Abstract
Aims/Introduction: Visceral obesity has been suggested to be an independent risk factor for cardiovascular disease (CVD); the role of adipokines in the risk for CVD is less clear. Aim of this study was to investigate the relationship between parameters of visceral obesity and index of CVD risk factors.
Materials and Methods: A cross‐sectional analysis of healthy males (n = 116) and females (n = 175) for evaluation of clinical, laboratory and anthropometric parameters were undertaken. Abdominal subcutaneous (SAT) and visceral adipose tissues (VAT) were measured by computed tomography. Adipokines, including retinol‐binding protein 4 (RBP4) and adiponectin, were determined. The risk for CVD was estimated using the 10‐year Framingham Coronary Heart Disease Risk Point scale (Framingham score).
Results: The Framingham score was increased in subjects with metabolic syndrome, and significantly increased with various indices of obesity, traditional risk factors of CVD, C‐reactive protein (CRP) and RBP4, but decreased with adiponectin. With multiple linear regression analysis, the Framingham score independently associated with age, smoking status, body mass index, triglyceride and RBP4. The magnitude of the Framingham score showed a linear trend of increase with CRP, VAT and RBP4 (all P < 0.001), but of decrease with SAT and adiponectin (all P < 0.05) at stratified levels of obesity.
Conclusions: RBP4 is increased with visceral fat accumulation and associated with CVD risk factors independent of obesity or traditional risk factors. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2012.00213.x, 2012)
Keywords: Visceral obesity, Adipokines, Retinol binding protein 4
Introduction
Visceral obesity is associated with the metabolic syndrome (MetS), type 2 diabetes and subsequent increased cardiovascular disease (CVD) morbidity and mortality1. However, the exact mechanisms accounting for the deleterious effects of visceral fat on CVD remain unknown2.
It is now recognized that adipocytes are endocrine cells, secreting a number of molecules collectively referred to as adipokines that function as hormones, regulating the biological activities of different tissues and organs. Although many of these proteins remain uncharacterized, leptin, retinol‐binding protein 4 (RBP4) and adiponectin have been identified as molecules responsible for the association between visceral obesity and insulin resistance (IR)3,4.
Although many studies have documented the association between abdominal visceral adipose tissue (VAT) or CVD risk in patients who are obese and have type 2 diabetes5, confirmation of this relationship, after taking into account direct measurements of adipose tissue distribution in healthy individuals, is very limited. Furthermore, the role of adipokines in the association between body fat distribution and CVD risk has not been reported.
The aim of the present study was to determine the relationship of CVD risk, as defined by the Framingham risk analysis method6, with body fat distribution (measured by clinical examination, bioelectrical impedance analysis [BIA], fat computed tomography [CT] and dual energy absorptiometry [DXA]). In addition, we determined the possible role of adipokines, such as RBP4 and adiponectin, in the association between CVD risk and body fat distribution.
Materials And Methods
Study Population and Design
The present study was designed to explore the associations of adiposity and adipokines in the Korean population, and part of this had been presented before7. In the present study, we recruited healthy adult volunteers (120 men and 180 women) by advertising. Volunteers participating in the present study had to meet the flowing criteria: (i) aged 19–70 years; (ii) judged as healthy by a responsible physician with no abnormality identified on a medical evaluation, including medical history and physical examination; (iii) not pregnant in the case of females; and (iv) not taking any medication at the time of the study. Participants who were not suitable to participate in the present study for any reason, in the opinion of the responsible physician, were excluded. The analysis excluded participants for whom information was missing (1 man and 2 women), and with incidentally diagnosed diabetes with repeated testing (3 men and 3 women). As a result, the final study population comprised 291 participants (116 men and 175 women). The Institutional Review Boards at the Ilsan Paik Hospital approved the study protocol according to the Declaration of Helsinki; all participants provided informed consent. All participants completed a self‐administered questionnaire that included demographic characteristics, general health status, smoking history and current medications.
