Abstract
Aims/Introduction: The computed tomography (CT) value of the pancreas was examined across the range of glucose tolerance, and the relationships between pancreatic CT values and factors responsible for glucose intolerance were analyzed.
Materials and Methods: A total of 167 health‐check examinees were classified into normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and diabetes mellitus (DM) according to 75 g oral glucose tolerance test (OGTT). Pancreatic and hepatic CT values were estimated at decreasing stages of glucose tolerance. The association of CT values of the pancreas and the indices of glucose tolerance were analyzed.
Results: Insulinogenic index (II) was decreased from NGT through IGT to DM. Mean pancreatic CT value was decreased significantly from NGT through IGT to DM. Mean area under the curves of glucose (AUC‐G) was significantly associated with II and insulin sensitivity index (ISI) composite in univariate analysis. In multiple regression analysis, II was most strongly inversely correlated with mean AUC‐G, suggesting that II is the strongest determinant of glucose tolerance in Japanese. In addition, II was significantly associated with mean pancreatic CT value in univariate analysis. In multiple regression analysis, mean pancreatic CT value was strongly correlated with II.
Conclusions: Pancreatic CT values were significantly decreased from NGT through IGT to DM. II was the strongest determinant of glucose tolerance, and was significantly influenced by pancreatic CT values. Thus, pancreatic fat deposition might impair insulin secretion in the early stage of development of type 2 diabetes, before overt deterioration of glucose tolerance. (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2012.00212.x, 2012)
Keywords: Computed tomography, Insulin secretion, Pancreas
Introduction
The development of type 2 diabetes is characterized by impaired insulin secretion and/or action that interferes with plasma glucose homeostasis1,2, but the mechanism is not fully clarified. In a histopathological autopsy study, diabetic patients showed more frequent and severe intralobular and interacinar fibrosis, fat deposition, and atrophy of the parenchyma than control subjects3. Fat deposition in the pancreas is one of the most frequent histological changes in aging and overweight. Schmitz‐Moormann et al.4 found that the adipose tissue and total weight of the pancreas was positively correlated with age and bodyweight in 50 autoptic pancreases. In diabetic patients, studies of abdominal ultrasonography reported increased pancreatic echogenicity induced by pancreatic fat deposition5. In rodent models of obesity and diabetes, pancreatic steatosis was found to result in hyperglycemia with failure of β‐cell function6,7. A human study suggested that pancreatic lipid content, as measured by proton‐magnetic resonance spectroscopy (1H‐MRS), might contribute to reduced β‐cell function8. We show here that pancreatic steatosis might also play a role in the pathogenesis of type 2 diabetes as a result of β‐cell dysfunction.
Computed tomography (CT) is widely used for imaging of abdominal organs. Diagnostic criteria of hepatic steatosis assessed by attenuation of liver CT value has been well documented. However, there are few studies that compare pancreatic CT values at the different stages of glucose tolerance in relation to the factors responsible for glucose tolerance, insulin secretory capacity and insulin sensitivity.
In the present study, we examined the CT values of pancreas across the range of glucose tolerance, and analyzed the relationship of CT values of the pancreas with insulin secretory capacity and insulin sensitivity.
Materials and Methods
A total of 167 Japanese participants aged 36–87 years were recruited to undergo 75 g oral glucose tolerance test (OGTT) because of having a positive urine glucose test, >5.6% glycated hemoglobin (HbA1c) level, >100 mg/dL fasting plasma glucose (FPG) level or a family history of diabetes at initial examination for a medical check‐up at Takasago Municipal Hospital. The HbA1c value was estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value calculated by the formula HbA1c = HbA1c (Japan Diabetes Society; JDS) +0.4%, considering the relational expression of HbA1c (JDS) measured by the previous Japanese standard substance and measurement methods, and HbA1c (NGSP)9. Standard OGTT was administered according to the National Diabetes Data Group recommendations, which require the subjects to fast overnight for 10–16 h. Fasting, 0.5, 1, 1.5 and 2 h blood samples for determination of plasma glucose and serum insulin levels after oral administration of 75 g OGTT were obtained. For international comparisons, World Health Organization criteria (1998) were used to evaluate the results of OGTT. Subjects in the three categories of glucose tolerance, normal glucose tolerance (NGT; n = 53: FPG level <110 mg/dL and 2 h‐PG level <140 mg/dL), impaired glucose tolerance (IGT; n = 52: FPG level <126 mg/dL and 140 mg/dL ≤ 2h‐PG level < 200 mg/dL) and diabetes mellitus (DM; n = 62: FPG level ≥126 mg/dL and/or 2h‐PG level ≥200 mg/dL) were enrolled in the study. Subjects who had significant pancreatic disease, hepatic or renal dysfunction, endocrine or malignant disease, a history of heavy exercise, or a history of gastrectomy were excluded.
