Abstract
Aims/Introduction
Recent studies have pointed to the effectiveness of combination therapy with an angiotensin‐converting‐enzyme inhibitor (ACEI) and an angiotensin receptor blocker (ARB) for diabetic nephropathy. However, some controversy over this combination treatment remains and the mechanisms underlying its renoprotective effects have not been fully clarified. Therefore, we compared the renoprotective effects of imidapril (ACEI) and losartan (ARB) combination therapy with losartan monotherapy in patients with diabetic nephropathy. We also compared the anti‐inflammatory and anti‐oxidative stress effects of these two treatments.
Materials and Methods
A total of 32 Japanese patients with type 2 diabetes and nephropathy were enrolled. Patients were randomized to either 100 mg/day losartan (n = 16) or 50 mg/day losartan plus 5 mg/day imidapril (n = 16). We evaluated clinical parameters, serum concentrations of high‐sensitivity C‐reactive protein (hs‐CRP), soluble intercellular adhesion molecule‐1 (sICAM‐1), interleukin‐18 (IL‐18) and monocyte chemotactic protein‐1 (MCP‐1), and the urinary concentrations of IL‐18, MCP‐1 and 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) at 24 and 48 weeks after starting treatment.
Results
Blood pressure was not significantly different between the two groups. The serum levels of hs‐CRP, sICAM‐1 and IL‐18, as well as urinary excretion of albumin, IL‐18 and 8‐OHdG decreased significantly in the combination therapy group at 48 weeks. The percent decreases in serum IL‐18 concentrations and urinary IL‐18 and 8‐OHdG were significantly greater in the combination therapy group than in the monotherapy group.
Conclusions
Combination therapy with an ACEI and an ARB could be beneficial for treating diabetic nephropathy through its anti‐inflammatory and anti‐oxidative stress effects.
Keywords: Combination, Diabetic nephropathy, Renin–angiotensin system
Introduction
It is widely accepted that chronic inflammation is profoundly involved in the development of atherosclerosis1. Adhesion molecules, pro‐inflammatory cytokines and chemokines, including soluble intercellular adhesion molecule‐1 (sICAM‐1), interleukin‐18 (IL‐18) and monocyte chemotactic protein‐1 (MCP‐1), are involved in the pathogenesis of diabetic nephropathy as well as atherosclerosis2. C‐reactive protein (CRP) is a good marker for cardiovascular risk4, and is a precipitating factor for diabetic nephropathy5. Angiotensin II, which is produced by the renin–angiotensin system (RAS), is known to promote inflammation6. Inhibition of the RAS and associated inflammation might be renoprotective in chronic renal diseases, including diabetic nephropathy6.
Oxidative stress is a critical pathogenic component of atherosclerosis and diabetic nephropathy8. After the onset of renal disorders, the levels of pro‐inflammatory cytokines and oxidative stress begin to increase, inducing cardiovascular diseases through vascular endothelial dysfunction9. Furthermore, an increase in oxidative stress has been reported in hyperglycemic rats10. While activation of the RAS increases oxidative stress, angiotensin‐converting enzyme inhibitors (ACEI)11 and angiotensin II type 1 receptor blockers (ARB)12 inhibit oxidative stress.
Combination therapy with an ACEI and an ARB has been considered in several renal diseases to protect the kidney by potently inhibiting RAS activity13. In the context of diabetic nephropathy6, combination therapy was found to reduce albuminuria. However, most of the studies testing ACEI/ARB combination therapy in diabetic nephropathy were short‐term observational studies, and clinical studies attempting to elucidate the mechanisms underlying these renoprotective effects are not sufficient15.
In patients with diabetic nephropathy, ACEI/ARB combination therapy is expected to have more potent anti‐inflammatory and anti‐oxidative stress effects than monotherapy at the systemic and local levels in the kidney. Combination therapy might also inhibit the development or progression of atherosclerosis and diabetic nephropathy more potently than monotherapy. However, to our knowledge, no clinical studies have evaluated the anti‐inflammatory and anti‐oxidative stress effects of combination therapy. Therefore, we carried out a randomized controlled study of ACEI/ARB combination therapy vs ARB monotherapy in patients with type 2 diabetes and early nephropathy to compare the anti‐inflammatory and anti‐oxidative stress effects of these therapies.
