Abstract
Aims/Introduction
Pancreatic cancer (PC) is related to diabetes. Long‐standing diabetes should be a prerequisite, whereas new‐onset hyperglycemia might be a result of PC. However, the association between diabetes and PC is still in dispute.
Materials and Methods
We investigated the relationship between glucose metabolism and other factors by retrospectively analyzing the clinical data of 331 PC patients. Any histopathological type was eligible. The patients were divided into three groups: group A, normal glucose metabolism; group B, hyperglycemia duration≤6 months; and group C, diabetes duration >24 months.
Results
The prevalence of hyperglycemia was 59.5%. Most patients were diagnosed with diabetes mellitus either concomitantly with cancer (39.0%) or within 6 months before cancer diagnosis (6.9%). There were more females in group C than group A (P = 0.005) and B (P = 0.018). Patients in group A were younger (A vs B, P < 0.001; A vs C, P = 0.032) and thinner (A vs B, P = 0.013; A vs C, P = 0.027). In group C, more individuals shared a family history of diabetes (A vs C, P = 0.004; B vs C, P = 0.023), but fewer smoked (A vs C, P = 0.027; B vs C, P = 0.020). Patients in group C had a larger proportion of poorly differentiated cancer (A vs C, P = 0.002; B vs C, P = 0.012). No differences in glucose metabolism were found among the different histological types.
Conclusions
We further support the notion that diabetes duration >24 months might not be cancer related. Older and fatter PC patients were more likely to develop hyperglycemia. More patients with long‐standing diabetes had poor tumor differentiation. We speculate that smoking and alcohol intake might advance PC onset.
Keywords: Diabetes duration, Pancreatic cancer, Risk factors
Introduction
More than one century ago, people began to notice that pancreatic cancer (PC) might be correlated with diabetes mellitus (DM). The increased prevalence of DM in PC patients has been well established. However, as both diseases have long latencies, the association between diabetes and PC, causal or consequential, is still in dispute.
The results of previous studies show that long‐standing diabetes is a risk factor for the development of PC. The strength of this claim is only moderate1. In a recent meta‐analysis of 17 case–control and 19 cohort or nested case–control studies published between 1966 and 2005, the combined age and sex‐adjusted odds ratio (OR) for PC associated with type 2 diabetes was 1.82 (95% confidence interval [CI] 1.66–1.89). Meanwhile, there is increasingly more evidence to support the notion that impaired glucose metabolism (IGM) might be an early symptom of PC. For individuals with a shorter history of diabetes (<4 years), the risk of PC was approximately 50% greater than the patients with more than 5 years of history (relative risk 1.5 vs 2.1; P = 0.005)2. New data show that diabetes or hyperglycemia is present in up to 40% of PC3, which is usually detected soon before the clinical manifestations of PC4, and can be improved or remitted after cancer resection3.
Previous studies, pathologically, were mostly confined to pancreatic ductal adenocarcinoma (PDAC). In contrast, in the present study, any histopathological type was eligible. The present study was designed to investigate the relationship between glucose metabolism and other factors (e.g. pathological type, differentiation, tumor location, tumor markers, etc.) by retrospectively analyzing the clinical data of 331 PC patients. The differences between the clinical profiles of patients who do and do not develop DM might provide clues to the pathogenesis of pancreatic cancer‐related DM.
Materials and Methods
Study Population
Of the 904 PC patients hospitalized in Huashan Hospital, College of Medicine, Fudan University (Shanghai, China) from January 2000 to June 2010, 449 newly diagnosed patients who had undergone an operation, including radical operation, palliative operation with biopsy or exploratory operation, were considered eligible. Excluded patients included the following: (i) those who had neither preoperative fasting plasma glucose (FPG) test results nor a prior history of DM, impaired glucose tolerance (IGT) and impaired fasting glucose (IFG; n = 8); (ii) those who had no postoperative histopathological diagnosis (n = 18); (iii) those who had a previous history of cancer at another organ site (n = 23); (iv) primary tumor was not in the pancreas (n = 5); (v) those who had PC recurrence (n = 3); (vi) those who had borderline tumor (n = 3); or (vii) those who were lacking other important information (n = 58). Finally, 331 cases with histopathologically confirmed primary PC were included in the present clinical study, without any age or sex restriction (Figure 1).
