Abstract
Aims/Introduction
The efficacy and safety of sitagliptin, a highly selective dipeptidyl peptidase‐4 inhibitor, when added to metformin monotherapy was examined in Japanese patients with type 2 diabetes.
Materials and Methods
In this 52‐week, add‐on to metformin study, 149 patients were randomly assigned to receive sitagliptin 50 mg or placebo once daily in a double‐blind fashion for 12 weeks. Thereafter, all patients who completed the double‐blind period of the study received open‐label sitagliptin 50 mg once daily for 40 weeks, with the investigator option of increasing sitagliptin to 100 mg once daily for patients who met predefined glycemic thresholds.
Results
After 12 weeks of treatment, the mean change from baseline in glycated hemoglobin (HbA1c) significantly decreased with sitagliptin relative to placebo (between‐group difference [95% confidence interval] = −0.7% [−0.9 to −0.5] P < 0.001). At week 12, the mean changes in 2‐h post‐meal glucose (−2.6 mmol/L [−3.5 to −1.7]) and fasting plasma glucose (−1.0 mmol/L [−1.3 to −0.6]) also decreased significantly with sitagliptin relative to placebo (P < 0.001 for both). Significant improvements from baseline in glycemic control were also observed in the open‐label period through to week 52. There were no differences between treatment groups in the incidence of adverse events (AEs), including hypoglycemia and predefined gastrointestinal AEs (nausea, vomiting and diarrhea) during the double‐blind period, with similar findings in the open‐label period.
Conclusions
Over a period of 52 weeks, the addition of sitagliptin once‐daily to ongoing metformin therapy was efficacious and generally well tolerated in Japanese patients with type 2 diabetes. This trial was registered with ClinicalTrials.gov (no. NCT00363948).
Keywords: Metformin, Sitagliptin, Type 2 diabetes
Introduction
Type 2 diabetes mellitus is a progressive metabolic disorder characterized by persistent high levels of blood glucose1. The etiology of type 2 diabetes principally includes impaired insulin secretion, increased insulin resistance and increased hepatic glucose production2. Therapy with a single oral hypoglycemic agent (OHA), in addition to diet and exercise, is often insufficient to provide or maintain adequate glycemic control and patients with type 2 diabetes frequently require treatment with multiple OHAs3.
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors are a new class of OHAs that stabilize the circulating levels of active incretins, glucagon‐like peptide 1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), thereby increasing glucose‐mediated insulin secretion; GLP‐1 also reduces glucagon secretion4. Sitagliptin is an oral, highly selective DPP‐4 inhibitor that has been shown in multinational studies to improve glycemic control and measures of β‐cell function, and to be well tolerated as monotherapy5, in combination with other OHAs, including metformin6, and in combination with insulin9.
Metformin is an OHA that reduces insulin resistance in muscle and adipose tissue, suppresses hepatic glucose overproduction11, and increases total GLP‐1 levels12. Metformin is recommended as a first‐line treatment for type 2 diabetes in the latest guidelines published by American Diabetes Association/European Association for the Study of Diabetes and International Diabetes Federation13.
Type 2 diabetes in Japanese patients is characterized mainly by β‐cell dysfunction; in particular, a decrease in early phase insulin secretion15. DPP‐4 inhibitors as monotherapy have also been shown to be as efficacious and well tolerated in other Asian patients with type 2 diabetes who have reduced β‐cell function as in their Japanese counterparts17. Therefore, DPP‐4 inhibitors might be a suitable option for Japanese patients with type 2 diabetes. Furthermore, given that sitagliptin and metformin have different mechanisms of action that have been shown to be complementary to reducing blood glucose levels12, the use of these two agents in combination is of interest. The present phase III study evaluated the efficacy and safety of sitagliptin as an add‐on therapy to ongoing metformin over a period of 52 weeks in Japanese patients with type 2 diabetes.
Methods
This was a multicenter, double‐blind, placebo‐controlled, randomized clinical trial carried out at 39 sites in Japan from August 2006 to February 2008. The present study was designed and carried out in accordance with the principles of Good Clinical Practice. The study protocol was approved by the institutional review board at each study site and all patients provided written informed consent before entering the study.
