Abstract
Aims/Introduction
Liraglutide, a glucagon‐like peptide‐1 receptor agonist, is expected to provide a new treatment option for diabetes. However, the suitable timing of liraglutide administration in type 2 diabetic patients has not yet been clarified.
Materials and Methods
We reviewed type 2 diabetic patients (n = 155) who visited the Osaka Red Cross Hospital for glycemic control, with administration of liraglutide at a dose of 0.6 mg (average glycated hemoglobin [HbA1c] level, 8.7 ± 0.1%). The effect of liraglutide based on the pretreatment status was compared. We also analyzed the background factors of both a successful and failed group of patients who switched to liraglutide from insulin.
Results
An improvement in blood glucose levels was confirmed in 122 of 155 patients. During the 4‐month observation period, the improvement in HbA1c levels was significantly greater in the group of drug‐naïve/previous oral hypoglycemic agent (9.1 ± 0.2 to 7.2 ± 0.2%) than that in the group switching from insulin (8.6 ± 0.2 to 7.8 ± 0.2%). In addition, C‐peptide immunoreactivity levels (fasting > 2.2 ng/mL; delta >1.6 ng/mL; urine > 70 μg/day), younger age and a smaller number of insulin units used per day were considered important when deciding on switching to liraglutide from insulin.
Conclusions
Liraglutide was more effective in patients who had not been treated previously or received oral hypoglycemic agents than in patients switching from insulin. With respect to switching to liraglutide from insulin, the most important factors to be considered were C‐peptide immunoreactivity levels, age, and the number of insulin units used per day.
Keywords: Glucagon‐like peptide‐1, Incretin, Type 2 diabetes
Introduction
Incretin‐related drugs include glucagon‐like peptide‐1 (GLP‐1) receptor agonists and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, which suppress glucagon secretion1, confer a β‐cell protective effect3 and do not alter bodyweight6. The aforementioned effects have not been observed with conventional drugs, and these drugs are expected to provide an entirely new treatment option for diabetes. Liraglutide, a GLP‐1 receptor agonist, was approved in Europe in June 2009 and in Japan and the USA in January 2010. In the phase III clinical trial of the Liraglutide Effect and Action in Diabetes (LEAD) study, the safety and effectiveness of liraglutide, as a monotherapy or in various combination therapies with oral hypoglycemic agents (OHAs), was evaluated8. In both studies, liraglutide has shown good blood glucose control compared with control groups. Furthermore, effects such as weight loss, decrease in systolic blood pressure, improved pancreatic β‐cell function and improved cardiovascular markers have been confirmed. In the clinical development program for liraglutide in Japan, two confirmatory tests were carried out15. One study compared the efficacy and safety of liraglutide monotherapy and glibenclamide monotherapy15, and the other compared the efficacy and safety of liraglutide combination therapy with sulfonylurea drugs, and sulfonylurea drug monotherapy17. Both have confirmed the superior hypoglycemic action and safety of liraglutide.
Liraglutide has excellent hypoglycemic effects and can be used in hyperglycemic patients, who have maintained some insulin secretion capacity in response to hyperglycemia. From a long‐term perspective, liraglutide should be aggressively used shortly after diabetes onset because of its protective effects on pancreatic β‐cells that could prevent their dysfunction3. In the present study, we compared liraglutide effects between the following two groups: (i) drug‐naïve patients or those with a relatively short diabetic history who switched from OHAs; and (ii) those with a measureable diabetic progression who switched from insulin. In addition, the suitable timing of liraglutide administration in patients with type 2 diabetes in Japan was evaluated.
Insulin has the most potent hypoglycemic effects and is used widely in diabetes management. However, insulin induces hypoglycemia and weight gain because of its fat accumulation effect19. Because liraglutide treatment can reduce bodyweight6 and is associated with reduced hypoglycemia, weight loss and a lower risk of hypoglycemia are expected on switching to liraglutide from insulin. To establish standards for liraglutide therapy, we compared background characteristics of both a successful and failed group of patients who switched to liraglutide from insulin.