Figure 1.
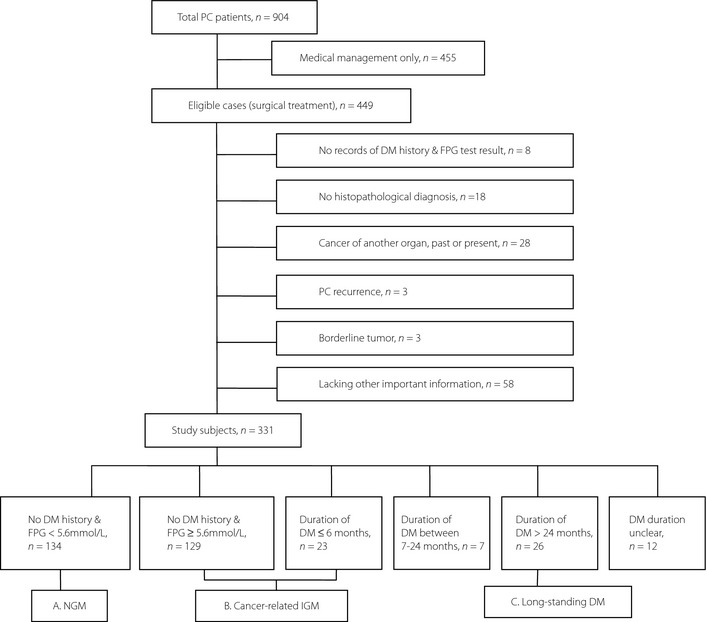
Flowchart of patients' selection and grouping. FPG, fasting plasma glucose; DM, diabetes mellitus; IGM, impaired glucose metabolism; NGM, normal glucose metabolism; PC, pancreatic cancer.
Study Conduct and Data Collection
According to the DM course, the study population can be divided into six groups: (i) normal glucose metabolism (NGM), that is FPG < 5.6 mmol/L on admission without DM history (n = 134); (ii) FPG ≥ 5.6 mmol/L on admission without DM history (n = 129); (iii) duration of DM ≤ 6 months (n = 23); (iv) duration of DM ranges from 7 to 24 months (n = 7); (v) duration of DM > 24 months (n = 26); and (vi) duration of DM unclear (n = 12; Figure 1).
In previous studies, DM duration ≤2 years was defined as ‘new‐onset diabetes’4. However, a new concept of cancer‐related IGM (i.e. hyperglycemia course ≤6 months) was more reasonable for the present study. First, the analysis of the chronology between hyperglycemia diagnosis and the clinical manifestation of PC supported this statement. For patients with DM duration >24 months, at the time they met criteria for DM, none had previous cancer‐related symptoms. For the patients with IGM duration ≤6 months, most (93.6%) already had cancer‐related symptoms. Whereas for the middle part, half received the DM diagnosis first, and half had cancer‐related symptoms first. Second, it was reported that PC was frequently undetectable or resectable on CT scans carried out ≥6 months before clinical diagnosis and seldom could be detected before 24 months9. Third, the natural history of PC was situated at 5.57 ± 2.63 months11. A total of 30% of the patients died within 1 month of diagnosis, and 90% died within 1 year12. Therefore, the use of 6 months as the division to define cancer‐related IGM might increase the specificity of the analysis. Accordingly, we regrouped the study population into three parts: group A, normal glucose metabolism (NGM), FPG < 5.6 mmol/L on admission without DM history (n = 134); group B, cancer‐related IGM, duration of DM ≤ 6 months or FPG ≥ 5.6 mmol/L on admission (n = 152); and group C, long‐standing DM, duration of DM > 24 months (n = 26; Figure 1). The patients with DM duration that ranged from 7 to 24 months (n = 7) were not included in the analysis between these three groups, as the relationship between DM and PC was obscure for them, and these patients only made up a small proportion in totality.