Materials and Methods
Study Design
Patients meeting the following inclusion criteria were eligible for the present study: age 30–74 years, diagnosed with type 2 diabetes, disease duration ≥7 years, diagnosis of diabetic neuropathy and retinopathy, creatinine clearance (CCr; determined using the Cockcroft–Gault formula) >60 mL/min and urinary albumin/creatinine ratio (ACR) >30 mg/gCr. The diagnosis of type 2 diabetes was made according to the World Health Organization criteria.
Patients meeting any of the following criteria were excluded from the present the study: patients with chronic inflammatory disease or malignancies, pregnant women or women who wished to become pregnant, patients with renovascular hypertension, patients with hemoglobin A1C (A1C; National Glycohemoglobin Standardization Program) >9.4%; patients with blood pressure (BP) >180/110 mmHg and patients who had participated in another clinical trial within 3 months before enrolling in the present study. At the discretion of the investigator, patients who had previously used an ACEI or ARB that could not be washed out were also excluded. Concomitant use of steroids, potassium‐sparing diuretics, digoxin, or anti‐arrhythmic drugs except for β‐blockers and calcium channel blockers was not allowed. Concomitant use of new statins or thiazolidinedione derivatives was not allowed during the washout or observation periods. Blood glucose levels were to be controlled by adjusting the dose of medications already in use.
Written informed consent was obtained from all participants. The present study was approved by the Ethics Committee of Okayama Saiseikai General Hospital.
The design of the present study is summarized in Figure 1a. During the washout period, previous ACEIs or ARBs were discontinued and switched to amlodipine (5 mg/day). The observation period was started 8 weeks after starting treatment with amlodipine. In the present study, we treated patients with losartan (ARB) and imidapril (ACEI) or losartan alone during the observation period. Amlodipine was stopped when starting combination or monotherapy.
Figure 1.
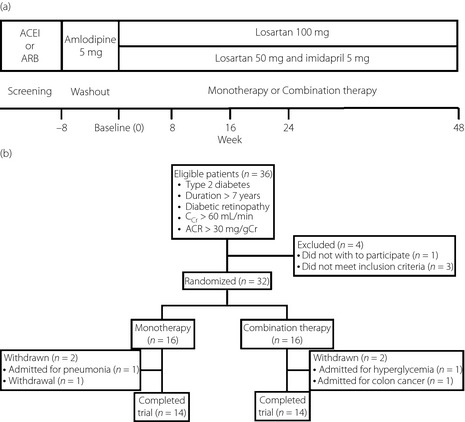
(a) Study design. During the washout period, angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) were discontinued and switched to amlodipine (5 mg/day). At the end of the washout period, patients were allocated to either monotherapy (100 mg/day losartan) or combination therapy (50 mg/day losartan plus 5 mg/day imidapril) and followed for 48 weeks. (b) Patient disposition. Four patients were excluded from this study during the washout period, including one patient who refused to participate in the study, two patients with an ACR < 30 mg/gCr and one patient with CCr < 60 mL/min. Of the 32 patients who started the observation period, 28 were treated for 48 weeks and were included in the analysis.
For losartan monotherapy, the losartan dose was 100 mg/day. In combination therapy, the doses of losartan and imidapril were 50 and 5 mg/day, respectively. The first patient enrolled in the study was allocated to monotherapy, with subsequent patients allocated to one of the two therapies.
During the observation period, patients were to be discontinued or withdrawn from the study if BP could not be maintained at <180/110 mmHg, CCr decreased to 60 mL/min, serum creatinine increased from baseline by ≥30%, or if an adverse drug reaction possibly related to therapy occurred.
Data Collection
After the baseline visit, patients were instructed to visit the hospital at 8, 16, 24 and 48 weeks. At each visit, physical findings, BP, hematological parameters, urinalysis parameters and complications were assessed (Figure 1b). Venous blood and urine samples were obtained in the early morning after an overnight fast. BP was measured by the same person in the morning. After a 5‐min rest in a sitting position, BP was measured in triplicate to calculate the mean for assessment.