Estimation of Insulin Secretion and Insulin Sensitivity
Early‐phase insulin secretion was evaluated by insulinogenic index (II) and insulin sensitivity index (ISI) composite was used to evaluate systemic insulin sensitivity during OGTT. The disposition index was expressed as the multiplex of the indices of insulin secretion and insulin sensitivity. The calculations were as follows:
 |
Pancreas and Liver CT Values
CT images were acquired with standard clinical abdominal CT protocol utilizing a multi‐slice CT scanner (LightSpeed Pro16; GE Healthcare UK Ltd, Little Chalfont, England). Pancreatic CT attenuations were determined by calculating the mean Hounsfield unit of two regions in the pancreas (one in the pancreas head, and the other in the pancreas body and tail); hepatic CT attenuations were determined similarly on the basis of the mean Hounsfield unit of two regions (one in the right lobe and one in the left lobe of the liver).
Statistical Analysis
Data are presented as mean values ± SE. Statistical analyses were carried out using SPSS (Statistical Package for Social Science) II for Windows, version 11.01 J. Differences between groups were calculated by Student’s t‐test for unpaired comparisons. The association of mean pancreas CT value and each measure of the variables was assessed by univariate and multiple regression analysis. P < 0.05 was considered statistically significant.
Results
Table 1 shows the clinical and metabolic characteristics of 167 Japanese subjects classified with NGT, IGT and DM according to the results of OGTT. There were no significant differences in respect to age and body mass index (BMI). In respect to free fatty acid (FFA), that in DM was significantly higher than that in NGT. There were no significant differences in history of alcohol intake, blood pressure, hepatobiliary enzyme and cholinesterase (ChE) among the three subgroups. FPG and the mean area under the curves of glucose (AUC‐G) were significantly increased from NGT through IGT to DM.
Table 1. Clinical characteristics of normal glucose tolerance, impaired glucose tolerance and diabetes mellitus.
| NGT | IGT | DM | |
|---|---|---|---|
| n | 53 | 52 | 62 |
| Male/female | 29/24 | 28/24 | 39/23 |
| Age (years) | 61.2 ± 1.4 | 64.8 ± 1.4 | 64.6 ± 1.1 |
| BMI (kg/m2) | 24.4 ± 0.5 | 24.5 ± 0.5 | 24.8 ± 0.4 |
| Abdominal circumference (cm) | 86.8 ± 1.4 | 87.3 ± 1.3 | 88.2 ± 1.2 |
| TG (mg/dL) | 124.8 ± 10.7 | 123.3 ± 9.1 | 131.4 ± 6.0 |
| LDL (mg/dL) | 132.3 ± 3.7 | 127.1 ± 3.8 | 129.1 ± 4.4 |
| HDL (mg/dL) | 67.5 ± 2.7 | 68.1 ± 2.5 | 64.9 ± 2.3 |
| FFA (mg/dL) | 387.4 ± 28.0 | 451.3 ± 26.6 | 491.6 ± 32.7* |
| Fasting PG (mg/dL) | 101.1 ± 0.8 | 107.8 ± 1.4** | 128.3 ± 2.8##** |
| mean AUC‐G (mg/dL) | 135.7 ± 2.5 | 169.4 ± 3.7** | 225.1 ± 5.7##** |
| Fasting IRI (ull/mL) | 7.0 ± 0.6 | 6.7 ± 0.8 | 8.1 ± 0.6 |
| MeanAUC‐l (uU/mL) | 54.9 ± 5.3 | 46.5 ± 4.5 | 53.6 ± 4.8 |
| HbA1C (%) | 5.75 ± 0.05 | 5.94 ± 0.04** | 6.58 ± 0.10##** |
| ISI composite | 5.85 ± 0.41 | 5.64 ± 0.38 | 4.36 ± 0.41#* |
| insulinogenic index | 1.18 ± 0.20 | 0.71 ± 0.21 | 0.39 ± 0.05** |
| disposition index | 5.87 ± 0.99 | 3.37 ± 0.91 | 1.38 ± 0.22#** |
HDL, high‐density lipoprotein cholesterol; IRI, immunoreactive insulin; LDL, low‐density lipoprotein cholesterol; TG, triglyceride.