DM was defined as FPG ≥ 7.0 mmol/L on admission or a previous diagnosis of DM, according to the American Diabetes Association criteria of 2010. IFG was defined as FPG level ≥ 5.6 mmol/L and <7.0 mmol/L. NGM was defined as FPG level < 5.6 mmol/L without DM history. IGM was the general name for DM and IFG. The course of DM was calculated from the date of DM diagnosis to the date of PC diagnosis. For those diagnosed on admission, the course was recorded as less than 6 months. Serum glucose measurements were obtained after fasting for routine clinical purposes. Alcohol intake was estimated according to the patient's report of duration and frequency. It was defined as consumption of beer, wine or liquor at least once per week for 1 year or more. Family history of PC was restricted to first‐degree or second‐degree relatives. The measurement of tumor size came from the greatest tumor diameter of the histopathological report. The histological classification was based on the World Health Organization classification of tumors of 200013.
Statistical Analysis
Comparisons of continuous variables among the three groups were carried out with one‐way anova test for normally distributed variables, and the Kruskal–Wallis test for non‐normally distributed variables; comparisons of categorical variables among the three groups were carried out using the Fisher's exact test or chi‐square test. Then the post‐tests between each two subgroups were carried out with Bonferroni's corrections. Statistical significance was defined as P < 0.05, and all tests were two‐sided. These tests were carried out with SPSS software, version 15.0 (SPSS, Chicago, IL, USA).
Results
Characteristics of the Study Population
Of the 331 PC patients we studied (188 male [56.8%] and 143 female [43.2%]), the mean age was 60.85 years (range 20–83), and the mean body mass index (BMI) was 22.84 ± 3.00 kg/m2. A total of 51 patients (15.4%) had a history of alcohol intake. A total of nine patients (2.7%) reported to have a family history of DM. A large proportion (59.5%) met the criteria for DM (36.5%) or IFG (23.0%). Most were diagnosed with DM either concomitantly with cancer (39.0%) or within half a year before cancer diagnosis (6.9%); therefore, the prevalence of cancer‐related IGM was 45.9%. Long‐standing DM accounted for approximately 7.9%. NGM cases represented 40.5% (Figure 2). Glucose metabolism of different histological types is shown in detail in Table 1, and no statistically significant differences were found among groups (P = 0.354). Most histological reports were moderately (45.6%) or poorly (17.5%) differentiated. The median tumor diameter of the entire PC group was 40 mm (range 8–150 mm). A total of 27 patients (8.2%) were found to have hepatic metastasis during surgery. Of the 26 diabetics in group C, six had never been treated with any hypoglycemic agents; eight had used insulin by itself or with other drugs.
Figure 2.
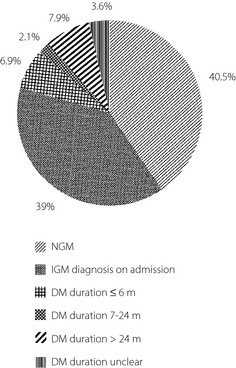
Glucose metabolism of pancreatic cancer (PC) patients. DM, diabetes mellitus; IGM, impaired glucose metabolism; NGM, normal glucose metabolism.
Table 1. Glucose metabolism of different histological type.
| Normal | ≤6 m | 7–24 months | >24 months | Total | |
|---|---|---|---|---|---|
| Cancers of the exocrine pancreas | |||||
| Adenocarcinoma (NOS) | 66 | 64 | 4 | 9 | 143 |
| Ductal adenocarcinoma | |||||
| Ductal adenocarcinoma (NOS) | 42 | 64 | 1 | 11 | 118 |
| Mucinous non‐cystic carcinoma | 4 | 4 | 1 | 9 | |
| Signet‐ring cell carcinoma | 1 | 1 | |||
| Adenosquamous carcinoma | 2 | 3 | 1 | 6 | |
| Undifferentiated carcinoma | 1 | 1 | 2 | ||
| Mixed ductal‐endocrine carcinoma | 1 | 1 | |||
| Serous cystadenocarcinoma | 1 | 1 | |||
| Mucinous cystadenocarcinoma | 3 | 1 | 1 | 5 | |
| Intraductal papillary mucinous carcinoma | 1 | 2 | 1 | 4 | |
| Acinar cell carcinoma | |||||
| Acinar cell carcinoma (NOS) | 2 | 4 | 6 | ||
| Mixed acinar‐endocrine carcinoma | 1 | 1 | |||
| Solid‐pseudopapillary carcinoma | 2 | 2 | |||
| Squamous cell carcinoma | 2 | 2 | |||
| Cancers of the endocrine pancreas | |||||
| Islet cell carcinoma | 1 | 1 | |||
| Neuroendocrine carcinoma | 8 | 5 | 1 | 14 | |
| Others | 1 | 2 | 3 | ||
| Total | 134 | 152 | 7 | 26 | 319 |
NOS, not otherwise specified.