Male and female patients with type 2 diabetes aged ≥20 and <75 years were eligible to participate in the present study. Patients with type 1 diabetes or with high serum creatinine levels (male: >100.8 μmol/L, female: >78.7 μmol/L) were excluded. Patients receiving an OHA other than metformin at screening with glycated hemoglobin (HbA1c) ≥6.4 and ≤9.4% required a 6‐week washout period for other OHAs and then entered a 2‐week, single‐blind, placebo run‐in period. The HbA1c values reported here have been converted to National Glycohemoglobin Standardization Program (NGSP) values as follows: HbA1c (NGSP) (%)19 = 1.02 × HbA1c (Japan Diabetes Society) (%) + 0.25%. Patients receiving only oral metformin at screening with HbA1c ≥6.9 and <10.5% directly entered the 2‐week, single‐blind, placebo run‐in period.
A design schematic for the study is shown in Figure S1. After completing the run‐in period, patients with HbA1c ≥6.9 and <10.5%, and fasting plasma glucose (FPG) ≤15.0 mmol/L at the beginning of the placebo run‐in period, who had been on metformin monotherapy for at least 12 weeks and on stable diet and exercise therapy for at least 8 weeks, were randomized to either sitagliptin 50 mg once daily or matching placebo in a 1:1 ratio, using a computer‐generated randomization scheme. Patients, clinical study personnel and sponsor study personnel were blinded to study treatment. All patients received diet and exercise education at the screening period, and were instructed to follow a stable program for the duration of the study.
The initial 12‐week, double‐blind treatment period was followed by a 40‐week, open‐label period, during which all patients received 50 mg of sitagliptin once daily. The patients who received sitagliptin for up to 52 weeks are identified henceforth as the S/S group (i.e. sitagliptin in the double‐blind period and the open‐label period) and those who received sitagliptin for up to 40 weeks after placebo are identified as the P/S group (i.e. placebo in the double‐blind period, switching to sitagliptin in the open‐label period). During the open‐label period, and irrespective of treatment group, the dose of sitagliptin could be increased to 100 mg once daily at the visit after a FPG level ≥7.8 mmol/L at or beyond week 16 or a HbA1c level ≥7.4% at or beyond week 24. Investigators were allowed to decrease the sitagliptin dose back to 50 mg/day if treatment with 100 mg/day was not considered to be well tolerated. After week 40, the sitagliptin dose remained stable until the completion of the study. The dose of metformin was to be maintained throughout the study.
Meal tolerance tests were carried out at weeks 0, 12 and 52, beginning 30 min after administration of the study drug (at week 0 patients received a dose of matching placebo). The test meal contained ~500 kcal (60% carbohydrate, 15% protein and 25% fat) and was to be consumed within 15 min. Blood samples were drawn before beginning the test meal, and 0.5, 1.0 and 2.0 h after beginning the meal.
End‐Points
The primary efficacy end‐point was the change from baseline in HbA1c at week 12. Secondary end‐points were the changes from baseline in 2‐h post‐meal glucose (PMG) and FPG at week 12. Additional end‐points included changes from baseline at week 12 in 1,5 anhydroglucitol (1,5‐AG), insulin, homeostasis model of β‐cell function (HOMA‐β), homeostasis model assessment of insulin resistance (HOMA‐IR), 2‐h post‐meal insulin, post‐meal glucose area under the concentration‐versus‐time curve (AUC), insulin AUC, C‐peptide AUC and insulinogenic index. In the open‐label period, the aforementioned end‐points were each evaluated for change from baseline at week 52. The proportions of patients with HbA1c values meeting the therapeutic goals of <7.4 and <6.9% were also assessed at weeks 12 and 52.
Safety end‐points included adverse events (AEs), vital signs (blood pressure, pulse rate), bodyweight, 12‐lead electrocardiogram (ECG) and laboratory results (hematology, serum chemistry and urinalysis). AEs of hypoglycemia and selected gastrointestinal (GI) AEs (nausea, vomiting and diarrhea) were considered of special interest, and were predefined for between‐group comparison. All laboratory assays were carried out at one central laboratory (Mitsubishi Chemical Medience Corporation, Tokyo, Japan).
Statistical Analyses
Efficacy
Analyses of all efficacy end‐points used the full analysis set (FAS), defined as all patients who had taken at least one dose of study medication and had both the baseline measurement and at least one post‐randomization measurement. Missing values for the double‐blind period were imputed using the method of last observation carried forward (LOCF). For the long‐term efficacy assessments, FAS was used as the primary efficacy population, but no data were imputed.