Clinical, Laboratory and Anthropometric Measurement
Anthropometric and body composition measurements were carried out in all study participants before breakfast, with the participants wearing light clothing and without shoes. In addition, height, waist circumference (WC) and the hip circumference (HC) were measured. The body mass index (BMI) was calculated as weight/height2 (kg/m2). WC was measured midway between the inferior margin of the last rib and the crest of the ileum in the horizontal plane. HC was measured around the pelvis at the point of maximal protrusion of the buttocks. The circumferences were measured to the nearest 0.1 cm. Blood pressure (BP) was measured from the right arm subsequent to the participant sitting at rest for a period of 20 min. The mean of two consecutive blood pressure recordings was used for statistical analysis.
Total body fat and muscle were then measured by BIA (Inbody 3.0; Biospace, Seoul, Korea)8. The total cross‐sectional abdominal and visceral fat areas were measured by CT scans (Somatom Plus 4; Siemens, Forchheim, Germany) using an established protocol9. A cross‐sectional scan, with 10‐mm thickness centered at the L4‐L5 vertebral disc space was obtained with the participant in the supine position using a radiograph of the skeleton as a reference; this was used to establish the position of the scans to the nearest millimeter. The abdominal subcutaneous adipose tissue (SAT) area was calculated by subtracting the abdominal visceral adipose tissue (VAT) area from the total area of adipose tissue. In addition, the body composition, including lean body mass and total body fat, was determined by a DXA (QDR 4500; Hologic, Bedford, MA, USA) carried out with a whole‐body scanner. The trunk fat was determined as the amount of fat measured by the DXA from below the neck to the pelvis, excluding the limbs.
Blood samples were collected from all participants after an overnight fast (10 h) between 08.30 h and 10.30 h, and the plasma were stored at −70° until used. Plasma RBP4 levels were measured with an enzyme immunoassay (EIA) kit (AdipoGen, Seoul, Korea), and inter‐ and intra‐assay variability were 7.2% and 5.5%, respectively. Plasma adiponectin was measured using a human adiponectin radioimmunoassay kit (Linco Research, St. Charles, MO, USA), with an intra‐assay coefficient of variation of 3.6%. The mean of two duplicated values was used for statistical analysis. Fasting plasma glucose, total cholesterol (TC), triglycerides (TG) and high‐density lipoprotein cholesterol (HDL‐C) were measured enzymatically using an autoanalyzer (ADVIA 1650; Bayer Ltd., Tokyo, Japan). High‐sensitivity C‐reactive protein (hsCRP) was measured by EIA (Modular P800; Roche, Basel, Switzerland). The plasma levels of insulin and leptin were measured by radioimmunoassay (Hitachi E170; Hitachi Ltd., Tokyo, Japan and Linco Research, St. Charles, MO, USA, respectively). The IR index was calculated from the fasting plasma insulin, and the plasma glucose level was estimated by the homeostasis model assessment (HOMA) where10: HOMA = fasting plasma insulin (lU/mL) × fasting plasma glucose (mmol/L)/22.5.
Metabolic Syndrome and CVD Risk Assessment
The Framingham risk score including age, TC, smoking status, HDL‐C and systolic BP, stratified by sex, was used to predict the 10‐year absolute risk of developing coronary heart disease (CHD)6. To calculate the score for an individual, a 10‐year Framingham Coronary Heart Disease Risk Point (10‐year FCRP) score was assigned for each risk factor. Participants that smoked regularly during the previous 12 months were classified as current smokers. The presence of metabolic syndrome was determined according to the 2005 revised National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria11. We defined visceral obesity as a WC ≥90 cm for males or ≥80 cm for females, as recommended by the revised NCEP criteria.