The characteristics of the three groups are presented in Table 2. The sex composition was different among the three groups; more females were found in group C (P = 0.016). For the patients without a previous history of DM, those who developed cancer‐related IGM were found to be older, fatter and larger in tumor size than the patients who did not (P = 0.001, 0.038 and 0.019, respectively). Additionally, group C had a higher level of carcinoembryonic antigen (CEA) and worse histological differentiation than the others.
Table 2. Features of pancreatic cancer patients among the three groups.
| Group A n = 134 | Group B n = 152 | Group C n = 26 | P (A,B,C) | P (A,B) | P (A,C) | P (B,C) | |
|---|---|---|---|---|---|---|---|
| Male:female | 1:0.63 | 1:0.73 | 1:2.25 | 0.016 | 1.000 | 0.015 | 0.053 |
| Age (years) | 58.00 ± 11.44 | 62.71 ± 9.95 | 63.08 ± 7.62 | <0.001 | 0.001 | 0.073 | 1.000 |
| BMI, kg/m2 (n) † | 22.23 ± 2.86 (120) | 23.15 ± 3.03 (143) | 23.64 ± 2.95 (25) | 0.015 | 0.038 | 0.090 | 1.000 |
| Weight loss (kg) ‡ |
0.00 (125) 0–10 |
0.00 (143) 0–9 |
1.00 (22) 0–14.4 |
0.218 | |||
| Weight loss/usual weight (%)‡ |
0.00 (118) 0–15.7 |
0.00 (138) 0–12.5 |
1.39 (22) 0–21.1 |
0.156 | |||
| Family history of DM (%)§ | 0 | 1.3 | 11.5 | <0.001 | 1.000 | 0.012 | 0.068 |
| Cigarette (%) | 35.1 | 23.0 | 11.5 | 0.013 | 0.078 | 0.059 | 0.881 |
| Alcohol intake (%)¶ | 19.4 | 13.2 | 3.8 | 0.084 | |||
| FPG (mmol/L)‡ |
5.1 (134) 4.1–5.5 |
6.4 (151) 5.6–11.7 |
8.0 (23) 4.1–16.5 |
<0.001 | <0.001 | <0.001 | <0.001 |
| Tumor size (cm) ‡,†† |
3.00 (134) 1.5–7.1 |
4.00 (151) 1.9–8.8 |
3.5 (25) 2.0–8.0 |
0.018 | 0.019 | 0.386 | 1.000 |
| Tumor marker | |||||||
| CA125 (U/mL)‡ |
35.0 (93) 7.1–82.6 |
35.0 (115) 6.6–168.2 |
35.0 (15) 7.2–175.8 |
0.436 | |||
| CA19‐9 (U/mL)‡ |
78.5 (115) 5.0–3786.0 |
168.0 (137) 12.8–3339.4 |
349.8 (22) 4.0–3521.6 |
0.035 | 0.081 | 0.162 | 1.000 |
| CEA (ug/L)‡ |
2.2 (104) 0.7–27.95 |
2.8 (113) 0.9–18.2 |
5.6 (17) 1.3–45.7 |
0.011 | 1.000 | 0.012 | 0.018 |
| CA50 (U/mL)‡ |
29.5 (85) 5.0–278.0 |
63.1 (99) 5.0–293.0 |
83.1 (15) 5.0–272.0 |
0.166 | |||
| CA242 (U/mL)‡ |
15.0 (60) 0.1–150.0 |
21.8 (72) 0.1–152.8 |
17.0 (9) 6.1–104.9 |
0.676 | |||
| Tumor location (n = 331) | |||||||
| Diffuse | 2 | 3 | 1 | 0.139 | |||
| Head | 58 | 75 | 13 | ||||
| Uncinate process | 20 | 24 | 0 | ||||
| Body and tail | 39 | 45 | 10 | ||||
| Ampulla of Vater | 15 | 5 | 2 | ||||
| Differentiation (n = 206) | |||||||
| Well | 6 | 7 | 4 | 0.013 | 0.855 | 0.005 | 0.035 |
| Moderate | 66 | 73 | 5 | ||||
| Poor | 28 | 23 | 7 | ||||
| Undifferentiated | 0 | 3 | 1 | ||||
| Hepatic metastasis (%) | 7.5 | 9.9 | 0 | 0.220 | |||
