Abstract
Aims/Introduction
An initial 26‐week, randomized, open‐label study compared the efficacy and safety of exenatide 10 mcg twice daily with exenatide 2 mg once weekly in Asian patients with type 2 diabetes who experienced inadequate glycemic control with oral antidiabetes medications. The aim of this study was to evaluate the safety of exenatide once weekly in Japanese patients, a subset of the initial patient population, who continued into this extension study for an additional 26 weeks of therapy on exenatide once weekly, followed by 10 weeks without exenatide once weekly.
Materials and Methods
Japanese patients initially assigned to exenatide twice daily (n = 62) switched to exenatide once weekly for the extended 26 weeks, and patients initially assigned to exenatide once weekly (n = 74) continued on this regimen for the remainder of the study (total treatment of 52 weeks).
Results
A total of 68% of patients reported one or more treatment‐emergent adverse events during the extension period; the most common of these were nasopharyngitis (14%) and vomiting (6%). No major hypoglycemic episodes were reported. Improvements in glycated hemoglobin, fasting plasma glucose and postprandial glucose were maintained over 52 weeks of treatment. At week 52, bodyweight remained reduced from baseline.
Conclusions
Exenatide once weekly added to oral antidiabetes medication was well tolerated in Japanese patients with type 2 diabetes, and was associated with glycemic control and weight loss through to 52 weeks, supporting the use of exenatide once weekly as an adjunctive treatment for type 2 diabetes in this patient population. The initial 26‐week portion of this trial was registered with ClinicalTrials.gov (no. NCT00917267).
Keywords: Blood glucose, Exenatide, Japanese
Introduction
The prevalence of diabetes has rapidly increased in Japan, with Japan now ranking among the top 10 countries with the highest prevalence of type 2 diabetes1. Although sulfonylureas (SUs) have been the most widely prescribed first‐line treatment for Japanese patients with type 2 diabetes2, these medications have been associated with hypoglycemia events, and primary failure rates of approximately 30% and secondary failure rates of up to 5% per year5.
A previous study evaluated the efficacy and safety of the glucagon‐like peptide‐1 (GLP‐1) receptor agonist, exenatide administered b.i.d. (EBID), over 24 weeks in Japanese patients with type 2 diabetes mellitus suboptimally controlled despite therapeutic doses of a SU alone or in combination with a biguanide or thiazolidinedione (TZD)6. In that study, exenatide 10 mcg b.i.d. was associated with a significantly greater reduction in glycated hemoglobin (HbA1c; −1.62%) and bodyweight (−1.54 kg) compared with placebo, and was well tolerated. The safety and efficacy findings for exenatide in Japanese patients6 were similar to results observed in studies of exenatide in mostly Caucasian patient populations7.
An extended‐release formulation of exenatide was recently approved by the European Medicines Agency and the US Food and Drug Administration for use as a once weekly (QW) subcutaneous injection for the treatment of type 2 diabetes10. Compared with EBID, 2 mg exenatide QW (EQW) was associated with greater reduction in HbA1c and fasting plasma glucose (FPG), with less nausea after 24–30 weeks of treatment in study populations that were predominately Caucasian12.
A recent phase 3, 26‐week, randomized, open‐label, multicenter, two‐arm study with 10 weeks of follow up compared the efficacy and safety of EQW with exenatide EBID in 678 Asian patients with type 2 diabetes who experienced inadequate glycemic control with SU, metformin (MET) or TZD, alone or in combination14. In that study, treatment with EQW showed weight loss and better glycemic control compared with EBID, with no new safety findings14. The current study, a 26‐week extension of the initial study,14 was carried out to evaluate the safety of EQW in Japanese patients (a subset of the initial patient population) for a total of 52 weeks of therapy on EQW and was followed by a 10‐week safety period without EQW.
Materials and Methods
This uncontrolled 26‐week extension study was designed to assess the safety of EQW exclusively in Japanese patients after a total of 52 weeks of exposure to EQW. This population was a subset of an initial 26‐week study comparing EBID with EQW in Asian patients14. The Japan extended analysis set included all Japan intent‐to‐treat (ITT) patients who received at least one EQW injection during the extension period; all analyses were based on this extended analysis set unless otherwise specified.