Statistical Analyses
Analysis was carried out with 291 participants for whom both RBP4 and adiponectin data were available. Data are expressed as mean ± SD. The distributions for fasting insulin, TG, HOMA‐IR, RBP4, adiponectin, hsCRP and leptin were normalized using log transformation, and transformed back for data presentation. We used the independent t‐test or chi‐squared‐test to analyze differences in categorical data (with and without metabolic syndrome). Pearson’s correlation analyses were used to describe the association between Framingham scores and continuous variables of interest. A multiple linear regression analysis was used to test the independent association of Framingham score and continuous variables. Multicollinearity was assessed using the variance inflation factor (VIF). To avoid multicollinearity among WC, HC, waist‐to‐hip ratio (WHR), total body fat and trunk fat, WHR was included as an independent variable. To assess the significance of a linear trend, continuous variables were stratified to tertile. And, we used a one‐way anova with post‐hoc analysis to assess the difference of mean values of the BMI‐adjusted VAT or SAT and levels of RBP4/or adiponectin with respect to the number of metabolic syndrome determinants. With two‐way anova using the general linear model (univariate), we tested the effects of interaction between adiposity (BMI or VAT) and the stratified variables (tertiles of CRP, VAT, SAT, RBP4 and adiponectin) on means of Framingham scores. A P‐value of <0.05 was considered statistically significant. Data were analyzed using SPSS for Windows (Version 12.0; SPSS Inc., Chicago, IL, USA).
Results
Individuals included 116 males (aged 40 ± 11 years) and 175 females (aged 40 ± 11 years). The BMI in men was higher than in women (25.4 ± 3.1 vs 23.6 ± 3.1 kg/m2, respectively, P < 0.001). When analyzed by presence of metabolic syndrome11, the participants with metabolic syndrome were more likely to be male, older, a smoker and obese. Details relating to clinical, laboratory and anthropometric characteristics are presented in Table 1. The two groups were significantly different for all the metabolic and anthropometric parameters, as expected (Table 1). Participants with metabolic syndrome showed higher levels of RBP4, CRP, and Framingham risk score and risk, whereas adiponectin values were lower than those without metabolic syndrome.
Table 1. Clinical, laboratory and anthropometric characteristics of the study participants with or without metabolic syndrome.
| n (%) | Metabolic syndrome (−) (234, 80.4) | Metabolic syndrome (+) (57, 19.6) |
|---|---|---|
| Female, n (%) | 158, 67.5 | 17, 29.8* |
| Age (years) | 38, 19 to 68 | 44, 26 to 70* |
| Smoker, n (%) | 60, 25.6 | 40, 70.2* |
| BMI (kg/m2) | 23.6 ± 3.0 | 27.0 ± 2.6* |
| WC (cm) | 82.0 ± 8.2 | 93.1 ± 7.5* |
| WHR | 0.83 ± 0.06 | 0.90 ± 0.05* |
| Systolic BP (mmHg) | 111.7 ± 13.2 | 126.7 ± 15.7* |
| Diastolic BP (mmHg) | 68.5 ± 8.4 | 79.9 ± 9.4* |
| Fasting plasma insulin (pmol/L)† | 34.7 ± 23.8 | 61.4 ± 36.2* |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.4 | 5.3 ± 0.6* |
| HOMA‐IR† | 1.0 ± 0.7 | 2.0 ± 1.2* |
| Total cholesterol (mmol/L) | 4.6 ± 0.8 | 4.8 ± 09 |
| Triglyceride (mmol/L)† | 1.4 ± 0.7 | 3.1 ± 1.7* |
| HDL‐C (mmol/L) | 1.3 ± 0.3 | 1.0 ± 0.2* |
| Total body fat (kg) | 18.2 ± 5.0 | 21.2 ± 5.0* |
| Lean mass (kg) | 42.3 ± 8.7 | 51.1 ± 10.6* |
| Trunk fat (kg) | 8.3 ± 2.8 | 10.8 ± 2.8* |