Values of P<0.05 are considered statistically significant (in bold). †Body mass index (BMI) is the weight in kilograms divided by the square of the height in meters. ‡Variables are reported as medians, n (in parentheses) and 5–95% percentiles for these non‐normally distributed continuous variables. §Family history of PC is restricted to first‐degree or second‐degree relatives. ¶Alcohol intake is defined as consumption of beer, wine or liquor at least once per week for 1 year or more according to patient's report. ††The measurement of tumor size comes from the greatest tumor diameter of histopathological report. CEA, carcinoembryonic antigen; DM, diabetes mellitus; FPG, fasting plasma glucose.
As pancreatic endocrine tumors might have different characteristics based on the cellular origin and affect the glucose metabolism independently through endocrinological processes, we repeated the comparison after ruling out these pancreatic endocrine tumors from groups A, B and C. Then there were 125, 147 and 26 patients left in groups A', B' and C', respectively (Table 3), and the results of comparison were quite similar to the previous comparison (Table 2).
Table 3. Features of pancreatic exocrine tumor patients among the three groups.
| Group A' n = 125 | Group B' n = 147 | Group C' n = 26 | P (A' B' C' ) | P(A' B' ) | P(A' C' ) | P (B' C' ) | |
|---|---|---|---|---|---|---|---|
| Male:female | 1:0.62 | 1:0.71 | 1:2.25 | 0.012 | 1.000 | 0.009 | 0.026 |
| Age | 58.01 ± 11.46 | 62.81 ± 9.92 | 63.08 ± 7.62 | <0.001 | 0.001 | 0.075 | 1.000 |
| BMI, kg/m2 (n)† | 22.18 ± 2.96 (113) | 23.12 ± 3.04 (138) | 23.64 ± 2.95(25) | 0.015 | 0.039 | 0.080 | 1.000 |
| Weight loss (kg)‡ |
0.00 (117) 0–10.1 |
0.00 (138) 0–9.05 |
1.00 (22) 0–14.4 |
0.176 | |||
| Weight loss/usual weight (%)‡ |
0.00 (111) 0–15.8 |
0.00 (133) 0–12.75 |
1.39 (22) 0–21.1 |
0.117 | |||
| Family history of DM (%)§ | 0 | 1.4 | 11.5 | <0.001 | 1.000 | <0.001 | 0.013 |
| Smoking (%) | 36.0 | 23.8 | 11.5 | 0.012 | 0.082 | 0.044 | 0.483 |
| Alcohol intake (%)¶ | 19.2 | 12.9 | 3.8 | 0.090 | |||
| FPG (mmol/L)‡ |
5.1 (125) 4.1–5.5 |
6.5 (146) 5.6–11.7 |
8.0 (23) 4.1–16.5 |
<0.001 | <0.001 | <0.001 | <0.001 |
| Tumor size (cm)‡,†† |
3.00 (125) 1.5–7.4 |
4.00 (146) 1.87–9.24 |
3.5 (25) 2.0–8.0 |
0.030 | 0.015 | 0.383 | 1.000 |
| Tumor marker | |||||||
| CA125 (U/mL)‡ |
35.0 (90) 7.8–84.5 |
35.0 (112) 6.6–170.8 |
35.0 (15) 7.2–175.8 |
0.427 | |||
| CA19‐9 (U/mL)‡ |
84.6 (111) 5.0–4332 |
191.5 (133) 16.1–3348.2 |
349.8 (22) 4.0–3521.6 |
0.028 | 0.052 | 0.191 | 1.000 |
| CEA (ug/L)‡ |
2.6 (100) 0.70–29.42 |
2.8 (110) 0.86–18.91 |
5.6 (17) 1.30–45.7 |
0.012 | 1.000 | 0.014 | 0.020 |
| CA50 (U/mL)‡ |
29.3 (84) 5.0–280.5 |
67.4 (96) 5.0–294.1 |
83.1 (15) 5.0–272.0 |
0.089 | |||