In the initial 26‐week study (week 0 through to week 26), Asian patients from five countries were stratified by oral antidiabetes medication (OAM) treatment (i.e. with or without SU), then randomized to add EQW or EBID to their current regimen. Patients self‐administered EQW 2 mg for 26 weeks or EBID 5 mcg for the first 4 weeks followed by EBID 10 mcg for the remaining 22 weeks. All patients continued their usual doses of MET or TZD, whereas SU dosages were decreased to the country‐specific minimum from the beginning of the 26‐week treatment period.
Patients from Japan only (n = 136) entered the extension period: patients assigned to EBID (n = 62) during the initial 26‐week study were switched to EQW for the extended 26 weeks (defined as the EBID→EQW group); patients initially assigned to EQW (n = 74) continued on this regimen for total treatment of 52 weeks. Additional safety data were collected approximately 10 weeks after the last visit of the extension period (week 62). During this 10‐week follow‐up period, patients were not permitted to use exenatide or any GLP‐1 receptor agonist.
Eligible patients were aged ≥20 years, had type 2 diabetes and had inadequate glycemic control (HbA1c ≥7 and ≤11%) while treated with stable doses of OAMs for at least 90 days before screening. Patients were excluded if they showed evidence of other clinically significant medical conditions within 4 weeks of screening, or had taken excluded medications (e.g. insulin, dipeptidylpeptidase‐4 inhibitors, pramlintide acetate, drugs promoting weight loss) within 90 days of screening.
Investigated outcome measures included measures of safety, glycemic control and metabolic control. Specifically, treatment‐emergent adverse events (TEAEs; defined as adverse events that occurred for the first time or existed before week 26 and worsened through to week 52) were recorded and assessed by antibody to exenatide status. Additionally, the incidence of hypoglycemic events; HbA1c over time; change in HbA1c from baseline to week 52; the proportion of patients achieving HbA1c ≤7 and ≤6.5%; fasting serum glucose (FSG); six‐point self‐monitored blood glucose (SMBG); change in bodyweight; and fasting serum lipids were evaluated.
Hypoglycemia episodes were categorized and defined as follows: minor – signs or symptoms of hypoglycemia with a concurrent blood glucose <54 mg/dL that resolved without treatment or with self‐treatment; major – any episode with symptoms of hypoglycemia resulting in loss of consciousness or seizures that showed prompt recovery in response to administration of glucagon or glucose, or a documented blood glucose <54 mg/dL, requiring the assistance of another person because of severe impairment in consciousness or behavior; symptoms – any reported hypoglycemic episode not categorized as minor or major episode. If hypoglycemia occurred, SU treatment could be discontinued, but could not recommence for the duration of the study.
Patients were discontinued from the study if treated with another diabetes therapeutic agent (e.g. insulin >7 days), if MET or TZD dosage changed for >14 days or in the case of evidence of pancreatitis or a serious adverse event (SAE), or abnormal laboratory value that warranted discontinuation.
The study protocol was approved by an ethical review board at each study site and was in accordance with the ethical principles described in the Declaration of Helsinki. Informed consent was obtained from each patient before starting the study. All informed consent documents were compliant with International Conference on Harmonization (ICH) guidelines on Good Clinical Practices.
Statistical Analysis
The analysis for this 26‐week extension study included data collected after the initial week 26 visit through to week 52, including early termination and excluding the 10‐week safety follow up. The safety follow up included data collected between the last treatment visit through to the final safety follow up approximately 10 weeks later.
The Japan ITT analysis set included all randomized patients from Japan who received at least one exenatide injection during the primary study period. The Japan extended analysis set included all Japan ITT patients who received at least one EQW injection during the extension period; all analyses were based on this extended analysis set unless otherwise specified. Data collected during the extension study were summarized descriptively, overall, and by treatment group assigned at randomization. No statistical comparisons were planned or carried out.
Actual and change from baseline values (week 0 of the initial 26‐week study) for the following variables were summarized using descriptive statistics at baseline and at the week 52 end‐point: HbA1c, FSG, bodyweight and SMBG (each time‐point). Patients who achieved HbA1c target values of ≤7 and ≤6.5% at the week 52 end‐point were summarized by frequencies and percentages. Changes from baseline in HbA1c to the week 52 end‐point were summarized using descriptive statistics by antibody to exenatide status at week 52. Treatment‐emergent antibody to exenatide status had three levels: negative, low titer (<625) and higher titer (≥625).
Results
Patient Disposition and Baseline Characteristics
Of the 155 Japanese ITT patients, 136 patients in 17 study sites completed the initial 26‐week study and entered the extension period (EBID→EQW 62; EQW 74). There were five patients from the EBID→EQW group and four patients from the EQW group who withdrew during the extension period, but returned for the follow‐up period. Both treatment groups had a similar rate of discontinuation from the extension period: EBID→EQW 8%; EQW 5% (Figure 1).