Data are means ± standard error.
* P < 0.05 vs normal glucose tolerance (NGT), **P < 0.01 vs NGT, #P < 0.05 vs impaired glucose tolerance (IGT), ##P < 0.01 vs IGT.
Insulin sensitivity was evaluated by ISI composite; insulin secretory capacity was evaluated by II. ISI composite was decreased gradually from NGT through IGT to DM, and showed a significant difference between NGT and DM (P < 0.05), and between IGT and DM (P < 0.05). II also was decreased from NGT through IGT to DM, and there were significant differences between NGT and DM (P < 0.01).
Figure 1 shows CT values of the liver and pancreas of the three subgroups. Mean pancreatic CT value was decreased significantly from NGT through IGT to DM (DM vs NGT P < 0.01, DM vs IGT P < 0.05, IGT vs NGT P < 0.05; Figure 1a). Pancreatic head CT value was decreased from NGT through IGT to DM, and diabetic patients had significantly lower values compared with those with NGT (P < 0.01) and IGT (P < 0.01) subjects (Figure 1b). Pancreatic body/tail CT value was decreased from NGT through IGT to DM (DM vs NGT P < 0.01, DM vs IGT P = 0.189, IGT vs NGT P < 0.01; Figure 1c).
Figure 1.
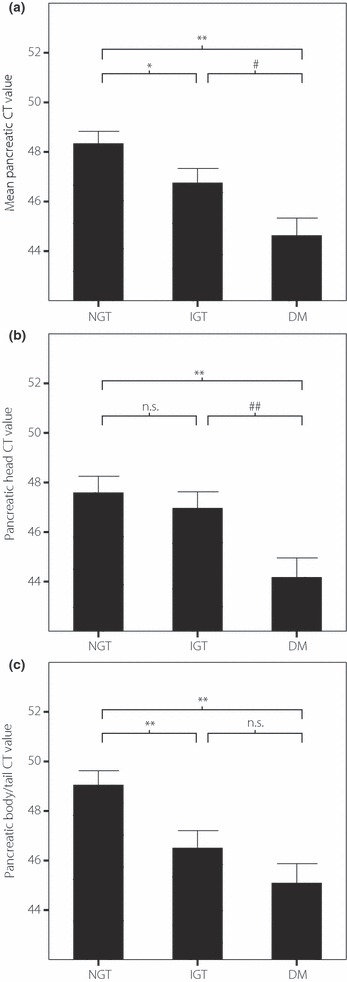
Pancreatic head, body/tail and mean computed tomography (CT) values of normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and diabetes mellitus (DM) are shown (*P < 0.05 vs NGT, **P < 0.01 vs NGT, #P < 0.05 vs IGT, ##P < 0.01 vs IGT). Pancreatic head and body/tail CT value are decreased from NGT through IGT to DM. In addition, mean pancreatic CT value is decreased significantly from NGT through IGT to DM (DM vs NGT P < 0.01, DM vs IGT P < 0.05, IGT vs NGT P < 0.05).
The correlation of II to measures of variables in univariate and multiple regression analysis are listed in Table 2. II was significantly associated with mean pancreatic CT value, triglyceride (TG), high‐density lipoprotein (HDL) cholesterol, abdominal circumference and BMI in univariate analysis. The relationship between mean pancreatic CT values and II are shown in Figure 2a. In multiple regression analysis, II was significantly associated with abdominal circumference and mean pancreatic CT value.
Table 2. Correlation of insulinogenic index to measures of variables.
| r | P | β | |
|---|---|---|---|
| Age | −0.02 | 0.799 | − |
| Sex | −0.073 | 0.345 | − |
| BMI | 0.248 | 0.001 | − |
| Abdominal circumference (cm) | 0.263 | 0.001 | 0.287 |
| TG (mg/dL) | 0.241 | 0.002 | − |
| LDL (mg/dL) | 0.032 | 0.678 | − |
| HDL (mg/dL) | −0.197 | 0.011 | − |
| FFA (mg/dL) | −0.129 | 0.158 | − |
| Mean pancreatic CT value | 0.247 | 0.001 | 0.272 |
FFA, free fatty acid; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; TG, triglyceride.
Figure 2.
