Abstract
This study was initiated to identify clinical and dietary parameters that predict efficacy of dipeptidyl peptidase‐4 inhibitors. A total of 72 untreated Japanese patients with type 2 diabetes who received DPP‐4 inhibitors (sitagliptin, alogliptin or vildagliptin) for 4 months were examined for changes of glycated hemoglobin (HbA1c) and body mass index (BMI), and self‐administered 3‐day food records, as well as serum levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). DPP‐4 inhibitors significantly reduced HbA1c (before initiation of DPP‐4 inhibitors 7.2 ± 0.7%, 4 months after initiation of DPP‐4 inhibitors 6.7 ± 0.6% [paired t‐test, P < 0.01 vs before]). Multiple regression analysis showed that changes of HbA1c were significantly correlated with baseline HbA1c, as well as estimated intake of fish. Furthermore, changes of HbA1c were significantly correlated with serum levels of EPA (r = −0.624, P < 0.01) and DHA (r = −0.577, P < 0.01). HbA1c reduction by DPP‐4 inhibitors is significantly correlated with estimated intake of fish and serum levels of EPA and DHA. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2012.00214.x, 2012)
Keywords: Dipeptidyl peptidase‐4 inhibitor, n‐3 Polyunsaturated fatty acid, Type 2 diabetes
Introduction
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors improve glycemic control in patients with type 2 diabetes by preventing degradation of two incretin hormones, glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), that are secreted from the intestine on ingestion of various nutrients1,2. Recent studies have shown associations of DPP‐4 inhibitors’ efficacy with age and fasting plasma glucose levels, as well as baseline glycated hemoglobin (HbA1c) and body mass index (BMI)3–5, but clinical parameters that predict the efficacy of DPP‐4 inhibitors are largely unknown. The present study showed that alterations in HbA1c level on administration of DPP‐4 inhibitors as monotherapy are associated with estimated intake of fish, estimated intake of dietary n‐3 polyunsaturated fatty acid (PUFA), and serum levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
Materials and Methods
The protocol was approved by the ethics committee of Kansai Electric Power Hospital, and carried out in accordance with the principles of the Declaration of Helsinki. A total of 72 untreated Japanese patients with type 2 diabetes (the Japan Diabetes Society criteria of 20106; age 64.6 ± 11.3 years; duration of diabetes 9.0 ± 8.9 years; baseline HbA1c 7.2 ± 0.7%; BMI 24.5 ± 4.3 kg/m2) who had been on diet and exercise therapies participated in the current study. DPP‐4 inhibitors (sitagliptin, alogliptin or vildagliptin) were given for 4 months and no other antidiabetic drugs were used during the period. A total of 59 patients received sitagliptin, 12 received alogliptin and one received vildagliptin. HbA1c levels were determined before the initiation of DPP‐4 inhibitors and 4 months after the initiation of DPP‐4 inhibitors, and were shown in National Glycohemoglobin Standardization Program values, as recommended by the Japan Diabetes Society6. Fasting serum levels of DHA, EPA and arachidonic acid (AA) were determined based on fatty acid composition of total lipids including phospholipids, triglycerides and cholesteryl esters in the serum of 20 patients (age 63.1 ± 11.7 years; duration of diabetes 6.7 ± 5.7 years; baseline HbA1c 7.0 ± 0.8%; BMI 24.2 ± 3.1 kg/m2) before initiation of DPP‐4 inhibitors. Self‐administered 3‐day food records, which were recorded during the 4‐month period, were analyzed for estimated intake of various nutrients using Healthy Maker Pro 501 (Mushroomsoft Co., Ltd., Okayama, Japan). All statistical calculations were carried out using PASW Statistics 18 (SAS Institute Inc., Cary, NC, USA), including linear regression analyses of the associations between changes in HbA1c levels and various parameters. Multiple linear regression analyses were carried out to identify the parameters potentially associated with HbA1c reduction, and simple regression analyses were carried out to evaluate their contributions. A P‐value of <0.05 was taken to show significant difference. Values are shown as mean ± SD.
Results
In the present study, DPP‐4 inhibitors, similarly to previous reports1, significantly reduced HbA1c levels, but not bodyweight (before initiation of DPP‐4 inhibitors 7.2 ± 0.7%; 4 months after initiation of DPP‐4 inhibitors 6.7 ± 0.6% [paired t‐test, P < 0.01 vs before]). Multiple regression analysis of HbA1c reduction (ΔHbA1c) taking into account sex, age, duration of diabetes, BMI, baseline HbA1c and estimated intake of various food categories in 3‐day food records showed that ΔHbA1c was well correlated with baseline HbA1c, but not with BMI (Table 1). ΔHbA1c also showed a significant association with estimated intake of fish and seafood in the food records (Figure 1a and Table 1). Among fish and seafood, estimated intake of fish, but not shellfish and other seafood, showed a significant association with ΔHbA1c (Table S1). The beneficial effects of fish on human health have been partly attributed to PUFA, such as EPA and DHA7. We therefore investigated the association of ΔHbA1c with estimated intake of PUFA. ΔHbA1c was significantly correlated with estimated intake of EPA and DHA, along with baseline HbA1c (Figure 1b and Table S2). As serum EPA and DHA levels might serve as markers for intake of corresponding fatty acids8 (Figure S1), we analyzed associations of ΔHbA1c with serum EPA and DHA levels. Serum levels of EPA and DHA, but not n‐6 PUFA arachidonic acid, were well correlated with ΔHbA1c (Figure 1c). Although ΔHbA1c reduction showed a significant association with estimated intake of milk products (Table 1), we were unable to find nutrients in milk products, including saturated fatty acids, that were responsible for the association.