Figure 1.
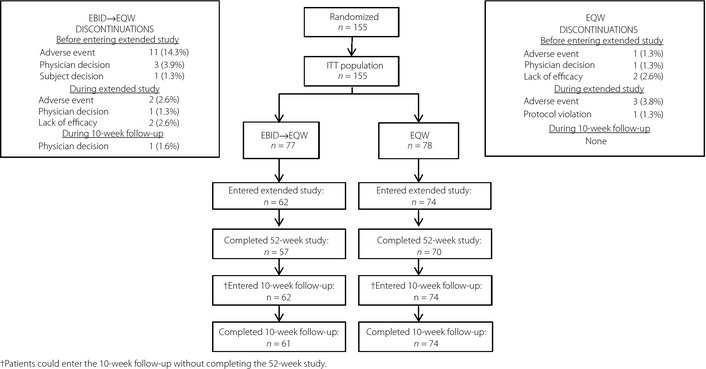
Patient flow diagram. Data shown for discontinuations are presented as n (%). EBID, 10 mcg exenatide b.i.d.; EBID→EQW, patients assigned to 10 mcg exenatide b.i.d. during the initial 26‐week study who were switched to 2 mg exenatide once weekly for the extended 26 weeks; EQW, 2 mg exenatide once weekly; ITT, intent‐to‐treat.
Baseline characteristics for the Japan ITT patients, including the 19 patients who did not enter the extension phase, were similar to those of the Japan extended study patients in either treatment group (Table 1). Most patients (>80%) in both treatment groups were taking a concomitant SU.
Table 1. Demographics and baseline characteristics.
| Japan ITT patients (n = 155) | Japan extension study patients | ||
|---|---|---|---|
| EBID→EQW | EQW | ||
| (n = 62) | (n = 74) | ||
| Age (years) | 61 (9) | 60 (9) | 61 (10) |
| Male, n (%) | 91 (59) | 38 (61) | 43 (58) |
| HbA1c (%) | 8.8 (0.9) | 8.8 (1.0) | 8.8 (1.0) |
| Duration of diabetes (years) | 10 (7) | 11 (7) | 10 (6) |
| FSG (mg/dL) | 166 (41) | 168 (41) | 167 (43) |
| Bodyweight (kg) | 69 (13) | 71 (13) | 68 (13) |
| BMI (kg/m2) | 26 (4) | 27 (4) | 26 (3) |
| Background OAM, n (%) | |||
| SU | 35 (23) | 11 (18) | 19 (26) |
| SU + MET | 79 (51) | 34 (55) | 37 (50) |
| SU + TZD | 18 (12) | 10 (16) | 6 (8) |
| MET | 19 (12) | 6 (10) | 10 (14) |
| MET + TZD | 2 (1) | 0 (0) | 1 (1) |
| TZD | 2 (1) | 1 (2) | 1 (1) |
Data are presented as mean (standard deviation), unless otherwise noted. BMI, body mass index; EBID, exenatide twice daily; EQW, exenatide once weekly; FSG, fasting serum glucose; HbA1c, glycated hemoglobin; ITT, intent‐to‐treat; MET, metformin; OAM, oral antidiabetes medication; SU, sulfonylurea; TZD, thiazolidinedione.
Safety and Tolerability
An overview of adverse events occurring in Japanese patients during the initial study period and the extension period is presented in Table 2. Of the 155 Japanese patients who entered the initial study, 121 (78%) reported at least one TEAE, and six (4%) reported at least one SAE during the first 26 weeks. During the extension period, the overall incidence of TEAEs decreased slightly to 68% (n = 92 of the 136 patients in the Japan extended group) and the incidence of SAEs remained at 4% (n = 6).