| CA242 (U/mL)‡ |
15.0 (60) 0.1–150.0 |
24.4 (71) 0.1–153.2 |
17.0 (9) 6.1–104.9 |
0.625 | |||
| Tumor location (n = 331) | |||||||
| Deffuse | 1 | 3 | 1 | 0.080 | |||
| Head | 55 | 73 | 13 | ||||
| Uncinate process | 19 | 24 | 0 | ||||
| Body and tail | 36 | 43 | 10 | ||||
| Ampulla of Vater | 14 | 4 | 2 | ||||
| Differentiation (n = 206) | |||||||
| Well | 6 | 6 | 4 | 0.010 | 0.844 | 0.006 | 0..22 |
| Moderate | 65 | 73 | 5 | ||||
| Poor | 28 | 23 | 7 | ||||
| Undifferentiated | 0 | 3 | 1 | ||||
| Hepatic metastasis (%) | 6.8 | 10.5 | 0 | 0.198 | |||
Values of P<0.05 are considered statistically significant (in bold). †Body mass index (BMI) is the weight in kilograms divided by the square of the height in meters. ‡Variables are reported as medians (n), and 5–95% percentiles for these non‐normally distributed continuous variables. §Family history of pancreatic cancer is restricted to first‐degree or second‐degree relatives. ¶Alcohol intake is defined as consumption of beer, wine or liquor at least once per week for 1 year or more according to patient's report. ††The measurement of tumor size comes from the greatest tumor diameter of histopathological report. DM, diabetes mellitus; FPG, fasting plasma glucose.
Besides the comparisons based on glucose metabolism,the mean age of smoking patients when diagnosed with PC was 59.04 years, whereas the mean age of non‐smoking patients was 61.51 years (P = 0.026). The mean age of alcoholic patients when diagnosed with PC was 57.96 years, and the mean age of non‐alcoholism was 61.37 years (P = 0.022). Undifferentiated carcinoma had the largest tumor diameter (median 8.5 cm) compared with well‐ (median 3.0 cm), moderately‐ (median 3.0 cm) and poorly‐differentiated (median 3.5 cm) carcinomas (P = 0.030). Tumors located in the pancreatic body and tail had a bigger size (median 4.33 cm) than those in the pancreatic head (median 3.65 cm) and the uncinate process (median 3.09 cm; P < 0.001). Focusing on adenocarcinoma, mucinous cystadenocarcinoma was larger in tumor size (median 6.5 cm) than PDAC (median 3.36 cm), serous cystadenocarcinoma (median 4.0 cm) and intraductal papillary mucinous carcinoma (median 3.0 cm; P = 0.023). PDAC had the highest level of carbohydrate antigen 19‐9 (median 153.9 U/mL) in adenocarcinoma (P = 0.015).
Discussion
A national study carried out in China in 2008 showed that 9.7% of adults were diabetics. In the present study, 59.5% of PC patients had IGM, which was much higher than in the general population. Thus, the high prevalence of IGM could not simply contribute to type 2 diabetes mellitus or type 1 diabetes mellitus, indicating that there must be some relationship with pancreatic cancer.