Table 1. Association of glycated hemoglobin reduction and estimated intake of various food categories.
| B | SE | β | P | |
|---|---|---|---|---|
| Sex | 0.065 | 0.130 | 0.065 | 0.621 |
| Age (years) | 0.005 | 0.010 | 0.103 | 0.583 |
| Duration of diabetes (years) | 0.007 | 0.009 | 0.119 | 0.402 |
| Baseline HbA1c (NGSP) | −0.375 | 0.120 | −0.451 | 0.005 |
| BMI | 0.013 | 0.018 | 0.100 | 0.474 |
| Cereals | 0.001 | 0.001 | 0.132 | 0.328 |
| Potatoes and starchy flours | 0.002 | 0.003 | 0.077 | 0.592 |
| Sugar and sweeteners | 0.013 | 0.016 | 0.109 | 0.414 |
| Beans | 0.002 | 0.001 | 0.262 | 0.075 |
| Nuts and seeds | −0.010 | 0.025 | −0.067 | 0.694 |
| Vegetables | 0.000 | 0.000 | 0.160 | 0.391 |
| Fruits | 0.000 | 0.001 | 0.014 | 0.940 |
| Mushrooms | 0.005 | 0.007 | 0.109 | 0.476 |
| Seaweeds | −0.005 | 0.024 | −0.031 | 0.842 |
| Fish and seafood | −0.006 | 0.002 | −0.475 | 0.003 |
| Meats | −0.004 | 0.002 | −0.297 | 0.077 |
| Eggs | 0.001 | 0.003 | 0.057 | 0.720 |
| Milk products | −0.002 | 0.001 | −0.343 | 0.042 |
| Lipids | 0.000 | 0.016 | −0.002 | 0.990 |
| Snacks | 0.004 | 0.002 | 0.230 | 0.104 |
| Beverages | 0.000 | 0.000 | −0.120 | 0.393 |
Multiple regression analysis regarding changes of glycated hemoglobin (HbA1c) levels (ΔHbA1c) by taking into account sex, age, duration of diabetes, body mass index (BMI), baseline HbA1c (National Glycohemoglobin Standardization Program [NGSP]) and estimated intake of various food categories in 3‐day food records in 72 patients with type 2 diabetes. Statistical calculation was carried out using PASW Statistics 18 (SAS Institute Inc.). B and β denote non‐standardized and standardized regression coefficients, respectively. For analysis of changes of HbA1c levels, the correlation coefficient squared (R2) was 0.550 and the F‐value with 15 degrees of freedom was 3.499 for a P‐value of 0.003.
Figure 1.
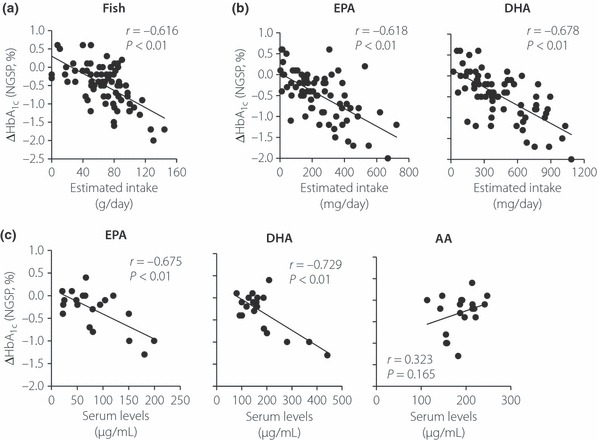
(a) Correlation between estimated intake of fish and seafood with glycated hemoglobin (HbA1c) reduction (National Glycohemoglobin Standardization Program [NGSP], %) 4 months after initiation of dipeptidyl peptidase‐4 inhibitors (ΔHbA1c; n = 72). (b) Correlation between estimated intake of eicosapentaenoic acid (EPA), docosahexaenoic (DHA) with ΔHbA1c (n = 72). (c) Correlation between serum levels of EPA, DHA and arachidonic acid (AA) with ΔHbA1c (n = 20). Linear regression analyses were carried out to calculate the correlation coefficient (r) and P‐values.