Analysis of Biomarkers
The primary objective of the present study was to evaluate the anti‐inflammatory and anti‐oxidative stress effects of imidapril/losartan combination therapy by measuring the serum concentrations of high‐sensitivity CRP (hs‐CRP), sICAM‐1, IL‐18, MCP‐1 and aldosterone, as well as the urinary concentrations of IL‐18, MCP‐1, aldosterone and 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG). Blood and urine samples were centrifuged immediately after collection, and the supernatants were stored at −80 and −30°C, respectively, until analysis. The concentrations of the pro‐inflammatory biomarkers were analyzed using samples obtained at baseline and at weeks 24 and 48. All samples were measured together once all specimens were collected.
An immunoturbidimetric assay was used for urinary albumin concentrations (Nitto Boseki Co. Ltd, Tokyo, Japan) and an immunonephelometric assay kit was used for hs‐CRP concentrations (Dade Behring, Marburg, Germany). Enzyme‐linked immunosorbent assay kits were used to measure IL‐18 (MBL, Nagoya, Japan), sICAM‐1 concentration and MCP‐1 concentrations (R&D Systems, Inc, Minneapolis, MN, USA), and 8‐OHdG concentration (Nikken SEIL Co. Ltd, Tokyo, Japan). A radioimmunoassay kit was used to measure aldosterone concentrations (TFB Co. Ltd, Tokyo, Japan). A1C was measured using a high‐pressure liquid chromatography method and is expressed in National Glycohemoglobin Standardization Program values. The urinary markers were divided by the urinary creatinine concentration to calculate the creatinine ratio for assessment.
Statistical Analysis
Comparisons of baseline factors between the two therapeutic groups were analyzed by Student's unpaired t‐test for continuous parameters or chi‐squared‐test for categorical parameters (sex and previous therapy). Baseline concentrations of pro‐inflammatory markers, serum and urinary aldosterone concentrations, and urinary 8‐OHdG concentrations were compared using Student's unpaired t‐test. The percent change (% change) from baseline for pro‐inflammatory biomarkers, serum and urinary aldosterone concentrations, urinary 8‐OHdG concentration, A1C, BP,and CCr at 24 and 48 weeks was calculated and analyzed by analysis of variance (anova). The differences in the percent change of these parameters at each time between the two therapeutic groups were analyzed by anova. All analyses were two‐sided with a significance level of 5%. Data are expressed as the number for categorical variables, mean change from baseline for percent changes and mean ± standard deviation for continuous variables.
Results
A total of 36 patients who met the inclusion criteria were screened (Figure 1b). Before screening, all of the patients had received an ACEI and/or ARB. Four patients were excluded from the study during the washout period, including one patient who refused to participate in the study, two patients with an ACR < 30 mg/gCr and one patient with CCr < 60 mL/min. Therefore, 32 patients started the study. In two patients allocated to combination therapy, the doses of losartan and imidapril were 25 and 2.5 mg/day, respectively, for the first 4 weeks because their systolic BP (SBP) was <120 mmHg. The doses in these patients were subsequently increased to 50 and 5 mg/day, respectively. During the observation period, two patients allocated to combination therapy were excluded from the study; one patient was hospitalized for hyperglycemia and one patient underwent surgery for colon carcinoma. In the monotherapy group, two patients were excluded during the observation period; one patient was hospitalized for pneumonia and one patient stopped visiting the hospital. Consequently, 14 patients in each therapeutic group, a total of 28 patients, completed 48 weeks of observation and were included in the analysis (Table 1). There were no significant differences in baseline parameters between the two therapeutic groups, including age, sex, duration of diabetes, body mass index, A1C, Ccr, SBP, diastolic BP (DBP), ACR and previous therapy.
Table 1. Baseline background of patients in each group.
| Mono | Combination | |
|---|---|---|
| No. patients | 14 | 14 |
| Age (years) | 61.4 ± 8.8 | 61.7 ± 5.3 |
| Sex (male/female) | 9/5 | 10/4 |
| Duration of diabetes (years) | 11.7 ± 4.7 | 15.6 ± 6.7 |
| Body mass index (kg/m2) | 25.4 ± 2.9 | 24.5 ± 5.1 |
| A1C (%) | 7.8 ± 1.5 | 7.8 ± 0.8 |
| Creatinine clearance (mL/min) | 85.4 ± 16.1 | 90.1 ± 36.1 |
| Systolic blood pressure (mmHg) | 136 ± 6 | 134 ± 12 |
| Diastolic blood pressure (mmHg) | 79 ± 6 | 79 ± 7 |
| Albumin creatinine ratio (mg/gCr) | 224 ± 197 | 270 ± 202 |
| Medication (n) | ||
| Insulin | 5 | 4 |
| Oral hyperglycemic agents | 5 | 9 |
| Statins | 5 | 4 |
A1C, hemoglobin A1C; Mono, monotherapy.