Comparisons between sitagliptin and placebo for continuous efficacy end‐points were carried out based on an analysis of covariance (ancova) model, including treatment group and prior use of other OHAs, other than metformin at screening, as factors, and relevant baseline level as a covariate. Between‐group comparisons for the proportion of patients with HbA1c values <7.4 or <6.9% were carried out using a logistic regression model with the same factors and covariate as the ancova model. Subgroup analyses in the primary efficacy end‐point were also carried out for predefined factors, including baseline HbA1c level (≤8.4, >8.4%), sex, age, body mass index (BMI), prior OHA other than metformin, baseline insulin, baseline HOMA‐IR, baseline HOMA‐β and duration of diabetes.
For long‐term efficacy assessment of sitagliptin, summary statistics for efficacy end‐points were calculated by treatment group at each time‐point in which the end‐point was measured over 52 weeks; the time‐course profiles were also investigated.
A post‐hoc analysis of the efficacy of uptitrating the sitagliptin dose from 50 to 100 mg once daily was carried out in the present study. Among patients with sitagliptin dose increases and HbA1c values ≥7.4% at the time of uptitration, the proportion of patients with HbA1c values meeting the glycemic goal of <7.4% 12 weeks after uptitration was calculated. Additionally, among patients who had their sitagliptin dose increased and who completed the study, the proportion of patients with HbA1c values <7.4% at week 52 was assessed. For these two analyses, missing values were not imputed.
Safety
Safety and tolerability analyses were carried out on the all‐patients‐as‐treated population, which included randomized patients who received at least one dose of the double‐blind study drug. In the double‐blind period, between‐group comparisons using Fisher's exact test were carried out for incidences of overall (one or more) AEs, drug‐related clinical AEs, hypoglycemia and prespecified gastrointestinal AEs. Differences in the incidences between the two groups and their 95% confidence intervals (CIs) were calculated using Wilson's score method. Summary statistics were calculated for the vital signs and laboratory results by treatment group at each time‐point over 12 weeks, and the changes from baseline were compared within groups using the paired t‐test or Wilcoxon signed rank test, depending on the parametric nature of the data.
For assessment of long‐term safety, the patient population included all patients who received at least one dose of sitagliptin after week 12. The incidences of overall AEs, drug‐related AEs, hypoglycemia and selected gastrointestinal AEs were summarized. Within‐group mean change from baseline in bodyweight was assessed using a paired t‐test at week 52. Summary statistics were calculated for the change from baseline in vital signs and laboratory tests by treatment group at each time‐point over 52 weeks, and the changes from baseline were compared within groups by the paired t‐test or Wilcoxon signed rank test, depending on the parametric nature of the data.
Results
Patients
Background demographic and disease characteristics were similar between groups (Table S1). At baseline, patients had a mean HbA1c of 8.3%, a mean FPG of 8.6 mmol/L and a mean 2‐h PMG of 13.6 mmol/L. The mean duration of diabetes since diagnosis was 7.5 years. In 95% of the patients, the metformin dose was between 500 and 750 mg a day, which was consistent with the approved metformin dosage for usual maintenance in Japan at the time the study was carried out.
The patient disposition is presented in Figure 1. A total of 149 patients were randomized in the present study. During the 12‐week, double‐blind period, 147 patients (76 in the sitagliptin 50 mg group and 71 in the placebo group) were included in the FAS and 149 patients (77 in the sitagliptin 50 mg group and 72 in the placebo group) were included in the safety analysis.
Figure 1.
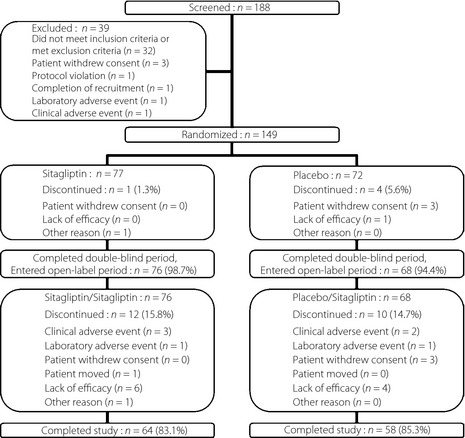
Patient disposition.