| Total extremity fat (kg) | 9.0 ± 2.6 | 9.3 ± 2.7 |
| Total abdominal fat (cm2) | 245.5 ± 85.4 | 335.2 ± 82.7* |
| Abdominal VAT (cm2) | 75.1 ± 39.6 | 134.6 ± 52.7* |
| Abdominal SAT (cm2) | 170.4 ± 64.8 | 200.7 ± 64.2* |
| Inbody‐weight (kg)‡ | 62.5 ± 10.4 | 75.4 ± 12.1* |
| Inbody‐fat (kg)‡ | 16.5 ± 4.2 | 20.4 ± 4.9* |
| Framingham score | 3.0, −9.0 to 18.0 | 9.0, −7.0 to 17.0* |
| Framingham risk | 0.5, 0.0 to 20.0 | 2.0, 0.0 to >30.0* |
| hsCRP (mg/dL)† | 0.04, 0.01 to 2.63 | 0.08, 0.02 to 0.52* |
| RBP4 (μg/mL)† | 52.2 ± 20.0 | 65.1 ± 26.8* |
| Adiponectin (μg/mL)† | 9.0 ± 6.9 | 6.0 ± 5.8* |
| Leptin (ng/mL)† | 7.3 ± 5.8 | 7.2 ± 5.5 |
Data are mean ± standard deviation or median (range), *P < 0.05. †Logarithmic transformation carried out before analysis. ‡Measured by bioimpedance analysis. BMI, body mass index; BP, blood pressure; HC, hip circumference; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; RBP4, retinol‐binding protein 4; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; WC, waist circumference; WHR, waist‐to‐hip ratio.
As shown in Table 2, Framingham score is significantly increased with age, BMI, WC, blood pressure, IR, TC/HDL, TG, obesity measured by BIA and DXA, CRP and RBP4, and, inversely, associated with adiponectin. The Framingham score was higher in men compared with women (5.2 ± 6.5 vs 0.8 ± 0.91, respectively, P < 0.001) and in smokers compared with non‐smokers (5.3 ± 6.9 vs 1.1 ± 1.4, respectively, P < 0.001).
Table 2. Pearson’s correlation coefficients between Framingham scores and various anthropometric and metabolic parameters.
| γ | P | |
|---|---|---|
| Age | 0.836 | <0.001 |
| BMI | 0.415 | <0.001 |
| WC | 0.465 | <0.001 |
| WHR | 0.478 | <0.001 |
| Systolic BP | 0.448 | <0.001 |
| Diastolic BP | 0.478 | <0.001 |
| Plasma insulin† | 0.136 | 0.020 |
| Plasma glucose | 0.288 | <0.001 |
| HOMA‐IR† | 0.169 | 0.004 |
| TC/HDL‐C | 0.348 | <0.001 |
| Triglyceride† | 0.363 | <0.001 |
| Total body fat | 0.210 | <0.001 |
| Lean mass | 0.133 | 0.023 |
| Trunk fat | 0.369 | <0.001 |
| Total extremity fat | −0.029 | 0.625 |
| Total abdominal fat | 0.422 | <0.001 |
| Abdominal VAT | 0.547 | <0.001 |
| Abdominal SAT | 0.186 | 0.01 |
| Inbody‐weight | 0.209 | <0.001 |
| Inbody‐fat | 0.261 | <0.001 |
| hsCRP† | 0.340 | <0.001 |
| RBP4† | 0.308 | <0.001 |
| Adiponectin† | −0.179 | 0.003 |
| Leptin† | 0.011 | 0.858 |
†Logarithmic transformation carried out before analysis. BMI, body mass index; BP, blood pressure; γ, correlation coefficients; HC, hip circumference; HDL‐C, high density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; RBP4, retinol‐binding protein 4; SAT, subcutaneous adipose tissue; TC, total cholesterol; VAT, visceral adipose tissue; WC, waist circumference; WHR, waist‐to‐hip ratio.
We carried out a stepwise multiple linear regression analysis using the Framingham score as the dependent variable. In this analysis, we included sex, smoking status and variables, which were statistically significantly correlated with Framingham score in Table 2 (P < 0.05) as independent variables. This model showed that age, smoking status, BMI, TG and RBP4 were independently associated with Framingham score; in addition, they accounted for 76.1% (adjusted R2) of the variance in the Framingham score of analyzed individuals (Table 3). In this model, no evidence of serious multicollinearity was observed (all VIF were below 1.5).