Data are n or mean ± standard deviation.
None of the patients developed new cerebrovascular disease, cardiovascular disease, arteriosclerosis obliterans, progression of nephropathy, excessive BP decrease or hyperkalemia during the study. Urinalysis showed no evidence of ketonuria, hematuria or urinary tract infection.
Time‐Course of A1C, BP and CCr
There were no significant differences in percent change in A1C at 8, 16, 24 or 48 weeks between the monotherapy (7.8 ± 1.6, 8.0 ± 1.2, 8.0 ± 1.3 and 7.9 ± 1.2%, respectively) and combination therapy groups (7.8 ± 0.6, 8.1 ± 1.0, 8.1 ± 1.0 and 7.8 ± 0.8%, respectively). Similarly, there were no significant differences in SBP (monotherapy 137 ± 7, 136 ± 8, 135 ± 7 and 134 ± 8 mmHg; combination therapy 134 ± 12, 133 ± 10, 137 ± 10 and 134 ± 9 mmHg), DBP (monotherapy 80 ± 6, 79 ± 7, 81 ± 7 and 79 ± 5 mmHg; combination therapy 79 ± 8, 80 ± 8, 80 ± 8 and 78 ± 8 mmHg) or CCr (monotherapy 84.6 ± 18.6, 85.2 ± 20.4, 82.8 ± 18.6 and 84.6 ± 21.6 mL/min; combination therapy 85.2 ± 31.8, 87.6 ± 33.6, 86.4 ± 34.2 and 86.4 ± 30.6 mL/min) at 8, 16, 24 and 48 weeks of treatment.
Time‐Course of Serum Concentrations of Pro‐Inflammatory Biomarkers and Aldosterone
At baseline, we found no significant differences in the serum concentrations of hs‐CRP, sICAM‐1, IL‐18, MCP‐1 or aldosterone between the two groups (Table 2).
Table 2. Change of the serum levels of inflammatory molecules and aldosterone.
| Serum molecules | Week 0 | Week 24 | Week 48 |
|---|---|---|---|
| hsCRP (mg/L) | |||
| All patients | 1.33 ± 0.77 | 1.04 ± 0.79 | 0.71 ± 0.60 |
| % change | −13.7 | −23.5* | |
| Mono | 1.39 ± 0.93 | 1.24 ± 1.01 | 1.04 ± 0.75 |
| % change | −0.4 | −16.6 | |
| Combination | 1.26 ± 0.56 | 0.83 ± 0.39 | 0.78 ± 0.36 |
| % change | −28.0* | −31.0‡ | |
| sICAM‐1 (pg/mL) | |||
| All patients | 275 ± 76 | 256 ± 74 | 250 ± 54 |
| % change | −5.9* | −6.4 | |
| Mono | 282 ± 96 | 265 ± 100 | 262 ± 65 |
| % change | −5.4 | −2.1 | |
| Combination | 269 ± 52 | 247 ± 35 | 238 ± 38 |
| % change | −6.4 | −10.6‡ | |
| IL‐18 (pg/mL) | |||
| All patients | 195 ± 62 | 209 ± 89 | 194 ± 75 |
| % change | +7.2 | 0.0 | |
| Mono | 180 ± 49 | 214 ± 107 | 207 ± 73 |
| % change | +16.4 | +11.6 | |
| Combination | 211 ± 72 | 204 ± 69 | 182 ± 53 |
| % change | −2.0 | −11.4*,§ | |
| MCP‐1 (pg/mL) | |||
| All patients | 308 ± 95 | 283 ± 78 | 282 ± 63 |
| % change | −6.3* | −5.6* | |
| Mono | 331 ± 114 | 295 ± 84 | 291 ± 71 |
| % change | −7.8 | −8.0 | |
| Combination | 285 ± 68 | 270 ± 74 | 273 ± 56 |
| % change | −4.8 | −3.2 | |
| Aldosterone (pmol/L) | |||
| All patients | 2470 ± 1180 | 2230 ± 930 | 1760 ± 950 |
| % change | −5.4 | −27.0‡ | |
| Mono | 2680 ± 1220 | 2510 ± 840 | 1860 ± 1210 |
| % change | +1.4 | −31.2‡ | |
| Combination | 2260 ± 1150 | 1960 ± 960 | 1650 ± 610 |
| % change | −12.1* | −22.7† | |
Data are means ± SD or frequencies (%).*P < 0.05, †P < 0.01, ‡P < 0.005 versus baseline. §P < 0.05 for combination versus monotherapy (Mono).