For the analysis of week 12 through to week 52, 144 patients (76 and 68 in the S/S and P/S groups, respectively) were included in FAS and a total of 145 patients (77 in the S/S group and 68 in the P/S group) were included in the safety analysis. The dose of sitagliptin was uptitrated to 100 mg once daily in 97 patients (67%) who met the uptitration criteria, 48 out of 77 (62%) in the S/S group and 49 out of 68 (72%) in the P/S group.
Efficacy
Double‐Blind Period
After 12 weeks of treatment, the mean change from baseline in HbA1c significantly decreased with the sitagliptin group relative to the placebo group (least squares [LS] mean difference [95% CI] was −0.7% [−0.9 to −0.5], P < 0.001, Table 1). The proportions of patients with HbA1c <7.4 or <6.9% were significantly (P < 0.05 for both) greater with sitagliptin (51.3 and 17.1%, respectively) than with placebo (22.1 and 4.4%, respectively).
Table 1. End‐points at week 12 in patients treated with sitagliptin or placebo added to ongoing metformin.
| End‐pointsa | n | Week 0 mean (SD) | Week 12 mean (SD) | Change from week 0 (baseline) to week 12 LS mean (95% CI) | Between group differences LS mean (95% CI) |
|---|---|---|---|---|---|
| HbA1c (%) | |||||
| Placebo | 71 | 8.3 (1.0) | 8.5 (1.2) | 0.3 (0.1 to 0.5) | −0.7 (−0.9 to −0.5)*** |
| Sitagliptin | 76 | 8.1 (0.9) | 7.5 (0.9) | −0.4 (−0.6 to −0.2) | |
| Fasting plasma glucose (mmol/L) | |||||
| Placebo | 71 | 8.9 (2.3) | 9.1 (2.2) | 0.4 (0.0 to 0.7) | −1.0 (−1.3 to −0.6)*** |
| Sitagliptin | 76 | 8.3 (1.7) | 7.7 (1.5) | −0.6 (−0.9 to −0.3) | |
| 1,5‐anhydroglucitol (μg/mL) | |||||
| Placebo | 70 | 6.3 (4.3) | 6.0 (4.7) | −0.4 (−1.1 to 0.4) | 3.8 (2.9 to 4.7)*** |
| Sitagliptin | 76 | 7.0 (5.4) | 10.6 (6.9) | 3.5 (2.7 to 4.2) | |
| Fasting insulin (pmol/L) | |||||
| Placebo | 70 | 39.0 (23.3) | 41.1 (25.0) | 1.5 (−3.7 to 6.7) | 6.9 (0.8 to 13.0)* |
| Sitagliptin | 76 | 44.6 (30.9) | 53.0 (35.3) | 8.4 (3.2 to 13.6) | |
| HOMA‐IR | |||||
| Placebo | 70 | 2.5 (1.6) | 2.8 (1.8) | 0.2 (−0.1 to 0.6) | 0.2 (−0.3 to 0.6) |
| Sitagliptin | 76 | 2.7 (1.8) | 3.1 (2.2) | 0.4 (0.0 to 0.7) | |
| HOMA‐β | |||||
| Placebo | 70 | 28.7 (22.7) | 27.9 (20.7) | −2.1 (−6.1 to 1.9) | 11.3 (6.5 to 16.0)*** |
| Sitagliptin | 76 | 35.6 (27.7) | 45.1 (29.5) | 9.1 (5.1 to 13.2) | |
CI, confidence interval; HbA1c, glycated hemoglobin; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; LS, least squares; SD, standard deviation.
Missing data were imputed using the last‐observation‐carried‐forward method.
***P < 0.001 for sitagliptin vs placebo; **P < 0.01, *P < 0.05.
The HbA1c change from baseline at week 12 was consistent across subgroups defined by sex, age, BMI, prior OHA other than metformin, baseline insulin, baseline HOMA‐β and duration of type 2 diabetes (treatment‐by‐subgroup interaction P‐values > 0.05). However, there was a significant P‐value (P = 0.006) for the interaction for baseline HbA1c level, consistent with a difference in HbA1c response between the treatment subgroups (−0.5% in ≤8.4% and −1.0% in >8.4%), suggesting that patients with a higher baseline HbA1c might have a greater reduction in HbA1c relative to placebo in response to sitagliptin therapy than patients with a lower baseline HbA1c. There was also a significant P‐value (P = 0.012) for the interaction for baseline HOMA‐IR, consistent with a difference in HbA1c reduction between the treatment subgroups (−0.4% in <median vs −0.9% in ≥median subgroup).
