundelöf J, et al Impaired insulin secretion increased the risk of Alzheimer disease. Neurology 2008; 71: 1065–1071 [DOI] [PubMed] [Google Scholar]
- 47.Rönnenmaa E, Zethelius B, Sundelöf J, et al Glucose metabolism and the risk of Alzheimer's disease and dementia: a population‐based 12 years follow‐up study in 71‐years‐old men. Diabetologia 2009; 52: 1504–1510 [DOI] [PubMed] [Google Scholar]
- 48.Profenno LA, Porsteinsson AP, Faraone SV. Meta‐analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry 2010; 67: 505–512 [DOI] [PubMed] [Google Scholar]
- 49.Sturman MT, Mendes de Leon CF, Bienias JL, et al Body mass index and cognitive decline in a biracial community population. Neurology 2008; 70: 360–367 [DOI] [PubMed] [Google Scholar]
- 50.Siervo M, Arnold R, Wells JCK, et al Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta‐analysis. Obes Rev 2011; 12: 968–983 [DOI] [PubMed] [Google Scholar]
- 51.Abbatecola AM, Lattanzio F, Molinari AM, et al Rosiglitazone and cognitive stability in older individuals with Type 2 diabetes and mild cognitive impairment. Diabetes Care 2010; 33: 1706–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Hanyu H, Hirao K, et al Efficacy of PPAR‐γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 2011; 32: 1626–1633 [DOI] [PubMed] [Google Scholar]
- 53.Shemesh E, Rudich A, Harman‐Boehm I, et al Effect of intranasal insulin on cognitive function‐A systematic review. J Clin Endocrinol Metab 2012; 97: 366–376 [DOI] [PubMed] [Google Scholar]
- 54.Craft S, Baker LD, Montine TJ, et al Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment. Arch Neurol 2012; 69: 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruce DG, Davis WA, Casey GP, et al Severe hypoglycemia and cognitive impairment in older patients with diabetes: the Fremantle Diabetes Study. Diabetologia 2009; 52: 1808–1815 [DOI] [PubMed] [Google Scholar]
- 56.Whitmer RA, Karter AJ, Yaffe K, et al Hypoglycemic episodes and risk of dementia in older patients with Type 2 diabetes mellitus. JAMA 2009; 301: 1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Åsvold BO, Sand T, Hestad K, et al Cognitive function in Type 1 diabetic adults with early exposed to severe hypoglycemia. A 16‐year follow‐up study. Diabetes Care 2010; 33: 1945–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Punthakee Z, Miller ME, Launer LJ, et al Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes. Post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012; 35: 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wessels AM, Rombouts SARB, Simsek S, et al Microvascular disease in type 1 diabetes alters brain activation. A functional magnetic resonance imaging study. Diabetes 2006; 55: 334–340 [DOI] [PubMed] [Google Scholar]
- 60.Wessels AM, Simsek S, Remijinse PL, et al Voxel‐based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 2006; 49: 2474–2480 [DOI] [PubMed] [Google Scholar]
- 61.Ding J, Strachan MWJ, Reynolds RM, et al Diabetic retinopathy and cognitive decline in older people with type 2 diabetes. The Edinburgh type 2 diabetes study. Diabetes 2010; 59: 2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Bresser J, Reijmer YD, van den Berg E, et al Microvascular determinants of cognitive decline and brain volume change in elderly patients with type2 diabetes. Dement Geriatr Cogn Disord 2010; 30: 381–386 [DOI] [PubMed] [Google Scholar]
- 63.Kwa VI, van der Sande JJ, Stam J, et al Retinal arterial changes correlate with cerebral small‐vessel disease. Neurology 2002; 59: 1536–1540 [DOI] [PubMed] [Google Scholar]
- 64.Lesage SR, Mosley TH, Wong TY, et al Retinal microvasuclar abnormalities and cognitive decline. The ARIC 14‐year follow‐up study. Neurology 2009; 73: 862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haan M, Espeland MA, Klein BE, et al Cognitive function and retinal and ischemic brain changes. The Women's Health Initiative. Neurology 2012; 78: 942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiner DE, Bartolomei K, Scott T, et al Albuminuria, cognitive functioning, and white matter hyperintensities in Homebound Elders. Am J Kidney Dis 2009; 53: 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito S, Nagasawa T, Abe M, et al Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro‐cardiovascular risk. Hypertens Res 2009; 32: 115–121 [DOI] [PubMed] [Google Scholar]