In all patients, the serum hs‐CRP concentration decreased significantly from baseline by 23.5% at 48 weeks. In the combination therapy group, the serum hs‐CRP concentration decreased significantly from baseline by 28.0 and 31.0% at 24 and 48 weeks, respectively. The serum hs‐CRP concentration decreased by 16.6% at 48 weeks in the monotherapy group, although not significantly (Table 2). The serum sICAM‐1 concentration decreased significantly by 10.6% at 48 weeks in the combination therapy group, but did not change significantly in the monotherapy group (Table 2). The serum IL‐18 concentration decreased significantly by 11.4% at 48 weeks in the combination therapy group, but tended to increase in the monotherapy group, resulting in a significant difference in the percent change between the two groups at 48 weeks (Table 2). In all patients, the serum MCP‐1 concentration decreased significantly from baseline by 6.3 and 5.6% at 24 and 48 weeks, respectively. The serum MCP‐1 concentration decreased in both therapeutic groups, although the percent change was not significant. The serum aldosterone concentration decreased significantly from baseline at 48 weeks in all patients and in both groups (Table 2).
Time‐Course of ACR and Urinary Concentrations of IL‐18, MCP‐1, Aldosterone and 8‐OHdG
At baseline, there were no significant differences between the two groups in ACR or urinary concentrations of IL‐18, MCP‐1, aldosterone or 8‐OHdG (Table 3).
Table 3. Change of the urinary levels of albumin/creatinine ratio, inflammatory molecules, aldosterone and 8‐hydroxy‐2′‐deoxyguanosine.
| Urinary molecules | Week 0 | Week 24 | Week 48 |
|---|---|---|---|
| ACR (mg/gCr) | |||
| All patients | 252 ± 197 | 200 ± 202 | 175 ± 159 |
| % change | −8.3‡ | −11.0‡ | |
| Mono | 234 ± 197 | 209 ± 221 | 182 ± 128 |
| % change | −3.5 | −6.2* | |
| Combination | 270 ± 202 | 192 ± 189 | 168 ± 191 |
| % change | −12.8‡,§ | −15.4‡,§ | |
| IL‐18 (pg/mL/Cr) | |||
| All patients | 57 ± 62 | 33 ± 34 | 13 ± 14 |
| % change | −15.7 | −24.3 | |
| Mono | 39 ± 35 | 25 ± 17 | 19 ± 17 |
| % change | −7.3 | +6.8 | |
| Combination | 75 ± 77 | 40 ± 45 | 8 ± 8 |
| % change | −24.1 | −55.3‡,§ | |
| MCP‐1 (pg/mL/Cr) | |||
| All patients | 318 ± 192 | 205 ± 101 | 340 ± 244 |
| % change | −15.4 | +22.4* | |
| Mono | 341 ± 220 | 216 ± 109 | 351 ± 216 |
| % change | −15.4 | +32.8 | |
| Combination | 294 ± 164 | 195 ± 95 | 329 ± 277 |
| % change | −15.4 | +12.0 | |
| Aldosterone (pmol/L/Cr) | |||
| All patients | 100 ± 70 | 48 ± 45 | 68 ± 54 |
| % change | −50.9‡ | −29.1‡ | |
| Mono | 125 ± 79 | 68 ± 52 | 89 ± 59 |
| % change | −35.7* | −22.6* | |
| Combination | 77 ± 68 | 29 ± 28 | 47 ± 41 |
| % change | −65.1‡ | −35.2 | |
| 8‐OHdG (pg/mL/Cr) | |||
| All patients | 12.8 ± 5.0 | 8.2 ± 4.2 | 8.6 ± 3.4 |
| % change | −28.6‡ | −26.6‡ | |
| Mono | 13.7 ± 3.8 | 9.7 ± 4.3 | 10.7 ± 3.1 |
| % change | −23.8* | −15.4‡ | |
| Combination | 12.0 ± 5.9 | 6.8 ± 3.6 | 6.5 ± 2.3 |
| % change | −33.5† | −37.8‡,§ | |
Data are means ± SD or frequencies (%). *P < 0.05, †P < 0.01, ‡P < 0.005 versus baseline. §P < 0.05 for combination versus monotherapy (Mono).