Table 3. Multiple linear regression analysis using Framingham scores as a dependent variable.
| Unstandardized coefficients | Standardized coefficients | P | ||
|---|---|---|---|---|
| β | SE | β | ||
| Constant | −27.435 | 2.672 | <0.001 | |
| Age (years) | 0.452 | 0.019 | 0.753 | <0.001 |
| Smoker† | 1.153 | 0.465 | 0.084 | 0.014 |
| BMI | 0.256 | 0.069 | 0.127 | <0.001 |
| Triglyceride‡ | 1.240 | 0.441 | 0.103 | 0.005 |
| RBP4‡ | 0.123 | 0.594 | 0.097 | 0.005 |
†Reference to non‐smoker. ‡Logarithmic transformation performed before analysis. BMI, body mass index; RBP4, retinol‐binding protein 4; SE, standard error.
When participants were stratified according to the number of the determinants of the metabolic syndrome as defined by the revised NCEP ATP III criteria11, BMI‐adjusted VAT increased with a number of determinants of metabolic syndrome (P‐value for trend <0.001; Figure 1a). SAT also increased with a number of determinants of metabolic syndrome (data not shown). However, the BMI‐adjusted value did not show these linear relationships (P = 0.326 for trend; Figure 1b). The RBP4 concentration was increased linearly according to the number of determinants of metabolic syndrome (P = 0.008 for trend), whereas the adiponectin decreased (P = 0.003 for trend; Figure 1c,d).
Figure 1.
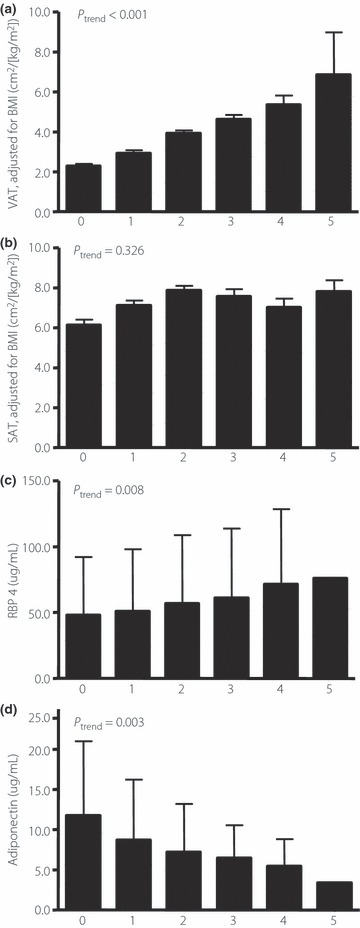
Means of body mass index‐adjusted abdominal (a) visceral adipose tissue (a) and (b) subcutaneous adipose tissue (SAT), plasma levels of (c) retinol binding protein 4 (RBP4), and (d) adiponectin with an increasing number of the determinant of metabolic syndrome (n for each number of determinant are 62, 94, 78, 36, 19 and 2, respectively). (a,b) Bars signify means; error bar, SD. (c,d) Bars signify means; error bar, 95% confidence interval of means.
Figure 2 shows the distribution of Framingham score by tertile CRP, VAT and SAT according to BMI (Figure 2a), and CRP, RBP4 and adiponectin according to VAT adjustment of BMI (Figure 2b). The mean values of Framingham scores were increased along the tertiles of CRP and VAT with increases of the tertiles of BMI (all P < 0.001) without any interaction (P = 0.509 and 0.054, respectively). However, inverse relationships were observed in the case of SAT values (P < 0.001) without interaction (P = 0.151; Figure 2a). The Framingham score for each RBP4 tertile was significantly different at each level of BMI‐adjusted VAT (P = 0.035), as well as adiponectin (P = 0.049) or CRP (P = 0.015) without any interaction (P‐value ranged from 0.133 to 0.976; Figure 2b).
Figure 2.