Table 2. Overview of adverse events.
| EBID→EQW | EQW | |||
|---|---|---|---|---|
| 0–26 Weeks | 26–52 Weeks | 0–26 Weeks | 26–52 Weeks | |
| (n = 77) | (n = 62) | (n = 78) | (n = 74) | |
| TEAEs†, n (%) | 60 (78) | 38 (61) | 61 (78) | 54 (73) |
| SAEs‡, n (%) | 3 (4)§ | 2 (3)¶ | 3 (4)†† | 4 (5)‡‡ |
| Discontinuation due to AE, n (%) | 11 (14) | 2 (3) | 1 (1) | 3 (4) |
| TEAEs occurring in ≥5% of patients in either treatment group | ||||
| Nausea, n (%) | 27 (35) | 3 (5) | 8 (10) | 3 (4) |
| Nasopharyngitis, n (%) | 9 (12) | 11 (18) | 8 (10) | 8 (11) |
| Injection site induration, n (%) | 1 (1) | 5 (8) | 25 (32) | 1 (1) |
| Vomiting, n (%) | 10 (13) | 2 (3) | 8 (10) | 6 (8) |
| Constipation, n (%) | 15 (20) | 2 (3) | 10 (13) | 2 (3) |
| Diarrhea, n (%) | 7 (9) | 1 (2) | 6 (8) | 0 (0) |
| Injection site pruritus, n (%) | 4 (5) | Not reported | 7 (9) | Not reported |
| Decreased appetite, n (%) | 8 (10) | 0 (0) | 2 (3) | 2 (3) |
| Abdominal discomfort, n (%) | 8 (10) | 0 (0) | 0 (0) | 1 (1) |
| Injection site erythema, n (%) | 1 (1) | 0 (0) | 5 (6) | 1 (1) |
| Tinea pedis, n (%) | Not reported | 3 (5) | Not reported | 2 (3) |
| Abdominal pain upper, n (%) | Not reported | 3 (5) | Not reported | 1 (1) |
| Cellulitis, n (%) | Not reported | 3 (5) | Not reported | 0 (0) |
†Treatment‐emergent adverse events (TEAEs) during weeks 26–52 were defined as adverse events that occurred for the first time or existed before week 26 and worsened after the first injection at week 26 through to study termination. ‡A patient might have had more than one serious adverse event (SAE). §Ileus (n = 1), pneumonia (n = 1), rotator cuff syndrome (n = 1). ¶Cellulitis (n = 1), rotator cuff syndrome (n = 1), subcutaneous abscess (n = 1). ††Atrial tachycardia (n = 1), diplegia (n = 1), pneumonia hemophilus (n = 1), small intestinal hemorrhage (n = 1). ‡‡Atrial tachycardia (n = 1), cerebral infarction (n = 1), mechanical ileus (n = 1), pneumonia hemophilus (n = 1). AE, adverse events; EBID, 10 mcg exenatide b.i.d.; EBID→EQW, patients assigned to 10 mcg exenatide b.i.d. during the initial 26‐week study who were switched to 2 mg exenatide once weekly for the extended 26 weeks; EQW, 2 mg exenatide once weekly; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Among patients in the EBID→EQW group, the majority of discontinuations as a result of adverse events (primarily due to nausea [7 of 11 discontinuations]) occurred during the first 26 weeks of the study, when patients were on EBID. The incidences of most gastrointestinal related events (nausea, vomiting, constipation, diarrhea) decreased when patients switched from EBID to EQW. However, the incidence of injection‐site induration increased on switching from EBID to EQW. One patient in the EBID→EQW group whose pancreatic enzymes were above the normal range at baseline discontinued during the extension study period because of an adverse event of abnormal pancreatic enzymes.
For patients who remained on EQW for the entire study, the incidence of overall TEAEs was consistent across the initial and the extension study periods. The incidences of most gastrointestinal related events (nausea, constipation, diarrhea) decreased during the extension period compared with the initial 26 weeks, as did injection‐site related events (induration, pruritus, erythema). No major hypoglycemic episodes were reported. The incidence of minor hypoglycemia decreased after patients in the EBID→EQW group switched to EQW (15 patients reported 35 total episodes during the first 26 weeks; 5 patients reported six total episodes during the extension period). For patients who remained on EQW throughout the entire study, the incidence of minor hypoglycemia remained relatively constant across the two study periods (six patients [eight episodes] during the first 26 weeks; eight patients [nine episodes] during the extension period). All patients who reported minor hypoglycemia were taking a SU. The incidences of symptoms of hypoglycemia decreased for both treatment groups during the extension period (EBID→EQW 11% [seven patients]; EQW 7% [five patients]) compared with the initial 26 weeks (EBID→EQW 32% [25 patients]; EQW 26% [20 patients]).