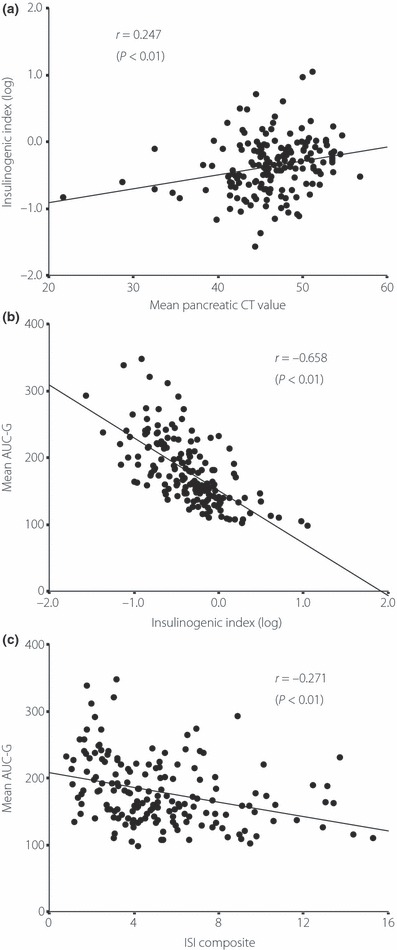
(a) Mean pancreatic computed tomography (CT) values were significantly positively correlated with insulinogenic index (II) in multiple regression analysis (r = 0.247, P < 0.01). (b) Insulinogenic index and (c) ISI composite were significantly inversely correlated with area under the curves of glucose (AUC‐G); however, II most strongly inversely correlated with AUC‐G.
The correlation between mean AUC‐G representing glucose tolerance and each measure of the factors was investigated (Table 3). Mean AUC‐G had a significant association with II, ISI composite and FFA. In multiple regression analysis, II and ISI composite showed significant correlation with mean AUC‐G. ISI composite representing insulin sensitivity was significantly inversely correlated with mean AUC‐G, but II was most strongly inversely correlated with AUC‐G (Figure 2b,c).
Table 3. Correlation of mean area under the curves of glucose to measures of variables.
| r | P | β | |
|---|---|---|---|
| Age | 0.007 | 0.931 | − |
| Sex | 0.134 | 0.085 | − |
| BMI | 0.063 | 0.421 | − |
| Abdominal circumference (cm) | 0.051 | 0.509 | − |
| TG (mg/dL) | 0.055 | 0.480 | − |
| LDL (mg/dL) | 0.01 | 0.902 | − |
| HDL (mg/dL) | −0.046 | 0.555 | − |
| FFA (mg/dL) | 0.263 | 0.003 | − |
| insulinogenic index (log) | −0.658 | <0.001 | −0.788 |
| ISI composite | −0.271 | <0.001 | −0.387 |
BMI, body mass index; FFA, free fatty acid; HDL, high‐density lipoprotein cholesterol; ISI, insulin sensitivity index; LDL, low‐density lipoprotein cholesterol; TG, triglyceride.
Discussion
In the present study, we found that pancreatic CT values are significantly decreased from NGT through IGT to DM in the early stage of the development of diabetes. Pancreatic CT values also had a significant correlation with II, which was the strongest determinant of glucose tolerance and was significantly influenced by pancreatic CT values. Thus, pancreatic fat deposition might impair insulin secretion early in the development of glucose intolerance.
Ectopic fat accumulation in skeletal muscle, liver and heart is associated with dysfunction of these organs10,11. However, it is not yet known whether fat accumulation in the pancreas causes β‐cell dysfunction. Saisho et al.12 found that pancreatic fat content increased with aging and obesity (mean age was approximately 65 years and mean BMI was 27.5 kg/m2), but was not further increased in type 2 diabetes. In the present study, BMI resulted in having no significant correlation with mean pancreatic CT value in univariate analysis (mean pancreatic CT value vs BMI; r = −0.104, P = 0.183). This might be a result of the different profile of the subjects examined between the studies. The mean BMI of our subjects was 24–25 kg/m2, which is the common feature of Japanese diabetic patients who do not show morbid obesity. It is comparable with the mean BMI of the representative epidemiological studies in Japanese being approximately 24 kg/m2. In addition, aging did not have a significant correlation with mean pancreatic CT value in the present study (mean pancreatic CT value vs age; r = −0.096, P = 0.215). The reason might be because the main subjects in the present study were 40–75 years‐of‐age, which is the common feature of exhibiting type 2 diabetes in Japanese; in contrast, the age groups of their study12 were widely distributed from birth to 100 years‐of‐age. It is still possible pancreatic fat deposition by aging results in the decrease of insulin secretion and glucose intolerance.