The LS mean difference between groups (95% CI) in changes from baseline in 2‐h PMG and FPG at week 12 were −2.6 mmol/L (−3.5 to −1.7) and −1.0 mmol/L (−1.3 to −0.6), respectively, in favor of sitagliptin (both P < 0.001). Statistically significant improvements with sitagliptin relative to placebo were also observed at week 12 in measures of β‐cell function (HOMA‐β, insulinogenic index after meal load), 1,5‐AG, and 2‐h post‐meal C‐peptide and insulin after meal load (Table 1 and Table S2).
Open‐Label Period
Regarding the evaluation of long‐term efficacy of sitagliptin, patients in the S/S group maintained a statistically significant improvement in HbA1c at all time‐points up to week 52 (P < 0.001 vs baseline; Figure 2). Additionally, the proportions of patients with HbA1c values below 7.4 and 6.9% at week 52 were 67.2 and 37.5%, respectively. Statistically significant improvements in 2‐h PMG were observed at week 52 (P < 0.001 vs baseline; Figure S2), consistent with those observed at week 12. In addition, significantly improved FPG values were observed at all measured time‐points up to week 52 (P < 0.001 vs baseline; Figure 3).
Figure 2.
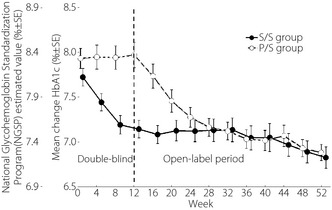
Changes from baseline in glycated hemoglobin (HbA1c). After week 20, uptitration of sitagliptin to 100 mg had been allowed. P/S, patients who received placebo in the double‐blind period and sitagliptin in the non‐blinded period; S/S, patients who received sitagliptin 50 mg in the double‐blind period and the open‐label period.
Figure 3.
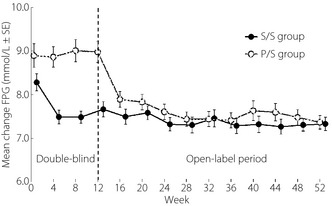
Change from baseline in fasting plasma glucose (FPG). After week 20, uptitration of sitagliptin to 100 mg had been allowed. P/S, patients who received placebo in the double‐blind period and sitagliptin in the open‐label period; S/S patients who received sitagliptin 50 mg in the double‐blind and open‐label periods.
In the P/S group, significant reductions from baseline in HbA1c at week 52 were observed (Figure 2). The proportions of patients with HbA1c values at week 52 meeting the therapeutic goals of <7.4 and <6.9% were 60.3 and 36.2%, respectively. The 2‐h PMG values at week 52 were also significantly lower than the baseline and the week 12 values (Figure S2). Additionally, FPG was significantly lower than baseline at all time‐points during the open‐label period (Figure 3). The results for other efficacy parameters were generally supportive of those for the key efficacy parameters (Tables 2 and S3).
Table 2. End‐points at week 52 in Japanese patients with type 2 diabetes in placebo/sitagliptin and sitagliptin/sitagliptin groups.