A total of 13 patients (21.0%) in the EBID→EQW group and 23 patients (31.1%) in the EQW group reported at least one adverse event that started during the 10‐week follow‐up period. Of these, the most frequently reported adverse events were nasopharyngitis (EBID→EQW 1.6%; EQW 5.4%), diabetic retinopathy (EBID→EQW 0.0%; QW 4.1%), bronchitis (EBID→EQW 1.6%; QW 1.4%), constipation (EBID→EQW 3.2%; QW 0.0%) and dizziness (EBID→EQW 1.6%; QW 1.4%).
Antibody Status and Treatment Emergent Adverse Events by Antibody Status
For both groups, the incidence of antibodies to exenatide increased through to approximately 38 weeks and decreased thereafter. At the 52‐week end‐point, 40% of patients in the EBID→EQW group had negative titers and 60% were antibody‐positive, with the majority (51% of all EBID→EQW patients) showing low antibody titers (<625) and a minority (9%) showing higher titers (≥625). A similar trend was observed in the EQW group, with 51% of patients antibody‐positive (36% low; 15% higher titer) at the end‐point.
The potential impact of the formation of antibodies to exenatide on safety was examined by summarizing TEAEs by antibody status. The incidence of potentially immune‐related TEAEs was determined over the entire study period and broken down by antibody status (three‐level) measured at 52 weeks15. At the end‐point, for both treatment groups, the incidence of patients with at least one potentially immune‐related TEAE was the highest within the groups of patients with low titer antibodies (11–19% of patients with low titer antibodies). The most frequent immune‐related TEAE reported was injection‐site induration, observed in four EBID→EQW patients and two EQW patients with low titer antibodies, and in one EBID→EQW patient with higher titer antibodies.
Glycemic Control
Improvements in HbA1c were maintained for 52 weeks of treatment in both treatment groups (Figure 2). Patients who switched from EBID to EQW at 26 weeks initially experienced a rise in HbA1c from 7.5% at 26 weeks to 7.8% at 30 weeks. This was followed by a gradual decline in HbA1c over the remainder of the study to a final value of 7.3% at 52 weeks. Patients who took EQW throughout the study maintained relatively consistent HbA1c values between 26 and 46 weeks (7.3%), followed by a slight increase to 7.4% at 52 weeks. At weeks 26 and 52, mean (standard deviation) decreases from baseline in HbA1c were −1.3% (1.0) and −1.4% (1.2) in the EBID→EQW group, and −1.4% (1.0) and −1.3% (1.0) in the EQW group.
Figure 2.
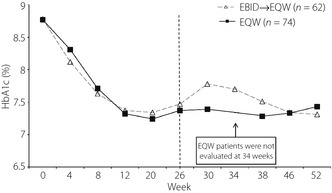
Mean glycated hemoglobin (HbA1c) values by week of study for both treatment groups. EBID, 10 mcg exenatide b.i.d.; EBID→EQW, patients assigned to 10 mcg exenatide b.i.d. during the initial 26‐week study who were switched to 2 mg exenatide once weekly for the extended 26 weeks; EQW, 2 mg exenatide once weekly; HbA1c, glycated hemoglobin.
Analyses of the proportions of patients achieving HbA1c ≤7 and ≤6.5%, at 26 and 52 weeks included only patients whose HbA1c was above the specific target HbA1c level at baseline. At week 26, the proportions of patients who achieved HbA1c ≤7 and ≤6.5% were 36 and 18%, respectively, for the EBID→EQW group; and were 42 and 15%, respectively, for the EQW group. By week 52, the proportions of patients who achieved HbA1c ≤7, and ≤6.5% increased from the week 26 end‐point to 45 and 21%, respectively, for the EBID→EQW group; and to 43 and 24%, respectively, for the EQW group.
Baseline FSG value was 167 mg/dL for both groups. Changes from baseline to end‐point in FSG were similar for patients in the EBID→EQW group (−29.3 mg/dL) and for those who remained on EQW (−31.4 mg/dL) during the extension study. Patients in the EBID→EQW group experienced a slight decrease in FSG from 143 mg/dL at week 26 to 138 mg/dL at end‐point, and patients who remained on EQW during the extension study experienced a slight increase in FSG between week 26 (130 mg/dL) and the end‐point (136 mg/dL).
Blood glucose values, as measured by SMBG profiles (Figure 3) decreased from baseline for both groups. During the first 26 weeks, patients initially taking EBID showed much improved morning and evening meal postprandial glucose control compared with baseline. After switching at week 26 to EQW, the EBID→EQW group showed increases in 2‐h postprandial glucose values at 52 weeks compared with 26 weeks, but these remained reduced from baseline values by 31–72 mg/dL. Improvements in the SMBG profile observed at 26 weeks for patients in the EQW group were maintained over the duration of the extension study.