In all patients, ACR decreased significantly by 8.3 and 11.0% at 24 and 48 weeks, respectively. ACR decreased significantly by 6.2% at 48 weeks in the monotherapy group and by 12.8 and 15.4% at 24 and 48 weeks, respectively, in the combination therapy group. The percent change (decrease) in ACR was significantly greater in the combination therapy group than in the monotherapy group at both times (Table 3). In all patients, the urinary IL‐18 concentration tended to decrease over time, although not significantly. In the combination therapy group, the urinary IL‐18 concentration decreased significantly by 55.3% at 48 weeks, and the percent change was significantly different from that in the monotherapy group (Table 3). In all patients, the urinary MCP‐1 concentration decreased by 15.4% at 24 weeks, but increased significantly by 22.4% at 48 weeks. In both groups, the urinary MCP‐1 concentration was increased at 48 weeks. The urinary aldosterone concentration was significantly decreased at all times in all patients and in both treatment groups; however, there were no significant differences in the percent changes between the two groups (Table 3). In all patients, the urinary 8‐OHdG concentration decreased significantly from baseline by 28.6 and 26.6% at 24 and 48 weeks, respectively. The urinary 8‐OHdG concentration decreased significantly by 23.8% at 24 weeks in the monotherapy group and by 33.5 and 37.8% at 24 and 48 weeks, respectively, in the combination therapy group. The percent change in 8‐OHdG concentration at 48 weeks was significantly greater in the combination therapy group than in the monotherapy group (Table 3).
Discussion
The present study showed that treatment with losartan and imidapril was more effective than treatment with amlodipine in decreasing ACR as well as inflammatory and oxidative stress markers in serum and urine in Japanese patients with type 2 diabetes and nephropathy. These effects were maintained for 48 week. Combination therapy with losartan and imidapril was more useful in decreasing ACR, and pro‐inflammatory and oxidative stress markers than losartan administered at a double dose, without differences in BP or glycemic control. These results suggest that the losartan/imidapril combination might be useful to prevent the progression of atherosclerosis and nephropathy in patients with type 2 diabetes by exerting anti‐inflammatory and anti‐oxidative effects through inhibition of the RAS.
The RAS and downstream inflammatory activities are involved in the progression of atherosclerosis16. In the present study, treatment with losartan and imidapril did not significantly change A1C or BP, but hs‐CRP levels at 24 and 48 weeks, and sICAM‐1 levels at 48 weeks were significantly lower than those at baseline; these changes were not observed in the monotherapy group. Amlodipine was reported to have anti‐inflammatory and anti‐oxidative stress effects, and to inhibit the production of hs‐CRP and sICAM‐117. RAS inhibitors also have anti‐inflammatory effects, which are favorable cardiovascular effects, independent of their antihypertensive effects19. The present study showed that losartan and imidapril could inhibit the progression of atherosclerosis by their anti‐inflammatory effects through inhibition of the RAS more potently than amlodipine.
The serum IL‐18 concentration tended to be increased in the monotherapy group, but decreased significantly in the combination therapy group, resulting in a significant difference between the two groups at 48 weeks. Serum IL‐18 is an important prognostic predictor of diabetic nephropathy and atherosclerosis21. It has been reported that ARBs and ACEIs decrease IL‐18 by inhibiting the RAS22. In the present study, amlodipine was only administered during the washout period. Therefore, the efficacy of losartan and imidapril represented the change from treatment with amlodipine. Based on our searches of the literature, amlodipine has no known effect on serum IL‐18 concentrations. These results indicate that imidapril/losartan combination therapy could have better anti‐inflammatory efficacy in patients with type 2 diabetes with nephropathy, as compared with amlodipine or losartan monotherapy.