In the present study, the proportion of long‐standing diabetes was 7.9% of all the cases. There were 12 diabetics for whom we could not determine their DM course from the clinical records. Taking them into consideration, the real proportion of long‐standing diabetes should range from 7.9 to 11.5%, which was quite close to the prevalence of DM in the general population. Additionally, we noticed that more patients have family histories of DM in group C than in both A and B. This observation implied that there was a genetic component in long‐standing DM rather than it arising as a result of PC. Both results support the notion that long‐standing DM (>24 months) is a pre‐existing condition belonging to type 2 diabetes mellitus, and is not likely to be cancer related.
The prevalence of cancer‐related IGM in the present study was 45.9%, which was lower than expected. The reasons were as follows. First, as 2‐h plasma glucose was not routinely tested, glucose tolerance was not well evaluated. Thus, many IGT cases might have been ignored. Second, the diagnosis of IGM was based on a single FPG test without a glycated hemoglobin value. It was difficult for us to obtain a general view of each patient's glucose metabolism. Third, the reported incidence of ectopic pancreas ranged from 0.55 to 13.7% based on autopsy or laparotomy14. β‐cells were proved to exist in the ectopic pancreas by histological examination, which might be supposed to benefit glycemic regulation. Therefore, for PC patients, the existence of ectopic pancreas might conceal the true extent of IGM resulting from a tumor.
From the analysis of sex differences, both groups A and B had more males than females, whereas group C was the opposite. According to the annual Surveillance, Epidemiology and End Results (SEER; a premier source for cancer statistics in the USA) program on PC, males always make up the majority. We speculate that females with long‐standing DM have a greater risk in developing PC.
The analysis of age and BMI showed that patients with hyperglycemia were found to be older and fatter. For PC patients without long‐standing DM, it was supposed that older and fatter patients were more likely to have β‐cell function deficiency and insulin resistance, so they were more prone to develop IGM as a result of PC.
Fewer smokers were found in group C, which might be because of the larger proportion of female patients; and these patients were more likely to quit smoking and refrain from drinking after being diagnosed with DM. Even though no significant differences were noted among groups on alcohol intake, we still noticed that fewer patients in group C drank alcohol. Smoking and alcohol intake are risk factors of many diseases. The age of PC patients with the addiction to smoking and alcohol was shown to be younger than those without. Therefore, smoking and alcohol could have an effect of advancing PC onset.
In previous studies, tumor size was thought to be related to IGM in PC patients. The bigger the tumor size was, the greater influence there would be on glucose metabolism. Later on, however, one study reported that 60.8% of the patients with small pancreatic cancers (<20 mm in size) had abnormal glucose tolerance16, bringing the claim of tumor size into question. Until recently, it appeared that the development of IGM was associated with peripheral insulin resistance, abnormal inflammatory mediators and impaired islet cell function, either directly or indirectly induced by cancer cells5. In the present study, the median tumor size of group B was significantly bigger than group A (P = 0.015), so we supported that tumor size could be one mechanism behind the development of IGM in PC.
We also noted the distinctions of tumor differentiation among groups. There was a larger proportion of poorly‐differentiated cases in group C than the other groups. Thus, it appears that the long duration of hyperglycemia might have a negative effect on tumor differentiation.
All of the tumor markers investigated in the present study are glycoproteins. As glycoproteins, they could be affected by plasma glucose level and be increased in a hyperglycemia situation. A higher level of CEA was found in group C than the other two groups, which might be attributed to long‐standing hyperglycemia. However, no correlation was observed between CEA and tumor differentiation in the present study.
The strengths of the present study included a large sample size with a clear histological diagnosis and detailed surgery information. As the present study of clinical data was retrospective, the main limitation was the inevitable loss of some information (e.g. the evaluation of glucose tolerance, the postoperation follow up).