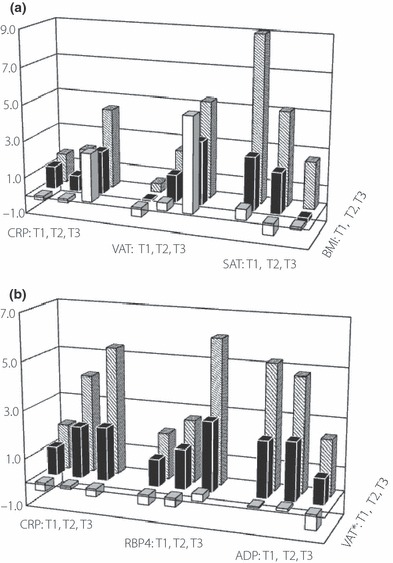
(a) Mean values of the Framingham score for each tertile of high‐sensitivity C‐reactive protein (hsCRP), visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) along the body mass index (BMI) tertile, (b) and those for each tertile of hsCRP, RBP4 and adiponectin along the VAT tertile. *BMI adjusted values. 95% confidence interval of each variables tertiles (n = 97, each) are following: BMI 20.6–21.2, 24.0–24.3 and 27.5–28.3 kg/m2; CRP 0.022–0.025, 0.047–0.051 mg/dL and 0.152–0.279; VAT 36.8–41.6, 76.5–82.0 and 134.4–149.4 cm2; SAT 103.8∼114.9, 168.5–173.6 and 238.9–258.2; VAT* 1.7–1.9, 3.1–3.3 and 5.1–5.3 cm2/[kg/m2]; RBP4 33.1–35.4, 49.6–51.6 and 75.4–83.1 μg/mL; adiponectin 2.9–3.3, 6.3–6.8 and 14.0–17.1; 149.4; SAT 103.8–114.9, 168.5–173.6 and 238.9–258.2 μg/mL.
Discussion
The unique contribution of the present study is the evaluation of a wide range of traditional and non‐traditional risk factors for CVD in well‐characterized subjects. Furthermore, we documented the significant, independent associations of the direct measurements of VAT and the Framingham score with variation in the levels of RBP4 after adjustment for confounding factors.
Individuals with visceral obesity are at an increased risk of type 2 diabetes and CVD1,12. Furthermore, these individuals had increased metabolic risk factors including IR, dyslipidemia, elevated free fatty acids and subclinical inflammation compared with individuals with lower body obesity12. Although paradigms, such as the portal hypothesis and the endocrine hypothesis, have been suggested, the role of adipokines remains unknown. However, it is possible that adipokines might become more important with longer‐term obesity or visceral fat deposit itself, when a pro‐ or an anti‐inflammatory role of these adipokines might become an important factor13. The present study showed that an increased plasma level of RBP4 in visceral fat accumulation, measured by CT, was associated with CVD risk factors independent of traditional CVD risk factors (age, smoking, triglyceride, obesity)14.
In the present study, we found a strong positive relationship between RBP4 and Framingham score, composite of CVD risk factors, at the degree of visceral adiposity. In addition, the plasma level of RBP4 increased along the increase of determinants of metabolic syndrome, consistent with previous reports (Figure 1a)15. However, data resulting from human studies are conflicting. RBP4 is not increased in obesity or not associated with IR in some situations. These inconsistencies most likely result from variations in studied subjects: genetic background, sex ratio and age, sample size, effects of retinol status, iron status, kidney function, and assay methods used16. In the present study, with multiple linear regression analysis of RBP4 as a dependent variable, plasma levels of RBP4 were independently associated with men (standardized coefficients β = 0.288, P < 0.001), triglyceride (β = 0.239, P < 0.001) and VAT (β = 0.152, P = 0.013) in participants without metabolic syndrome, and TG (β = 0.398, P = 0.002) and VAT (β = 0.390, P = 0.002) in participants with metabolic syndrome (data not shown). Thus, we had observed a significant association between RBP4 and VAT in all participants. This association was more prominent in participants with metabolic syndrome in whom RBP4 was independently associated with VAT, even after accounting for sex, age and BMI. Consistent with a previous study, in which a correlation of RBP4 with abdominal obesity in participants with a wide range of BMI was shown15, these findings let us suggest the possibility of an association between circulating RBP4 with specific fat deposits and a role of RBP4 in the associations of CVD risk factors. In addition, Framingham scores in participants with the highest tertile of VAT were not different between those with the lowest tertile of BMI and those with the highest tertile of BMI (mean [95% CI], 5.6 [3.0, 11.9] and 5.5 [7.7, 10.4], respectively, P = 0.129). However, there was weak, but not significant, interaction between visceral fat deposit (i.e. VAT) and overall obesity (i.e. BMI) on the Framingham score (P = 0.054). These findings are similar to that studied in an Asian population study17,18, and also agree with evidence linking specific visceral fat deposit to the increased risk of CVD19.
