Self‐monitoring of blood glucose (SMBG) has increasingly been shown as one of the most powerful tools for achieving ideal glycemic control in patients with diabetes irrespective of diet control, oral hypoglycemic agents or insulin therapy1. It provides a timely reading of blood glucose levels and enables diabetes patients to more safely manage their condition, to detect hyperglycemia and hypoglycemia, and to generate information for adjusting medications, dietary content and physical activities. To date, results from most meta‐analyses have shown the clinical benefits of SMBG on glycemic control1. People with type 2 diabetes who carried out SMBG were reported to have lower rates of non‐fatal micro‐ and macrovascular events than those who did not carry out SMBG2. Thus, several academic bodies, including the International Diabetes Federation (IDF)3, have issued recommendations for the use of SMBG. However, patients as well as diabetes care professionals have found that carrying out the so‐called standardized or structured SMBG, including frequencies, patterns and times, and matching it with individual clinical needs is very confusing and impractical. The clinical suggestions are even more difficult to implement in areas where diabetes patients receive no reimbursement for test strips and glucometers. In this regard, a simple, easy and structured SMBG, such as the so‐called meals‐based testing or paired testing that covers both preprandial and postprandial glucose levels and minimizes glycemic variability, is clearly preferable.
It is widely acknowledged that proper use of SMBG is associated with a significant reduction of glycated hemoglobin (HbA1c), as compared with those not using SMBG. Likewise, structured SMBG, using a standardized approach, has been shown to be superior to free testing. In a study carried out in Japan, diabetes patients who were treated without insulin were randomized to carry out SMBG with no more than 10 blood glucose tests per month, specifically in the postprandial phase, as compared with those who carried out SMBG in a random manner. The data showed HbA1c reduction was significantly greater in those with a fixed structure as compared with those with free testing, showing the structured test was more beneficial4. Recently published results from the Structured Testing Program (STeP) study also confirmed that after a 12‐month intervention in insulin‐naïve type 2 diabetes patients, greater and significant reduction in mean HbA1c in the structured testing group was observed compared with that in the active control group5.
In 2009, the IDF guideline for SMBG in non‐insulin treated type 2 diabetes recommended several focused regimens, including five‐ and seven‐point profiles, a staggered SMBG regimen, meal‐based testing, detection/assessment fasting hyperglycemia, and detection of asymptomatic hypoglycemia3. Among these recommendations, meal‐based SMBG (before and after selected meals), I believe, is the most appealing, because it helps people with diabetes understand the effects of their meals in association with treatment regimens, assists clinicians in identifying postprandial hyperglycemia, guides therapeutic adjustments and provides more timely feedback regarding medication changes.
Recently, a group of European experts recommended two schemes of SMBG, ranging from less intensive to more intensive testing1, depending on whether patients are in states of newly‐diagnosed diabetes by lifestyle control or by oral antidiabetic agents or by insulin therapies. The less intensive testing scheme involved one paired meal testing; that is, preprandial and postprandial testing, daily to 3–7 days per week, whereas the intensive testing recommended seven testing points a day with frequencies of a minimum of 3 days per week to 1 week per month. The expert group emphasized that intensity of testing should be adjusted based on the quality of metabolic controls, treatment approaches and the risk of hypoglycemia.
Given the complexities of the clinical scenario, I would advocate the use of paired glycemic testing (or meal‐based testing) before and 2 h after meals, preferably three times a week covering breakfast, lunch and dinner (Figure 1). These regimens could serve as a basic start for SMBG in patients with type 2 diabetes. These recommended regimens also enable the patient to monitor glycemic excursions and identify any effect of dietary content, physical activities or medications that might influence glycemic variability6. There is increasing evidence that postprandial hyperglycemia and glycemic variability are associated with impairment of endothelial function and subsequent macrovascular complications7. This phenomenon seems more critical for Asian diabetes patients, who might be more prone to having postprandial hyperglycemia8. In this regard, the IDF issued a revised version of the SMBG guideline in 2011 and recommended that glucose be measured 1–2 h after a meal with a target of 160 mg/dL (9.0 mmol/L)3.
Figure 1.
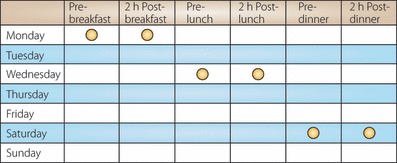
An example of paired glycemic testing of SMBG, covering breakfast, lunch and dinner, within 1 week.
It should be emphasized that even though SMBG can improve patients’ awareness of hyperglycemia or hypoglycemia, this information can be challenging and might lead to anxiety and even self‐blame1. Thus, SMBG can only be effective if reinforced by diabetes education. Only when the patient is fully informed about the treatment goals and how to achieve these goals can SMBG function as a tool to achieve better glycemic control.
In conclusion, SMBG has been proven to be a practical tool to improve glycemic control in patients with type 2 diabetes. However, SMBG should be implemented in a structured, standardized approach ranging from more intensive to less intensive schemes. Thus, for non‐insulin treated diabetes patients, a simple, acceptable alternative would be paired glycemic testing, measured before and 2 h after meals, covering three meals at least three times per week. A structured SMBG regimen, plus reinforcement through diabetes education, behavior and medication modifications might provide a useful measure for achieving better glycemic control. Further study to confirm that this regimen is practical and applicable is clearly warranted.
References
- 1.Schnell O, Alawi H, Battelino T, et al. Addressing schemes of self‐monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther 2011; 13: 959–965 [DOI] [PubMed] [Google Scholar]
- 2.Martin S, Schneider B, Heinemann L, et al. Self‐monitoring of blood glucose in type 2 diabetes and long‐term outcome: an epidemiological cohort study. Diabetologia 2006; 49: 271–278 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . Guideline on Self‐Monitoring of Blood Glucose in Non‐Insulin Treated Type 2 Diabetes. International Diabetes Federation, Brussels, 2009. [Google Scholar]
- 4.Shiraiwa T, Takahara M, Kaneto H, et al. Efficacy of occasional self‐monitoring of postprandial blood glucose levels in type 2 diabetic patients without insulin therapy. Diabetes Res Clin Pract 2010; 90: e91–e92 [DOI] [PubMed] [Google Scholar]
- 5.Polonsky WH, Fisher L, Schikman CH, et al. Structured self‐monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin‐treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care 2011; 34: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JS, Lin SD, Lee WJ, et al. Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24‐week, randomized, open‐label, parallel‐group comparison. Clin Ther 2011; 33: 1932–1942 [DOI] [PubMed] [Google Scholar]
- 7.Sheu WH, Rosman A, Mithal A, et al. Addressing the burden of type 2 diabetes and cardiovascular disease through the management of postprandial hyperglycaemia: an Asian‐Pacific perspective and expert recommendations. Diabetes Res Clin Pract 2011; 92: 312–321 [DOI] [PubMed] [Google Scholar]
- 8.Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev 2011; 27: 79–84 [DOI] [PubMed] [Google Scholar]