Figure 3.
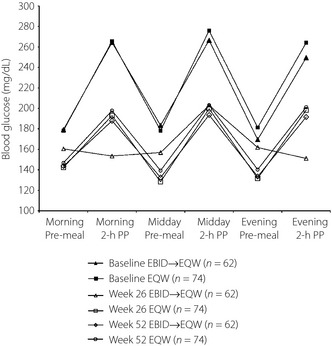
Self‐monitored blood glucose profiles. EBID, 10 mcg exenatide b.i.d.; EBID→EQW, patients assigned to 10 mcg exenatide b.i.d. during the initial 26‐week study who were switched to 2 mg exenatide once weekly for the extended 26 weeks; EQW, 2 mg exenatide once weekly; ITT, intent‐to‐treat; PP, postprandial.
HbA1c Changes by Antibody Status
The change in HbA1c from baseline to end‐point was analyzed by antibody to exenatide status (at end‐point). Patients in the EBID→EQW group who were antibody‐negative (n = 18) experienced a mean decrease in HbA1c of 1.7% (range +0.1 to −3.9%), and those who were antibody‐positive with low titers (<625; n = 36) had a mean decrease of 1.4% (range +0.8 to −4.2%). The small number of EBID→EQW patients who were antibody‐positive with higher titers (≥625; n = 7) experienced a smaller mean reduction in HbA1c of 0.4% (range +1.2 to −2.0%). The EQW group showed a similar pattern with HbA1c reductions for patients who were antibody‐negative (n = 37), antibody‐positive with low titers (n = 27) and antibody‐positive with higher titers (n = 12) of 1.6% (range 0 to −3.5%), 1.4% (range +1.2 to −3.5%) and 0.1% (range +1.5 to −1.7%), respectively.
Bodyweight
Baseline bodyweight was 70.8 kg for the EBID→EQW group and 68.2 kg for the EQW group. Patients who switched from EBID to EQW experienced a slight increase in bodyweight from 69.2 kg at week 26 to 69.4 kg at the end‐point, whereas patients who remained on EQW during the extension study experienced a slight decrease in bodyweight between week 26 (67.7 kg) and the end‐point (67.3 kg; Figure 4). Change in bodyweight from baseline to endpoint was greater for the EBID→EQW group (−1.4 kg) than the EQW group (−0.9 kg).
Figure 4.
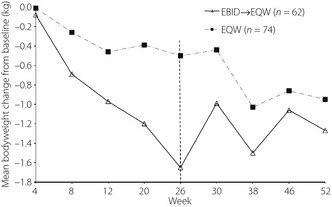
Mean change in bodyweight by week of study for both treatment groups. EBID, 10 mcg exenatide b.i.d.; EBID→EQW, patients assigned to 10 mcg exenatide b.i.d. during the initial 26‐week study who were switched to 2 mg exenatide once weekly for the extended 26 weeks; EQW, 2 mg exenatide once weekly.
Fasting Serum Lipids
Only slight changes in mean values for fasting serum lipids were observed for both groups of patients (Table 3).
Table 3. Changes in serum lipids.
| EBID→EQW (n = 62) | EQW (n = 74) | Total (n = 136) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change to W26 | Change to W52 | Baseline | Change to W26 | Change to W52 | Baseline | Change to W26 | Change to W52 | |
| Total cholesterol (mg/dL) | 195 (29) | −7 (26) | −10 (24) | 193 (32) | −8 (22) | 0 (28) | 194 (31) | −8 (24) | −5 (27) |
| HDLC (mg/dL) | 55 (13) | −3 (7) | −1 (7) | 56 (15) | −2 (7) | −1 (6) | 56 (14) | −2 (7) | −1 (6) |
| LDLC (mg/dL) | 113 (30) | −3 (22) | −9 (21) | 110 (30) | −5 (23) | +1 (25) | 111 (30) | −4 (22) | −3 (24) |
| Triglycerides(mg/dL) | 135 (60) | −3 (44) | 0 (45) | 138 (68) | −4 (55) | −4 (58) | 137 (64) | −4 (50) | −2 (53) |
Data are presented as mean (standard deviation). EBID, 10 mcg exenatide b.i.d.; EBID→EQW, patients assigned to 10 mcg exenatide b.i.d. during the initial 26‐week study who were switched to exenatide once weekly for the extended 26 weeks; EQW, 2 mcg exenatide once weekly; HDLC, high‐density lipoprotein cholesterol; LDLC, low‐density lipoprotein cholesterol; W26, week 26; W52, week 52.