| End‐pointsa | n | Week 0 mean (SD) | Week 52 mean (SD) | Change from week 0 (baseline) to week 52 (mean [95% CI]) |
|---|---|---|---|---|
| HbA1c (%) | ||||
| P/S | 58 | 8.1 (0.8) | 7.3 (0.7) | −0.9 (−1.1 to −0.7)*** |
| S/S | 64 | 8.0 (0.8) | 7.2 (1.0) | −0.8 (−1.0 to −0.6)*** |
| Fasting plasma glucose (mmol/L) | ||||
| P/S | 58 | 8.4 (1.8) | 7.4 (1.2) | −1.0 (−1.5 to −0.6)*** |
| S/S | 64 | 7.9 (1.3) | 7.3 (1.2) | −0.6 (−1.0 to −0.3)*** |
| 1,5‐anhydroglucitol (μg/mL) | ||||
| P/S | 58 | 7.0 (4.4) | 11.7 (5.8) | 4.7 (3.7 to 5.7)*** |
| S/S | 64 | 7.7 (5.5) | 12.8 (8.2) | 5.1 (4.1 to 6.1)*** |
| Fasting insulin (pmol/L) | ||||
| P/S | 58 | 39.2 (23.1) | 42.3 (30.6) | 3.1 (−2.5 to 8.7) |
| S/S | 64 | 44.9 (31.9) | 64.9 (99.0) | 20.1 (−3.3 to 43.5) |
| HOMA‐IR | ||||
| P/S | 58 | 2.4 (1.4) | 2.4 (2.1) | 0.0 (−0.4 to 0.4) |
| S/S | 64 | 2.6 (1.8) | 3.7 (5.7) | 1.1 (−0.2 to 2.4) |
| HOMA‐β | ||||
| P/S | 58 | 30.6 (23.8) | 37.7 (24.8) | 7.0 (3.0 to 11.1)*** |
| S/S | 64 | 37.6 (28.8) | 58.9 (87.9) | 21.3 (0.2 to 42.4)* |
CI, confidence interval; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; P/S, patients who received placebo in double‐blind period and sitagliptin in the open‐label period; SD, standard deviation; S/S, patients who received sitagliptin 50 mg in the double‐blind and open‐label periods.
Missing data were not imputed.
***P < 0.001, **P < 0.01, *P < 0.05.
After week 20 (based on assay values from the previous clinic visit), uptitration of sitagliptin to 100 mg once daily was allowed for the patients who met predefined glycemic criteria to provide additional glycemic improvement. The sitagliptin dose was uptitrated in 48 of the 76 patients (63%) in the S/S group and 49 of the 68 patients (72%) in the P/S group. Of the cohort of 97 patients in both groups who were uptitrated, 65 patients had both, HbA1c ≥7.4% at the time of uptitration, and had HbA1c values available 12 weeks after uptitration. Of these, 15 patients (23.1%) had HbA1c <7.4% 12 weeks later. Overall, 80 of the 97 uptitrated patients (82%) completed 52 weeks of the study. Among these, 38 patients (47.5%) and 18 patients (22.5%) had HbA1c values <7.4 and <6.9% at week 52, respectively.
Safety
Double‐Blind Period
Sitagliptin and placebo were similarly well tolerated (Tables S4 and S5). The incidence of drug related clinical AEs was lower in the sitagliptin group than in the placebo group; 1.3% vs 6.9%, respectively (Table S4). Two serious AEs of pneumonia were reported for one patient in each treatment arm; both events were considered by the investigators not to be related to the study drug, and both recovered while the patient was in the study (Table S4). No episodes of hypoglycemia were reported in either treatment group. The overall proportions of patients with predefined GI AEs were similar for both groups; 1.3% for sitagliptin and 2.8% for placebo (Table S4). Fewer patients in the sitagliptin group (7.8%) had an AE of nasopharyngitis compared with the placebo group (18.1%; Table S4). The incidence of laboratory AEs was low and similar between groups (Table S5).
At week 12, the addition of sitagliptin or placebo to ongoing therapy with metformin resulted in mean changes from baseline in bodyweight of 0.42 and −0.29 kg (P = 0.006 and P = 0.048, respectively). These changes were relatively small and not considered clinically meaningful.
Open‐Label Period
During the open‐label period, one or more clinical AEs were reported for most patients in both the S/S and P/S groups (Table S4). Drug‐related clinical AEs were reported in 3.9 and 4.4% of patients in the S/S and P/S groups, respectively; none of these events were reported to occur in more than one patient each. Serious AEs (7 events) were reported for six patients in the S/S group (colon cancer, aortic dissection, pneumonia, lumbar spinal stenosis, angina pectoris and duodenal ulcer hemorrhage reported in a single patient, and prostate cancer) compared with two serious AEs reported for two patients in the P/S group (loss of consciousness and overdose; Table S4). One patient in the S/S group died as a result of a serious AE of aortic dissection. All serious AEs were considered by the investigators not to be related to study medication. Only one mild episode of hypoglycemia (not requiring medical intervention) was reported in the open‐label period for one patient in the S/S group (Table S4). The proportion of patients with predefined GI AEs was numerically higher for the S/S groups compared with the P/S group; 7.8% vs 4.4%, respectively (Table S4). Clinical AEs reported with an incidence of ≥5% in either the S/S or P/S group included diabetic retinopathy, diarrhea, nasopharyngitis, upper respiratory tract inflammation and rash (Table S4). No laboratory AEs were reported with an incidence of ≥5% in either the S/S or P/S group; the incidences of specific laboratory AEs were similar between the two groups; and few were reported as drug‐related (Table S5). Laboratory AEs leading to discontinuation were reported for one patient in each treatment group (alanine aminotransferase increased [P/S] and blood creatinine increased [S/S]).