In the present study, we examined health‐check examinees who had not been diagnosed with overt diabetes or received any medication in relation to glucose intolerance. Thus, pancreatic steatosis might begin with a decline of insulin secretion from the very early stage of glucose intolerance. Tushuizen et al.8 reported that in overt type 2 diabetic patients (BMI of approximately 30 kg/m2) treated with diet and medication, pancreatic fat content measured by 1H‐MRS was higher than in non‐diabetic control subjects matched by age and BMI, and pancreatic fat content was significantly associated insulin secretory capacity in non‐diabetic subjects. Using 1H‐MRS, Heni et al.13 reported a negative association between pancreatic fat content and insulin secretion in subjects with impaired glucose regulation. These reports using 1H‐MRS agree with our data in the present study on pancreatic steatosis using pancreatic CT value.
The mechanism by which pancreatic steatosis might affect insulin secretion early in the disease is not clear at present. In non‐obese children with a heterozygous carboxyl‐ester lipase mutation that causes diabetes, pancreatic fat content measured by ultrasound and 1H‐MRS is increased, and the first‐phase insulin response to intravenous glucose is significantly decreased14. A rodent study reported that lipid deposition in pancreatic islet cells as a result of a high‐fat/high‐glucose diet can damage pancreatic β‐cells and elevate plasma glucose levels15. Abnormal fat deposition of the pancreas has many harmful effects, including oxidative stress, inflammation and apoptosis of the islets16.
As islet volume comprises just ∼2% of total pancreas mass, the CT attenuations of the pancreas induced by increased adipose mass are related to signals from outside the β‐cell. However, it is difficult to discern where fatty infiltration occurs within the pancreas using 1H‐MRS, CT or any other of the in vivo examinations. According to some studies of pathological observations in the human pancreas17,18, fat deposition appears mainly in interlobular septa rather than within cells. As the β‐cell was suggested to be differentiated from the area near the pancreatic duct, it is possible that β‐cell replication is decreased by the fatty infiltration that causes the decline in insulin secretion. Fatty replacement of the pancreas seems to occur primarily in the head of the pancreas where acinar tissue is most abundant, and it is reported that pancreatic islets are relatively more abundant in the caudal part than the head19,20. In the present study, we observed attenuations of CT values in the pancreatic head, body/tail and their mean from NGT through IGT to DM. However, the CT values in the pancreatic caudal part, where islets are more abundant, did not show a stronger decline than that in pancreatic head. Further studies using different populations and methods are required to clarify more precisely how regional fat distribution within the pancreas contributes to impaired insulin secretion. Previous studies have shown that liver steatosis can be evaluated by attenuation of liver CT value, and that fatty liver is associated with uptake of glucose in liver in type 2 diabetes21,22. In the present study, mean hepatic CT value decreased gradually from NGT through IGT to DM, and showed a significant difference between NGT and DM (P < 0.05). In addition, mean hepatic CT value was significantly associated with ISI composite (r = 0.233) in univariate analysis without a significant correlation with II (r = −0.137; data not shown). These results support evidence that liver CT attenuation has a closer relationship with insulin resistance than with insulin secretory capacity.
Several studies have found that insulin secretory capacity is lower in Japanese than in other populations23,24. We found that decreased insulin secretory capacity contributes more strongly to reduced glucose tolerance than insulin resistance in Japanese using minimal model analysis and OGTT25. Indeed, in the present study in Japanese, glucose tolerance assessed by AUC‐G was shown to be most strongly correlated with II.
We described significant pancreatic CT attenuations from NGT through IGT to DM in the very early stage of the development of diabetes. Additionally, there was a significant positive correlation between II and pancreatic CT values. Because II is the strongest determinant of glucose tolerance in the Japanese population, we propose that pancreatic fat infiltration early in the course of the development of type 2 diabetes might contribute to impaired insulin secretion that results in deterioration of glucose tolerance.
Acknowledgements
This research was supported by the Kobe Translational Research Cluster, the Knowledge Cluster Initiative and Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the Japanese Diabetes Society and Manpei Suzuki Diabetes Foundation. We thank Dr Toshiyuki Takemoto, Dr Takayuki Kawaguchi, Dr Yutaka Shigeyama, Dr Shinichi Kinoshita, Dr Kazuo Takazawa, Dr Yoshiaki Hirosue, Dr Takeo Goto and the staff of the central radiation section in Takasago Municipal Hospital, Use Techno Corporation, Ono Pharmaceutical Co. Ltd., Abbott Japan Co. Ltd., and Dainippon Sumitomo Pharma Co. Ltd. for their help in this study. The authors do not have any financial support or relationships that pose a conflict of interest.