- 68.Ryan CM. Diabetes, aging, and cognitive decline. Neurobiol Aging 2005; 26S: S21–S25 [DOI] [PubMed] [Google Scholar]
- 69.Manschot SM, Biessels GJ, Rutten GEHM, et al Peripheral and central neurologic complications in type 2 diabetes mellitus: no association in individual patients. J Neuro Sci 2008; 264: 157–162 [DOI] [PubMed] [Google Scholar]
- 70.Kawamura T, Umemura T. Is cognitive impairment the fourth diabetic microvascular complication? J Diabetes Invest 2011; 2: 351–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel B, Markus HS. Magnetic resonance imaging in ceregral small vessel disease and its use as a surrogate disease marker. Int J Stroke 2011; 6: 47–59 [DOI] [PubMed] [Google Scholar]
- 73.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci 2010; 299: 131–135 [DOI] [PubMed] [Google Scholar]
- 74.van Harten B, Scheltens P, de Leeuw FE, et al Brain imaging in patients with diabetes. A systematic review. Diabetes Care 2006; 29: 2539–2548 [DOI] [PubMed] [Google Scholar]
- 75.Smith EE, Salat DH, Jeng J, et al Correlation between MRI white matter lesion location and excutive function and episodic memory. Neurology 2011; 76: 1492–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farrall AJ, Wardlaw JM. Blood‐brain barrier: ageing and microvascular disease‐systematic review and meta‐analysis. Neurobiol Aging 2009; 30: 337–352 [DOI] [PubMed] [Google Scholar]
- 77.Kawamura T, Umemura T, Kanai A, et al The incidence and characteristics of silent cerebral infarction in elderly diabetic patients: association with serum‐soluble adhesion molecules. Diabetologia 1998; 41: 911–917 [DOI] [PubMed] [Google Scholar]
- 78.Kawamura T, Umemura T, Kanai A, et al Soluble adhesion molecules and C‐reactive protein in the progression of silent cerebral infarction in patients with type 2 diabetes mellitus. Metabolism 2006; 55: 461–466 [DOI] [PubMed] [Google Scholar]
- 79.Umemura T, Kawamura T, Umegaki H, et al Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small‐vessel disease and cognitive impairment: a 6‐year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Pshychiatry 2011; 82: 1186–1194 [DOI] [PubMed] [Google Scholar]
- 80.Hoth KF, Tate DF, Poppas A, et al Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke 2007; 38: 308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnes D, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011; 10: 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ritchie K, Carriérw I, Ritchie CW, et al Designing prevention program to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ 2010; 341: c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alonso A, Mosley TH Jr, Gottesman RF, et al Risk of dementia hospitalization associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009; 80: 1194–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu C, Winblad B, Fratiglioni L. The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005; 4: 487–499 [DOI] [PubMed] [Google Scholar]
- 85.Li N‐C, Lee A, Whitmer RA, et al Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 2010; 340: b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haag MDM, Hofman A, Koudstaal PJ, et al Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 2009; 80: 13–17 [DOI] [PubMed] [Google Scholar]
- 87.Wu JH, Hann MN, Liang J, et al Impact of diabetes on cognitive function among older Latinos. A population‐based cohort study. J Clin Epidemol 2003; 56: 686–693 [DOI] [PubMed] [Google Scholar]
- 88.Pan A, Lucas M, Sun Q, et al Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med 2010; 170: 1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katon W, Lyles CR, Parker MM, et al Association of depression with increased risk of dementia in patients with type 2 diabetes. The Diabetes and Aging Study. Arch Gen Psychiatry 2012; 69: 410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strachan MWJ, Reynolds RM, Marioni RE, et al Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 2011; 7: 108–114 [DOI] [PubMed] [Google Scholar]