In conclusion, from the present study, we found that the prevalence of hyperglycemia and cancer‐related IGM were 59.5 and 45.9%, respectively. We further support the notion that diabetes duration >24 months might not be cancer related. Older and fatter PC patients are more likely to develop hyperglycemia. More patients with long‐standing diabetes have poor tumor differentiation. Although the concomitance between asymptomatic PC and new‐onset IGM is not clear yet, IGM could still be an indication of PC. Further understanding of the radical mechanism of PC might help in discovering an early detection method or providing some suggestions that can improve the quality of life. When an elderly person is newly diagnosed with DM, especially for someone with other risk factors of PC, screening for PC is highly recommended.
Acknowledgements
This research did not receive any specific grant from any funding agency in the public, commercial or not‐for‐profit sector. We are indebted to the staff of the department of pathology (Shanghai Huashan Hospital) for their help in providing the histopathology reports.
(J Diabetes Invest, doi: 10.1111/j.2040-1124.2012.00237.x, 2013)
References
- 1.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta‐analysis. JAMA 1995; 273: 1605–1609 [PubMed] [Google Scholar]
- 2.Huxley R, Ansary‐Moghaddam A, Berrington DGA, et al Type‐II diabetes and pancreatic cancer: a meta‐analysis of 36 studies. Br J Cancer 2005; 92: 2076–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannala R, Leirness JB, Bamlet WR, et al Prevalence and clinical profile of pancreatic cancer‐associated diabetes mellitus. Gastroenterology 2008; 134: 981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chari ST, Leibson CL, Rabe KG, et al Pancreatic cancer‐associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008; 134: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Permert J, Ihse I, Jorfeldt L, et al Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg 1993; 80: 1047–1050 [DOI] [PubMed] [Google Scholar]
- 6.Pannala R, Leibson CL, Rabe KG, et al Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol 2009; 104: 2318–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Q, Cai Q, Li Z, et al The relationship between new‐onset diabetes mellitus and pancreatic cancer risk: a case‐control study. Eur J Cancer 2011; 47: 248–254 [DOI] [PubMed] [Google Scholar]
- 8.Pannala R, Basu A, Petersen GM, et al New‐onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009; 10: 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelaez‐Luna M, Takahashi N, Fletcher JG, et al Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol 2007; 102: 2157–2163 [DOI] [PubMed] [Google Scholar]
- 10.Gangi S, Fletcher JG, Nathan MA, et al Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol 2004; 182: 897–903 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez MJ, Truan AN, Turienzo E, et al Epidemiology of exocrine pancreatic cancer in the Principality of Asturias, 1973‐1992. Rev Esp Enferm Dig 1995; 87: 653–657 [PubMed] [Google Scholar]
- 12.Gudjonsson B, Livstone EM, Spiro HM. Cancer of the pancreas: diagnostic accuracy and survival statistics. Cancer 1978; 42: 2494–2506 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton SR, Aaltonen LE. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of the Digestive System. IARC Press, Lyon, 2000 [Google Scholar]
- 14.Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg 1974; 109: 762–765 [DOI] [PubMed] [Google Scholar]
- 15.Feldman M, Weinberg T. Aberrant pancreas: a cause of duodenal syndrome. JAMA 1952; 148: 893–898 [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya R, Noda T, Harada N, et al Collective review of small carcinomas of the pancreas. Ann Surg 1986; 203: 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso D, Brigato L, Veronesi A, et al The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res 1995; 15: 2585–2588 [PubMed] [Google Scholar]
- 18.Wang F, Larsson J, Abdiu A, et al Dissociated secretion of islet amyloid polypeptide and insulin in serum‐free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol 1997; 21: 157–164 [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Larsson J, Adrian TE, et al In vitro influences between pancreatic adenocarcinoma cells and pancreatic islets. J Surg Res 1998; 79: 13–19 [DOI] [PubMed] [Google Scholar]
- 20.Katsumichi I, Pour PM. Diabetes mellitus in pancreatic cancer: is it a causal relationship? Am J Surg 2007; 194: S71–S75 [DOI] [PubMed] [Google Scholar]
- 21.Fogar P, Basso D, Pasquali C, et al Portal but not peripheral serum levels of interleukin 6 could interfere with glucose metabolism in patients with pancreatic cancer. Clin Chim Acta 1998; 277: 181–189 [DOI] [PubMed] [Google Scholar]