At week 52, slight decreases from baseline (66.1 and 65.9 kg, respectively) in mean weight of 0.5 and 0.4 kg, respectively, were observed in the S/S and P/S groups; the differences in both groups were not statistically significant.
Discussion
The present study evaluated sitagliptin as an add‐on therapy in Japanese patients with type 2 diabetes who had inadequate glycemic control with metformin monotherapy in addition to diet and exercise counseling. The results showed that sitagliptin therapy added to ongoing metformin monotherapy significantly improved glycemic control as measured by HbA1c, 2‐h PMG and FPG relative to adding placebo for 12 weeks. The observed improvements from baseline were sustained up to week 52 with sitagliptin therapy. The greater reduction of HbA1c observed in the subgroup of patients with higher HbA1c at baseline has also been observed in previous studies18.
In the present study, uptitration of sitagliptin from 50 to 100 mg was allowed if a patient met predefined glycemic control targets during the open‐label period. The results show that the regimen of sitagliptin initiated at 50 mg and uptitrated to 100 mg provided improved HbA1c goal achievement over time in a meaningful proportion of patients. Although the results should be interpreted with caution because of the lack of a placebo arm or other comparator during this phase of the study, they suggest that sitagliptin uptitration to 100 mg might help some patients improve their glycemic control.
In addition to improvements in glycemic parameters, the present study showed that treatment with sitagliptin as an add‐on to metformin improved measures of β‐cell function (HOMA‐β, insulinogenic index after meal load). This is of particular importance for Japanese patients with type 2 diabetes, who tend to present with greater β‐cell dysfunction relative to their Western counterparts. This finding is consistent with the results of a previous sitagliptin monotherapy study in Asian patients with type 2 diabetes who had reduced β‐cell function as their Japanese counterparts17. The addition of sitagliptin to metformin has previously been shown to have an additive effect on active GLP‐1 levels12. Treatment with GLP‐1 and incretin‐based therapies, which increase active GLP‐1 levels, are likely to mediate β‐cell function, and the different mechanisms of action of these drugs seem to contribute to an enhanced antihyperglycemic effect.
Similar proportions of patients in the sitagliptin and placebo groups had AEs during the double‐blind treatment period, and no hypoglycemic events were observed in either treatment group. A numerically higher incidence of serious AEs was observed in the S/S group compared with the P/S group in the open‐label period, which appeared to be related to small differences for a range of AEs, with no discernable pattern. There were no serious, drug‐related AEs in the present study. Unlike sulfonylureas and insulin1, sitagliptin and metformin are associated with a low risk of hypoglycemia and a neutral effect on bodyweight. Sitagliptin added on to metformin for 52 weeks produced no meaningful change in bodyweight. Both treatment groups had a very low incidence of predefined gastrointestinal symptoms throughout the study period. These results are consistent with those observed in previous sitagliptin added on to metformin studies6.
In summary, the present study shows that sitagliptin added on to metformin for Japanese patients with type 2 diabetes provided significant improvements in HbA1c, 2‐h PMG and FPG that were sustained over the entire 52‐week study period. Sitagliptin added on to metformin was generally well tolerated. These results are consistent with the findings of previous multinational studies7.
Supplementary Material
Table S1. Patient demographic and disease characteristics of full analysis population.
Table S2. End‐points measured during meal tolerance test at week 12 in Japanese patients with type 2 diabetes with sitagliptin or placebo added to ongoing metformin.
Table S3. End‐points measured during meal tolerance test at week 52 in Japanese patients with type 2 diabetes in P/S and S/S group.
Table S4. Clinical adverse event results.
Table S5. Laboratory adverse event results.
Figure S1. Study design schematic.
Figure S2. Mean change from baseline in 2‐h post‐meal glucose test.
Appendix S1. MK‐0431/ONO5435‐08, MSD K.K. 057 Primary investigators listing.