- 91.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron 2009; 63: 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dore GA, Elias MF, Robbins MA, et al Presence of APOE ε4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine‐Syracuse Study. Diabetologia 2009; 52: 2551–2560 [DOI] [PubMed] [Google Scholar]
- 93.Irie F, Fitzpatrick AL, Lopez OL, et al Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE ε4. The Cardiovascular Health Study Cognition Study. Arch Neurol 2008; 65: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahtiluoto S, Polvikoski T, Peltonen M, et al Diabetes, Alzheimer disease, and vascular dementia. A population‐based neuropathologic study. Neurolgy 2010; 75: 1195–1202 [DOI] [PubMed] [Google Scholar]
- 95.Nielson KA, Nolan JH, Berchtold NC, et al Apolipoprotein‐E genotyping of diabetic dementia patients: is diabetes rare in Alzheimer's disease? J Am Geriatr Soc 1996; 44: 897–904 [DOI] [PubMed] [Google Scholar]
- 96.Blair CK, Folsom AR, Knopman DS, et al APOE genotype and cognitive decline in a middle‐aged cohort. Neurology 2005; 64: 268–276 [DOI] [PubMed] [Google Scholar]
- 97.Gæde P, Lund‐Andersen H, Parving H‐H, et al Effect of multifactorial intervention on mortality in Type 2 diabetes. N Engl J Med 2008; 358: 580–591 [DOI] [PubMed] [Google Scholar]
- 98.Noto H, Tsujimoto T, Noda M. Significantly increased risk of cancer in diabetes mellitus patients: a meta‐analysis of epidemiological evidence in Asia an non‐Asias. J Diabetes Invest 2012; 3: 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Driver JA, Beiser A, Au R, et al Inverse association between cancer and Alzheimer's disease: result from the Framingham Heart Study. BMJ 2012; 344: e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu W, Caracciolo B, Wang H‐X, et al Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 2010; 59: 2928–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruis C, Biessels GJ, Gorter KJ, et al Cognition in the early stage of type 2 diabetes. Diabetes Care 2009; 32: 1261–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alencar RC, Cobas RA, Gomes MB. Assessment of cognitive status in patients with type 2 diabetes through the mini‐mental status examination: a cross‐sectional study. Diabetol Metab Syndr 2010; 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamiyama K, Wada A, Sugiura M, et al Potential hippocampal region atrophy in diabetes mellitus type 2: a voxel‐based morphometry VSRAD study. Jpn J Radiol 2010; 28: 266–272 [DOI] [PubMed] [Google Scholar]
- 104.Anan F, Masaki T, Shimomura T, et al High‐sensitivity C‐reactive protein is associated with hippocampus volume in nondementia with type 2 diabetes mellitus. Metabolism 2011; 60: 460–466 [DOI] [PubMed] [Google Scholar]
- 105.Tan ZS, Beiser AS, Vasan RS, et al Inflammatory markers and the risk of Alzheimer disease. The Framingham Study. Neurology 2007; 68: 1902–1908 [DOI] [PubMed] [Google Scholar]
- 106.Hoshi T, Yamagami H, Furukado S, et al Serum inflammatory proteins and frontal lobe dysfunction in patients with cardiovascular risk factors. Eur J Neurol 2010; 17: 1134–1140 [DOI] [PubMed] [Google Scholar]
- 107.O'Bryant SE, Xiao G, Barber R, et al A serum protein‐based algorithm for the detection of Alzheimer disease. Arch Neurol 2010; 67: 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Himbergen TM, Beiser AS, Ai M, et al Biomarkers for insulin resistance and inflammation and the risk for all‐cause dementia and Alzheimer disease. Results from the Framingham Heart Study. Arch Neurol 2012; 69: 594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vidoni ED, Townley RA, Honea RA, et al Alzheimer disease biomarkers are associated with body mass index. Neurology 2011; 77: 1913–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]