Discussion
For both EQW and EBID treatment groups in this exclusively Japanese population, the incidences of gastrointestinal‐related adverse events decreased during the extension period relative to the initial 26 weeks. This is consistent with previous observations of decreased nausea incidence over time for both EBID16 and EQW17, as well as reports of lower incidence of nausea in EQW‐treated patients vs EBID‐treated patients12.
No patients in the extension study experienced major hypoglycemia. Few patients experienced minor hypoglycemia and this occurred only in patients using a concomitant SU. Concomitant use of SU is known to present an increased risk for hypoglycemia, so this observation was not unexpected.
Titers of antibody to exenatide varied across the study for both treatment groups. For either treatment group, the incidence of positive antibody titer status peaked between 12–38 weeks, then declined through to 52 weeks. Immune‐related adverse events were primarily related to injection‐site reactions and, for both treatment groups, occurred most frequently in the group of patients that showed low titer antibodies. These results are similar to those of Fineman et al., who recently carried out a post‐hoc pooled analysis of several EBID and EQW trials, and reported that mean titers peaked between 6–22 weeks and subsequently declined, and that treatment with EBID or EQW was associated with an increase in injection‐site reactions15.
The mean HbA1c reduction (approximately 1.3%) at 52 weeks was similar between treatment groups. Patients remaining on EQW for the entire 52 weeks maintained glucose control between weeks 26 and 52. These results are consistent with a previous study of EQW of similar duration in a mostly Caucasian study population18. The initial rise in HbA1c observed in patients who switched from EBID to EQW was likely a result of the difference in the pharmacokinetic profile of each exenatide formulation18. EBID must be administered within 60 min before morning and evening meals, and exerts its effects during the postprandial period, whereas EQW is released gradually and requires several weeks to achieve steady state18. Therefore, a temporary decline in glycemic control would not be unexpected during the time required to reach steady‐state plasma concentration after switching to EQW. An analogous trend was observed by Buse et al. for FPG in patients who switched to EQW after 30 weeks of treatment with EBID18. Patients who switched to EQW experienced a transient increase in FPG, which subsequently declined to a level similar to the level observed in patients who had remained on EQW throughout the entire study18.
For both treatment groups, the mean change in HbA1c was similar for patients who had negative or low titer antibodies; however, in the small group of patients who had higher titer antibodies, an attenuated mean response was observed. The Fineman et al. study found that exenatide treatment was often associated with low titer anti‐exenatide antibodies (32% of patients taking EBID, 45% of patients taking EQW), and that efficacy in the low titer group was relatively unaffected15. They also showed that, within the less common higher titer antibody group (5% EBID, 12% EQW), increasing antibody titer was associated with reduced average efficacy that was statistically significant for EQW15. However, it should be noted that, like the Fineman et al. study, we observed that the range of HbA1c responses was similar across antibody‐negative patients and antibody‐positive patients. Therefore, an individual's antibody status was not predictive of glycemic response to exenatide and is consequently not useful for making treatment decisions15.
Weight loss at 52 weeks appeared to be greater for patients originally treated with EBID (−1.4 kg) compared with patients originally on EQW (−0.9 kg). This was likely due to what appeared to be a faster rate of weight loss over the first 26 weeks in Japanese patients taking EBID compared with those taking EQW. This would be consistent with observations over the first 26 weeks in the overall Asian population in which treatment with EBID was associated with greater weight loss than treatment with EQW14.
One limitation of the present study is its open‐label design that might have influenced patients' expectations of treatment, biasing study results. Additionally, the extension period of the study was uncontrolled. As such, there was no comparator arm against which to judge EQW effects on safety and efficacy in this study population. Another possible limitation was the small number of patients included in this Japanese patient population, complicating the generalization of our results to Japanese patients as a whole.
In conclusion, this 26‐week uncontrolled extension study showed that EQW was generally safe and well tolerated, and maintained glycemic control over a total of 52 weeks of treatment in this population of Japanese patients with type 2 diabetes. Exenatide once weekly might offer a treatment option as an adjunctive therapy with OAMs, such as SU, MET, TZD or any two of these taken in combination.