Discussion
We find that changes of HbA1c levels on administration of DPP‐4 inhibitors are associated with estimated intake of fish and estimated intake of dietary n‐3 PUFA, as well as serum EPA and DHA levels. Despite being a retrospective cohort study with a limited sample size, these findings are clinically important in two respects: (i) the efficacy of DPP‐4 inhibitors can be predicted by serum EPA and DHA levels; and (ii) consuming more fish with diet therapy can enhance the efficacy of DPP‐4 inhibitors. Furthermore, the current findings suggest that the differing efficacies of DPP‐4 inhibitors found among different ethnicities2 might be partly a result of differences in fish consumption.
Although many studies have shown the beneficial effects of dietary n‐3 PUFA from fish7, the mechanisms involving dietary n‐3 PUFA in DPP‐4 inhibitor efficacy have not yet been investigated. Although EPA and DHA have been shown to prevent excessive adiposity, thereby ameliorating insulin resistance in animal models, the effects of n‐3 PUFA on glycemic control in type 2 diabetes itself are somewhat controversial9. Interestingly, it has been found that EPA and DHA enhance GLP‐1 secretion, possibly through free fatty acid receptors, such as GPR120, in GLP‐1‐secreting cells and mice10,11. It is thus possible that dietary n‐3 PUFA and DPP‐4 inhibitors synergistically increase biologically‐active GLP‐1 levels to facilitate maintenance of glycemic control, but it remains to be determined whether EPA and DHA enhance GLP‐1 secretion in patients with type 2 diabetes. In addition, whether the present findings hold true for DPP‐4 inhibitors in general is not known, as most of the patients in the current study received sitagliptin or alogliptin, and vildagliptin patients were limited.
We find that the reduction of HbA1c by DPP‐4 inhibitors significantly correlates with estimated intake of fish, estimated intake of EPA and DHA, and serum levels of EPA and DHA.
Supplementary Material
Figure S1 Correlation between estimated intakesand serum concentrations of eicosapentaenoic acid (EPA) anddocosahexaenoic (DHA; n = 16).
Table S1 Association of glycated hemoglobin(HbA1c) reduction and estimated intake of fish,shellfish and other seafood
Table S2 Association of glycated hemoglobin(HbA1c) reduction and estimated intake ofpolyunsaturated fatty acids
Supporting info item
Supporting info item
Acknowledgements
The authors thank Dr Kentaro Murotani of the Translational Research Informatics Center for discussion on statistics, and Takeshi Murakami and Takuro Yamaguchi of Kansai Electric Power Hospital for technical support. We also thank Aya Sanagi of the Kansai Electric Power Hospital for secretarial assistance and Dalmen Mayer for his help in preparation of this manuscript. YS reports receiving consulting and/or speaker fees from Eli Lilly, MSD, Sanofi‐Aventis and Takeda. KT reports receiving speaker fees from Eli Lilly, Novartis and Sanofi‐Aventis. DY reports receiving speaker fees from Eli Lilly, MSD, Sanofi‐Aventis and Takeda. No other potential conflict of interest relevant to this article is reported. This study was partly supported by grants from the Japan Diabetes Foundation and the Diabetes Masters Conference.
References
- 1.Dicker D. DPP‐4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 2011; 34(Suppl 2): S276–S278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the Two Incretin Hormones: Similarities and Differences. J Diabetes Invest 2010; 1: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda H, Kubota A, Tanaka Y, et al. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract 2012; 95: e20–e22 [DOI] [PubMed] [Google Scholar]
- 4.Monami M, Cremasco F, Lamanna C, et al. Predictors of response to dipeptidyl peptidase‐4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev 2011; 27: 362–372 [DOI] [PubMed] [Google Scholar]
- 5.Nomiyama T, Akehi Y, Takenoshita H, et al. Contributing factors related to efficacy of the dipeptidyl peptidase‐4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2012; 95: e27–e28 [DOI] [PubMed] [Google Scholar]
- 6.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006; 296: 1885–1899 [DOI] [PubMed] [Google Scholar]
- 8.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n‐3 fatty acids in humans. Am J Clin Nutr 2006; 83: 1467S–1476S [DOI] [PubMed] [Google Scholar]
- 9.Fedor D, Kelley DS. Prevention of insulin resistance by n‐3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2009; 12: 138–146 [DOI] [PubMed] [Google Scholar]
- 10.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon‐like peptide‐1 secretion through GPR120. Nat Med 2005; 11: 90–94 [DOI] [PubMed] [Google Scholar]
- 11.Morishita M, Tanaka T, Shida T, et al. Usefulness of colon targeted DHA and EPA as novel diabetes medications that promote intrinsic GLP‐1 secretion. J Control Release 2008; 132: 99–104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Correlation between estimated intakesand serum concentrations of eicosapentaenoic acid (EPA) anddocosahexaenoic (DHA; n = 16).
Table S1 Association of glycated hemoglobin(HbA1c) reduction and estimated intake of fish,shellfish and other seafood
Table S2 Association of glycated hemoglobin(HbA1c) reduction and estimated intake ofpolyunsaturated fatty acids
Supporting info item
Supporting info item