Materials and Methods
Patients with type 2 diabetes who visited to Osaka Red Cross Hospital (Osaka, Japan) between June 2010 and August 2011, and started liraglutide treatment were observed in the present study. Type 2 diabetic patients treated with diet therapy with or without OHAs and/or insulin, had glycated hemoglobin (HbA1c) concentrations >5.5 and <15.8%, were aged between 24 and 87 years, and had bodyweight >40 kg were included in the present study. Patients were excluded if they had detectable glutamic acid decarboxylase antibody, impaired hepatic function, significant cardiovascular disease (heart failure, coronary artery disease or uncontrolled hypertension) or non‐stabilized proliferative retinopathy. Before liraglutide administration, 82 patients were treated with insulin (64 patients with multiple daily injection and 18 with single basal injections), 128 patients were treated with OHAs, including sulfonylurea (62 patients), biguanide (70 patients), thiazolidinedione (30 patients), alpha‐glucosidase inhibitor (11 patients), phenylalanine derivative (7 patients) and DPP‐4 inhibitor (14 patients). In outpatients, the initial liraglutide dose was 0.3 mg, which was increased to 0.6 mg after 1 week. In hospitalized patients, after resolving glucose toxicity with intensive insulin therapy, the initial liraglutide dose was 0.3 mg, which was increased to 0.6 mg after 3 days. Insulin was discontinued on liraglutide administration, whereas OHAs were tapered or added based on each physician's recommendation. Responders included patients with improvement in HbA1c or glycosylated albumin (Gly‐A) levels measured during a 4‐month observation period, or those with HbA1c concentrations <6.5% after switching to liraglutide from insulin. HbA1c values were defined by the National Glycohemoglobin Standardization Program (NGSP) standards.
Ethics
Informed consent was obtained from all patients before study initiation. The present study was approved by the relevant ethics committee and was carried out in accordance with the Declaration of Helsinki.
Statistical Analyses
Statistical analyses were carried out using the StatView 5.0 software (SAS Institute, Inc., Cary, NC, USA). Differences between groups were assessed using paired or unpaired two‐tailed Student's t‐tests. Differences in glycemic control improvement and changes in bodyweight between the drug‐naïve/previous OHA group and the group that switched from insulin was analyzed by anova. Values of P < 0.05 were considered to be statistically significant.
Results
Patients included 74 males and 81 females (n = 155), and their characteristics are shown in Table 1. Compared with the drug‐naïve/previous OHA group, the group that switched from insulin showed longer diabetes history, lower body mass index (BMI) values and lower C‐peptide immunoreactivity (CPR) levels, suggesting diabetes progression. Improvement in blood glucose levels was confirmed in 122 of the 155 patients, and 37 of 122 (30.3%) and 60 of 122 (49.2%) patients achieved HbA1c levels of <6.5 and <7.0%, respectively. Of these 122 patients, 87 were treated with OHAs, including sulfonylurea in 49, biguanide in 35, thiazolidinedione in 13, alpha‐glucosidase inhibitor in four and phenylalanine derivative in four. Liraglutide was discontinued because of its side‐effects in 13 patients, and no improvement was observed in the remaining 20 patients. Side‐effects included nausea (n = 6), discomfort (n = 3), dizziness (n = 1), anorexia (n = 1), abdominal distension (n = 1) and stomach ache (n = 1). Significant hypoglycemia was not detected in any patients during this study. In the drug‐naïve group, improved blood glucose levels were observed in all nine patients (100%). In the group that switched from OHAs to liraglutide, 56 of 64 patients (88%) found liraglutide to be effective, 3 (5%) discontinued liraglutide because of its side‐effects and 5 (7%) discontinued liraglutide because of no improvement in glycemic control. In the group that switched to liraglutide from insulin, 57 of 82 patients (70%) found liraglutide effective, 10 (12%) discontinued liraglutide because of side‐effects and 15 (18%) discontinued liraglutide because they showed no improvement in glycemic control (Figure 1).
Table 1. Characteristics of patients with type 2 diabetes in three different groups divided according to pretreatment status (before administration of liraglutide).