Similar to microinflammation, oxidative stress is profoundly involved in the development of atherosclerosis and diabetic complications23. 8‐OHdG is a biomarker of systemic DNA damage in diabetic nephropathy24. In the present study, the urinary 8‐OHdG concentration decreased significantly after treatment with losartan and imidapril, as compared with baseline levels. The decrease in urinary 8‐OHdG concentrations was greater in the combination therapy group than in the monotherapy group. It has been reported that amlodipine17, ACEI11 and ARB12 suppress oxidative stress. However, it is unclear which of these agents or which combination of these drugs elicits the greatest reduction in oxidative stress in patients with diabetic nephropathy. The present results suggest that imidapril/losartan combination therapy might be more effective in preventing the development or progression of diabetic nephropathy because of its more potent inhibitory effects on oxidative stress than losartan monotherapy. These effects were maintained for up to 48 weeks.
The urinary aldosterone concentration was also decreased more markedly by combination therapy than monotherapy in the present study, which suggests that combination therapy more potently inhibits the RAS in the kidney. The urinary IL‐18 concentration was also significantly decreased by combination therapy as compared with monotherapy. We previously reported that serum and urine IL‐18 concentrations are predictors of diabetic nephropathy21. IL‐18 secretion in monocytes and macrophages is stimulated by inflammation and oxidative stress associated with hyperglycemia25. These results suggest that the combination of losartan and imidapril might be useful to prevent the development of early nephropathy in patients with type 2 diabetes by decreasing ACR, and reducing inflammation and oxidative stress in the kidney through more potent inhibition of the RAS.
The main limitation of the present study was that the observation period was not long enough to evaluate renal or cardiovascular outcomes. The Olmesartan Reducing Incidence of Endstage Renal Disease in Diabetic Nephropathy Trial (ORIENT)26, and Ongoing Telmisartan Alone and in Combination with Ramipril Global End Point Trial (ONTARGET)27 studies both showed that combination therapy with an ACEI and an ARB did not have significant benefits on renal outcomes. However, in the ORIENT study26, combination therapy with olmesartan and an ACEI reduced proteinuria, as observed in the present study, but did not further improve renal or cardiovascular outcomes. The reason for these discrepancies remains unclear. Further studies are required to confirm the beneficial effect of combination therapy with an ARB and an ACEI on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.
In the present study, the sample size of 28 patients was small, meaning larger studies are required to confirm these results. Although the serum and urinary concentrations of several biomarkers decreased after monotherapy or combination therapy, some changes were not statistically significant, perhaps because of the small sample size. Although amlodipine was used during the washout period to prevent possible changes in BP, we measured ambulatory BP at a single time, so we cannot exclude the possible effects of BP on the decrease in ACR. A larger clinical study using 24‐h BP monitoring might be required to verify the results of the present study.
In conclusion, a combination of an ACEI and an ARB could be beneficial for treating diabetic nephropathy through their anti‐inflammatory and anti‐oxidative stress effects.
Acknowledgements
This study was supported in part by a Grant‐in‐Aid for Scientific Research (C) to K Shikata (21591031), a Grant‐in‐Aid for Young Scientists (B) to D Ogawa (23790942) from the Ministry of Education, Culture, Sports, Science and Technology, and by a Grant‐in‐Aid for Diabetic Nephropathy Research from the Ministry of Health, Labour and Welfare of Japan. This work was also supported by the Takeda Science Foundation, the Naito Foundation, the Japan Foundation for Applied Enzymology, and the Ryobi Teien Memory Foundation. We thank the staff at Okayama Saiseikai General Hospital for their cooperation in this study. The authors have no potential conflicts of interest relevant to this study to report.