Acknowledgements
This study was sponsored by Ono Pharmaceutical Co., Ltd. and Merck Japan. T Kadowaki and N Tajima served as coordinating investigators in this study, and M Odawara served as a medical expert. M Nishii, T Taniguchi and JC Arjona Ferreira are current employees of Ono Pharmaceutical Co., Ltd., MSD K. K., and Merck Sharp & Dohme Corp., subsidiaries of Merck & Co., Inc., respectively. The authors would like to thank H Honda, M Kawashima and M Odani from Ono Pharmaceutical Co., Ltd, and Christine McCrary Sisk, William V Taggart, Kathleen Newcomb and Jennifer Rotonda from Merck for their contributions to the writing and editing of this manuscript. Primary investigators in this study are listed in Appendix S1.
(J Diabetes Invest, doi: 10.1111/jdi.12001, 2013)
References
- 1.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002; 287: 360–372 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta‐cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687 [DOI] [PubMed] [Google Scholar]
- 3.Cook MN, Girman CJ, Stein PP, et al Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med 2007; 24: 350–358 [DOI] [PubMed] [Google Scholar]
- 4.Herman GA, Bergman A, Stevens C, et al Effect of single oral doses of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006; 91: 4612–4619 [DOI] [PubMed] [Google Scholar]
- 5.Aschner P, Kipnes MS, Lunceford JK, et al Effect of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29: 2632–2637 [DOI] [PubMed] [Google Scholar]
- 6.Charbonnel B, Karasik A, Liu J, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638–2643 [DOI] [PubMed] [Google Scholar]
- 7.Hermansen K, Kipnes M, Luo E, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab 2007; 9: 733–745 [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Brazg R, Andryuk PJ, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clin Ther 2006; 28: 1556–1568 [DOI] [PubMed] [Google Scholar]
- 9.Vilsbøll T, Rosenstock J, Yki‐Järvinen H, et al Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 167–177 [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Tajima N, Odawara M, et al Addition of sitagliptin, an oral, highly‐selective dipeptidyl peptidase‐4 inhibitor, improved glycemic control and was well tolerated in Japanese patients with type 2 diabetes on insulin monotherapy. Diabetes 2011; 60(Suppl. 1): A279 [Google Scholar]
- 11.Hundal RS, Krssak M, Dufour S, et al Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000; 49: 2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migoya EM, Bergeron R, Miller JL, et al Dipeptidyl peptidase‐4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP‐1. Clin Pharmacol Ther 2010; 88: 801–808 [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med 2006; 23: 579–593 [DOI] [PubMed] [Google Scholar]
- 15.Kuroe A, Fukushima M, Usami M, et al Impaired β‐cell function and insulin sensitivity in Japanese subjects wih normal glucose tolerance. Diabetes Res Clin Pract 2003; 59: 71–77 [DOI] [PubMed] [Google Scholar]
- 16.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract 2004; 66S: S37–S43 [DOI] [PubMed] [Google Scholar]
- 17.Mohan V, Yang W, Son HY, et al Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract 2009; 83: 106–116 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein BJ, Feinglos MN, Lunceford JK, et al Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase‐4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007; 30: 1979–1987 [DOI] [PubMed] [Google Scholar]
- 19.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwamoto Y, Tajima N, Kadowaki T, et al Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double‐blind trial. Diabetes Obes Metab 2010; 12: 613–622 [DOI] [PubMed] [Google Scholar]
- 21.Raz I, Chen Y, Wu M, et al Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin 2008; 24: 537–550 [DOI] [PubMed] [Google Scholar]
- 22.Barnett A, Allsworth J, Jameson K, et al A review of the effects of antihyperglycaemic agents on body weight: the potential of incretin targeted therapies. Curr Med Res Opin 2007; 23: 1493–1507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient demographic and disease characteristics of full analysis population.
Table S2. End‐points measured during meal tolerance test at week 12 in Japanese patients with type 2 diabetes with sitagliptin or placebo added to ongoing metformin.
Table S3. End‐points measured during meal tolerance test at week 52 in Japanese patients with type 2 diabetes in P/S and S/S group.
Table S4. Clinical adverse event results.
Table S5. Laboratory adverse event results.
Figure S1. Study design schematic.
Figure S2. Mean change from baseline in 2‐h post‐meal glucose test.
Appendix S1. MK‐0431/ONO5435‐08, MSD K.K. 057 Primary investigators listing.