The opposite correlations of RBP4 and adiponectin for VAT were consistent with studies of cultured visceral and subcutaneous adipocytes from humans and animal models. Findings from these studies have shown that although both adiponectin gene expression and secretion are higher in visceral adipocytes20, adiponectin secretion from adipocyte deposition decreases with increasing visceral obesity21. This might partly explain the present results, in regard to a blunted protective effect of adiponectin on the CVD risk with increasing visceral obesity (Figure 2b). The underlying mechanism responsible for the interaction of these adipokines in situations of visceral fat deposit or metabolic syndrome with the CVD risk factors remains to be elucidated by future studies.
CRP has been identified as an effecter in the athero‐thrombotic process22, and as a predictor of CVD risk among representative inflammatory markers in the clinical setting23. However, the correlation between CRP and Framingham score did not reach statistical significance in multiple linear regression analysis. These findings suggest that correlations between CRP and various atherosclerotic risk factors should be corrected for adiposity or adipokines, because the adipocyte itself is a known source of various inflammatory cytokines24.
Although the present study has unique strength, it also has limitations. First, the Framingham risk (represented as %) could overestimate or underestimate the risk in populations other than the USA population25. Although it has not been established whether the Framingham risk is suggested as a predictor of CHD risk in Korea, reasonable accuracy in predicting CHD in an Asian population had been shown in the past26, and Koreans were found to have a comparable CVD risk profile and their estimated 10‐year CHD risk is currently almost as high as that in the general USA population27,28. Future validation studies are clearly required to assess the utility of this risk calculation. Second, there are no previous data available for determining sample size for the present study. Therefore, we could not estimate an appropriate sample size in the present study. Power calculations were based on the addition of a variable to an existing regression model with a R2 of 0.4–0.5. A sample size of the present study provided 99.8% power at the 5% significance level for detecting an increase in the R2 of 0.05 or greater Third, the participants in the present study were a clinically narrow range from the perspective of overall risk; as shown, 95.5% of study participants showed ‘low‐risk’ (Framingham risk <15%). Thus, someone might argue that this cohort was neither heterogeneous nor representative of the general population. However, the purpose of the present study and cohort was to investigate the association of adiposity and CVD risk in healthy subjects without any known CVD diseased condition. Despite the clinically narrow range from the perspective of overall risk, the study’s findings showed the relevant relationship between plasma levels of RBP4 and CVD risk factors, represented by Framingham score rather than Framingham risk. Finally, the cross‐sectional deign of the present study did not definitively establish the causal relationships. Additional human studies using longitudinal study designs are required for further clarification of these relationships.
In conclusion, RBP4 is increased with visceral fat accumulation and associated with CVD risk factors. Thus, RBP4 could play a role in the mediating deleterious effects of visceral obesity on the increased risk of CVD independent of traditional risk factors.
Acknowledgements
This study was supported by the Health Promotion Funds from the Korea Ministry of Health & Welfare; a grant from the IN‐SUNG Foundation for Medical Research, Inje University, 2010, Korean Diabetes Association, 2009; and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010‐0020224).