(J Diabetes Invest, doi: 10.1111/jdi.12004, 2013)
References
- 1.Ross R. Atherosclerosis‐an inflammatory disease. N Engl J Med 1990; 340: 115–126 [DOI] [PubMed] [Google Scholar]
- 2.Fornoni A, Ijaz A, Tejada T, et al Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 2008; 4: 10–17 [DOI] [PubMed] [Google Scholar]
- 3.Navarro‐González JF, Mora‐Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 433–442 [DOI] [PubMed] [Google Scholar]
- 4.Blake GJ, Rifai N, Buring JE, et al Blood pressure, C‐reactive protein, and risk of future cardiovascular events. Circulation 2003; 108: 2993–2999 [DOI] [PubMed] [Google Scholar]
- 5.Abrahamian H, Endler G, Exner M, et al Association of low‐grade inflammation with nephropathy in type 2 diabetic patients: role of elevated CRP‐levels and 2 different gene‐polymorphisms of proinflammatory cytokines. Exp Clin Endocrinol Diabetes 2007; 115: 38–41 [DOI] [PubMed] [Google Scholar]
- 6.Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int 2005; 67: 799–812 [DOI] [PubMed] [Google Scholar]
- 7.Ferrari P. Prescribing angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Nephrology 2007; 12: 81–89 [DOI] [PubMed] [Google Scholar]
- 8.Tomohiro T, Kumai T, Sato T, et al Hypertension aggravates glomerular dysfunction with oxidative stress in a rat model of diabetic nephropathy. Life Sci 2007; 80: 1364–1372 [DOI] [PubMed] [Google Scholar]
- 9.Descamps‐Latscha B, Witko‐Sarsat V, Nguyen‐Khoa T, et al Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis 2005; 45: 39–47 [DOI] [PubMed] [Google Scholar]
- 10.Mori T, Cowley AW Jr. Role of pressure in angiotensin II‐induced renal injury: chronic servo‐control of renal perfusion pressure in rats. Hypertension 2004; 43: 752–759 [DOI] [PubMed] [Google Scholar]
- 11.Tojo A, Onozato ML, Kobayashi N, et al Angiotensin II and oxidative stress in Dahl Salt‐sensitive rat with heart failure. Hypertension 2002; 40: 834–839 [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Mori T, Nako K, et al Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension 2006; 47: 699–705 [DOI] [PubMed] [Google Scholar]
- 13.Kunz R, Friedrich C, Wolbers M, et al Meta‐analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148: 30–48 [DOI] [PubMed] [Google Scholar]
- 14.Jennings DL, Kalus JS, Coleman CI, et al Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta‐analysis. Diabet Med 2007; 24: 486–493 [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Teo KK, Pogue J, et al Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559 [DOI] [PubMed] [Google Scholar]
- 16.Ferrario CM, Strawn WB. Role of the renin‐angiotensin‐aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 2006; 98: 121–128 [DOI] [PubMed] [Google Scholar]
- 17.Yoshii T, Iwai M, Li Z, et al Regression of atherosclerosis by amlodipine via anti‐inflammatory and anti‐oxidative stress actions. Hypertens Res 2006; 29: 457–466 [DOI] [PubMed] [Google Scholar]
- 18.Kataoka C, Egashira K, Ishibashi M, et al Novel anti‐inflammatory actions of amlodipine in a rat model of arteriosclerosis induced by long‐term inhibition of nitric oxide synthesis. Am J Physiol Heart Circ Physiol 2004; 286: 768–774 [DOI] [PubMed] [Google Scholar]
- 19.Koh KK, Ahn JY, Han SH, et al Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol 2003; 42: 905–910 [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Iwai M, Wu L, et al Important role of nitric oxide in the effect of angiotensin‐converting enzyme inhibitor imidapril on vascular injury. Hypertension 2003; 42: 542–547 [DOI] [PubMed] [Google Scholar]
- 21.Nakamura A, Shikata K, Hiramatsu M, et al Serum interleukin‐18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 2005; 28: 2890–2895 [DOI] [PubMed] [Google Scholar]
- 22.Lapteva N, Ide K, Nieda M, et al Activation and suppression of renin‐angiotensin system in human dendritic cells. Biochem Biophys Res Commun 2002; 296: 194–200 [DOI] [PubMed] [Google Scholar]
- 23.Pan HZ, Chang D, Feng LG, et al Oxidative damage to DNA and its relationship with diabetic complications. Biomed Environ Sci 2007; 20: 160–163 [PubMed] [Google Scholar]
- 24.Xu GW, Yao QH, Weng QF, et al Study of urinary 8‐hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal 2004; 36: 101–104 [DOI] [PubMed] [Google Scholar]
- 25.Arnalich F, Hernanz A, Lopez‐Maderuelo D, et al Enhanced acute‐phase response and oxidative stress in older adults with type II diabetes. Horm Metab Res 2000; 32: 407–412 [DOI] [PubMed] [Google Scholar]
- 26.Imai E, Chan JC, Ito S, et al Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo‐controlled study. Diabetologia 2011; 54: 2978–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559 [DOI] [PubMed] [Google Scholar]