References
- 1.Porte D Jr. Banting lecture 1990. Beta‐cells in type 2 diabetes mellitus. Diabetes 1991; 40: 166–180 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta‐cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687 [DOI] [PubMed] [Google Scholar]
- 3.Putzke HP, Friedrich G. Pancreatopathy in diabetes mellitus. Zentralbl Allg Pathol 1986; 131: 37–44 [PubMed] [Google Scholar]
- 4.Schmitz‐Moormann P, Pittner PM, Heinze W. Lipomatosis of the pancreas. A morphometrical investigation. Pathol Res Pract 1981; 173: 45–53 [DOI] [PubMed] [Google Scholar]
- 5.Morrison EY, Moule N, Ragoobirsingh D. Radiological evaluation of the pancreas in malnutrition‐related (phasic insulin dependent) diabetes mellitus. J Natl Med Assoc 1991; 83: 59–62 [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Hirose H, Ohneda M, et al. Beta‐cell lipotoxicity in the pathogenesis of non‐insulin‐dependent diabetes mellitus of obese rats: impairment in adipocyte‐beta‐cell relationships. Proc Natl Acad Sci USA 1994; 23: 10878–10882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milburn JL Jr, Hirose H, Lee YH, et al. Pancreatic beta‐cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem 1995; 3: 1295–1299 [DOI] [PubMed] [Google Scholar]
- 8.Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and beta‐cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30: 2916–2921 [DOI] [PubMed] [Google Scholar]
- 9.The Committee of Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krssak M, Roden M. The role of lipid accumulation in liver and muscle for insulin resistance and type 2 diabetes mellitus in humans. Rev Endocr Metab Disord 2004; 5: 127–134 [DOI] [PubMed] [Google Scholar]
- 11.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008; 52: 1793–1799 [DOI] [PubMed] [Google Scholar]
- 12.Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type‐2 diabetes. Clin Anat 2007; 20: 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heni M, Machann J, Staiger H, et al. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev 2010; 26: 200–205 [DOI] [PubMed] [Google Scholar]
- 14.Raeder H, Haldorsen IS, Ersland L, et al. Pancreatic lipomatosis is a structural marker in nondiabetic children with mutations in carboxyl‐ester lipase. Diabetes 2007; 56: 444–449 [DOI] [PubMed] [Google Scholar]
- 15.Yin W, Liao D, Kusunoki M, et al. NO‐1886 decreases ectopic lipid deposition and protects pancreatic beta cells in diet‐induced diabetic swine. J Endocrinol 2004; 180: 399–408 [DOI] [PubMed] [Google Scholar]
- 16.Robertson RP, Harmon J, Tran POT, et al. Beta‐cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004; 53(Suppl. 1): S119–S124 [DOI] [PubMed] [Google Scholar]
- 17.Nghiem DD, Olson PR, Ormond D. The “fatty pancreas allograft”: anatomopathologic findings and clinical experience. Transplant Proc 2004; 36: 1045–1047 [DOI] [PubMed] [Google Scholar]
- 18.Tham RT, Heyerman HG, Falke TH, et al. Cystic fibrosis: MR imaging of the pancreas. Radiology 1991; 179: 183–186 [DOI] [PubMed] [Google Scholar]
- 19.Walters MN. Adipose atrophy of the exocrine pancreas. J Pathol Bacteriol 1966; 92: 547–557 [DOI] [PubMed] [Google Scholar]
- 20.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol 1984; 15: 677–683 [DOI] [PubMed] [Google Scholar]
- 21.Longo R, Ricci C, Masutti F, et al. Fatty infiltration of the liver: quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol 1993; 28: 297–302 [PubMed] [Google Scholar]
- 22.Ryysy L, Hakkinen A‐M, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 2000; 49: 749–758 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Fukushima M, Usami M, et al. Factors responsible for development from normal glucose tolerance to isolated postchallenge hyperglycemia. Diabetes Care 2003; 26: 1211–1215 [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Miettinen H, Gaskill SP, et al. Decreased insulin secretion and increased insulin resistance are independently related to the 7‐year risk of NIDDM in Mexican‐Americans. Diabetes 1995; 44: 1386–1391 [DOI] [PubMed] [Google Scholar]
- 25.Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835 [DOI] [PubMed] [Google Scholar]