References
- 1.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887 [DOI] [PubMed] [Google Scholar]
- 2.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology 2003; 144: 2195–2200 [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Hennige AM, Staiger H et al. High circulating retinol‐binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care 2007; 30: 1173–1178 [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Graham TE, Mody N et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005; 436: 356–362 [DOI] [PubMed] [Google Scholar]
- 5.Bacha F, Saad R, Gungor N et al. Are obesity‐related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care 2006; 29: 1599–1604 [DOI] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) . Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002; 106: 3143–3421 [PubMed] [Google Scholar]
- 7.Won JC, Park CY, Lee WY et al. Association of plasma levels of resistin with subcutaneous fat mass and markers of inflammation but not with metabolic determinants or insulin resistance. J Korean Med Sci 2009; 24: 695–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha K, Chertow GM, Gonzalez J et al. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol 1995; 79: 1316–1319 [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L, Kvist H, Cederblad A et al. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol 1986; 250: E736–E745 [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752 [DOI] [PubMed] [Google Scholar]
- 12.Despres JP. Intra‐abdominal obesity: an untreated risk factor for Type 2 diabetes and cardiovascular disease. J Endocrinol Invest 2006; 29: 77–82 [PubMed] [Google Scholar]
- 13.Bergman RN, Kim SP, Hsu IR et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 2007; 120: S3–8, 29–32. [DOI] [PubMed] [Google Scholar]
- 14.Mokdad AH, Ford ES, Bowman BA et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA 2003; 289: 76–79 [DOI] [PubMed] [Google Scholar]
- 15.Graham TE, Yang Q, Bluher M et al. Retinol‐binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006; 354: 2552–2563 [DOI] [PubMed] [Google Scholar]
- 16.Kotnik P, Fischer‐Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol 2011; 165: 703–711 [DOI] [PubMed] [Google Scholar]
- 17.Ko GT, Chan JC, Woo J et al. Simple anthropometric indexes and cardiovascular risk factors in Chinese. Int J Obes Relat Metab Disord 1997; 21: 995–1001 [DOI] [PubMed] [Google Scholar]
- 18.Wildman RP, Gu D, Reynolds K et al. Are waist circumference and body mass index independently associated with cardiovascular disease risk in Chinese adults? Am J Clin Nutr 2005; 82: 1195–1202 [DOI] [PubMed] [Google Scholar]
- 19.Carr DB, Utzschneider KM, Hull RL et al. Intra‐abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004; 53: 2087–2094 [DOI] [PubMed] [Google Scholar]
- 20.Motoshima H, Wu X, Sinha MK et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab 2002; 87: 5662–5667 [DOI] [PubMed] [Google Scholar]
- 21.Fisher FM, McTernan PG, Valsamakis G et al. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res 2002; 34: 650–654 [DOI] [PubMed] [Google Scholar]
- 22.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C‐reactive protein on human endothelial cells. Circulation 2000; 102: 2165–2168 [DOI] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511 [DOI] [PubMed] [Google Scholar]
- 24.Rogowski O, Shapira I, Toker S et al. Obesity‐related correlation between C‐reactive protein and the calculated 10‐y Framingham Coronary Heart Disease Risk Score. Int J Obes (Lond) 2005; 29: 772–777 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Hong Y, D’Agostino RB Sr et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi‐Provincial Cohort Study. JAMA 2004; 291: 2591–2599 [DOI] [PubMed] [Google Scholar]
- 26.Barzi F, Patel A, Gu D; Asia Pacific Cohort Studies Collaboration et al. Cardiovascular risk prediction tools for populations in Asia. J Epidemiol Community Health 2007; 61: 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko M, Kim MT, Nam JJ. Assessing risk factors of coronary heart disease and its risk prediction among Korean adults: the 2001 Korea National Health and Nutrition Examination Survey. Int J Cardiol 2006; 110: 184–190 [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Giles WH, Mokdad AH. The distribution of 10‐Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 2004; 43: 1791–1796 [DOI] [PubMed] [Google Scholar]