Acknowledgements
This study was funded by Eli Lilly and Company, and Amylin Pharmaceuticals, Inc. The authors appreciate the contributions of all investigators, co‐investigators, clinical research coordinators and participants of this clinical study in Japan. The authors thank Magaly Perez for excellent oversight of operational aspects of the study, Kate Turner and Barbara McLean of PharmaNet/i3, an InVentiv Health Company, Indianapolis, USA, for writing and editorial assistance, respectively.
Yukiko Onishi has accepted lecturing fees from Eli Lilly and Company. Takeshi Imaoka is a full‐time employee of Eli Lilly Japan, and Marilyn K Boardman is a full‐time employee of Eli Lilly and Company; both are shareholders of Eli Lilly and Company. All other authors are full‐time employees of their respective institutions and have no competing interests to declare.
(J Diabetes Invest, doi: 10.1111/jdi.12000, 2013)
Glycated hemoglobin (HbA1c) values are expressed as NGSP values.
References
- 1.Wild S, Roglic G, Green A, et al Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Neville SE, Boye KS, Montgomery WS, et al Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25: 705–716 [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Yamazaki K, Hirao K, et al The status of diabetes control and antidiabetic drug therapy in Japan–a cross‐sectional survey of 17,000 patients with diabetes mellitus (JDDM 1). Diabetes Res Clin Pract 2006; 73: 198–204 [DOI] [PubMed] [Google Scholar]
- 4.Arai K, Matoba K, Hirao K, et al Present status of sulfonylurea treatment for type 2 diabetes in Japan: second report of a cross‐sectional survey of 15,652 patients. Endocr J 2010; 57: 499–507 [DOI] [PubMed] [Google Scholar]
- 5.Heine RJ. Role of sulfonylureas in non‐insulin dependent diabetes mellitus: part II – ‘The cons’. Horm Metab Res 1996; 28: 522–526 [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki T, Namba M, Imaoka T, et al Improved glycemic control and reduced bodyweight with exenatide: a double‐blind, randomized, phase 3 study in Japanese patients with suboptimally controlled type 2 diabetes over 24 weeks. J Diabetes Invest 2011; 2: 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buse JB, Henry RR, Han J, et al Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in sulfonylurea‐treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628–2635 [DOI] [PubMed] [Google Scholar]
- 8.Kendall DM, Riddle MC, Rosenstock J, et al Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Ratner RE, Han J, et al Effects of exenatide (exendin‐4) on glycemic control and weight over 30 weeks in metformin‐treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092–1100 [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency . Bydureon: EPAR – Summary for the Public. [Internet] [Updated 2011 May; accessed on 2012, February 14]. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002020/human_med_001457.jsp&mid=WC0b01ac058001d124&murl=menus/medicines/medicines.jsp&jsenabled=true
- 11.U.S. Food and Drug Administration . Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. [Internet] [Updated 2012 July; accessed on 2012, July 13]. Available at: http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=022200&TABLE1=OB_Rx [Google Scholar]
- 12.Drucker DJ, Buse JB, Taylor K, et al Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet 2008; 372: 1240–1250 [DOI] [PubMed] [Google Scholar]
- 13.Blevins T, Pullman J, Malloy J, et al DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 1301–1310 [DOI] [PubMed] [Google Scholar]
- 14.Ji L, Onishi Y, Chul Ahn CW, et al Efficacy and Safety of Exenatide Once Weekly Versus Exenatide Twice Daily in Asian Patients with Type 2 Diabetes. International Diabetes Federation; Dubia, Saudia Arabia: December 5, 2011: P1144. [Internet] [accessed on 2012, July 20] Available at: http://conference2.idf.org/dubai2011/CM.NET.WebUI/CM.NET.webUI.SCPR/SCPRfunctiondetail.aspx?confID=05000000-0000-0000-0000-000000000001&sesID=05000000-0000-0000-0000-000000000509&absID=07000000-0000-0000-0000-000000003708 [Google Scholar]
- 15.Fineman MS, Mace KF, Diamant M, et al Clinical relevance of anti‐exenatide antibodies: safety, efficacy and cross‐reactivity with long‐term treatment. Diabetes Obes Metab, 2012; 14: 546–554 [DOI] [PubMed] [Google Scholar]
- 16.Macconell L, Brown C, Gurney K, et al Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo‐controlled and comparator‐controlled clinical trials. Diabetes Metab Syndr Obes 2012; 5: 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamant M, Van Gaal L, Stranks S, et al Safety and efficacy of once‐weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care 2012; 35: 683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buse JB, Drucker DJ, Taylor KL, et al DURATION‐1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010; 33: 1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]