| Drug naive (n = 9) | Previous OHAs (n = 64) | Switched from insulin (n = 82) | |
|---|---|---|---|
| Male:female (n) | 4:5 | 35:29 | 35:47 |
| Inpatients (%) | 7/9 (77.8%) | 32/64 (50.0%) | 18/82 (22.0%) |
| Age (years) | 57.4 ± 4.7 | 62.7 ± 1.4 | 63.1 ± 1.5 |
| DM duration (years) | 5.8 ± 3.3 | 12.8 ± 1.0* | 15.5 ± 1.1**,† |
| BMI (kg/m2) | 30.3 ± 2.4 | 29.4 ± 0.7 | 26.2 ± 0.6*,‡ |
| HbA1c (%) | 10.3 ± 0.9 | 9.0 ± 0.2 | 8.6 ± 0.2* |
| Gly‐A (%) | 28.7 ± 4.6 | 24.1 ± 0.7 | 24.3 ± 0.7 |
| Fasting CPR (ng/mL) | 2.4 ± 0.6 | 2.5 ± 0.2 | 1.7 ± 0.1‡ |
| Maximum CPR (ng/mL) | 4.6 ± 0.8 | 5.1 ± 0.3 | 3.5 ± 0.2‡ |
| Delta CPR (ng/mL) | 2.0 ± 0.7 | 2.5 ± 0.3 | 1.7 ± 0.1 |
| Urine CPR (μg/day) | 82.1 ± 19.3 | 97.1 ± 8.1 | 63.6 ± 5.3‡ |
All values are expressed as mean ± SEM (n = 155).
*P < 0.05, **P < 0.01 vs group of drug naïve; †P < 0.05, ‡P < 0.01 vs group of previous oral hypoglycemic agents (OHAs).
BMI, body mass index; CPR, C‐peptide immunoreactivity; DM, diabetes mellitus; Gly‐A, glycosylated albumin; HbA1c, glycated hemoglobin.
Figure 1.
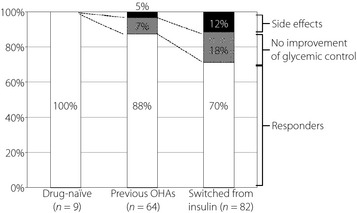
The effect on glycemic control in three different groups before administration of liraglutide. White bars indicate the group of responders. Gray and black bars indicate the group of non‐responders that discontinued liraglutide because glycemic control did not improve and the group that discontinued liraglutide because of side‐effects, respectively. OHAs, oral hypoglycemic agents.
We compared the effect on glycemic control of responders divided according to the pretreatment status (before liraglutide administration). Compared with the group that switched from insulin (n = 57), the group of drug‐naïve/previous OHAs (n = 65) showed no difference in age, but the diabetes history tended to be shorter (11.6 ± 1.0 vs 13.5 ± 1.2 years; P = 0.11), there was a high proportion of hospitalized patients (53.8 vs 21.1%), high BMI values (29.8 ± 0.7 vs 25.8 ± 0.7 kg/m2; P < 0.01), higher fasting CPR levels (2.5 ± 0.2 vs 1.8 ± 0.1 ng/mL; P < 0.01), higher maximum CPR levels (5.1 ± 0.3 vs 3.8 ± 0.2 ng/mL; P < 0.01), higher delta CPR levels before and after breakfast (2.5 ± 0.3 vs 1.9 ± 0.2 ng/mL; P < 0.05), and higher urine CPR levels (93.2 ± 8.0 vs 71.7 ± 6.4 μg/day; P < 0.05), respectively (Table 2). During the 4‐month observation period, improvement in HbA1c levels was observed in the drug‐naïve/previous OHA group (from 9.1 ± 0.2 to 7.2 ± 0.2%) and in the group that switched from insulin (from 8.6 ± 0.2 to 7.8 ± 0.2%). Considerable and significant improvements were observed in both groups, and the extent of improvement was significant in the former (Figure 2a, upper). The improvement in Gly‐A levels was also observed in the drug‐naïve/previous OHAs group (from 24.1 ± 0.9 to 18.7 ± 0.6%) and in the group that switched from insulin (from 24.3 ± 0.9 to 22.0 ± 0.7%). Considerable and significant improvements were observed in both groups, and the extent of improvement was significant in the former (Figure 2a, lower). With respect to bodyweight, a reduction of 4.5 ± 0.6% was observed in the group of drug‐naïve/previous OHAs, with a reduction of 5.1 ± 0.8% in the group that switched from insulin; a significant improvement was observed in both groups, and the extent was comparable between these two groups without significance (Figure 2b). The drug‐naïve/previous OHA group showed a higher proportion of hospitalized patients compared with those who switched from insulin (53.8 vs 21.1%); therefore, hospitalization might influence the effects of liraglutide treatment. We then divided patients into subgroups of hospitalized patients and outpatients, and compared the effects of liraglutide treatment in each. In hospitalized patients, substantial improvement was observed in HbA1c levels in the drug‐naïve/previous OHA group (from 9.8 ± 0.4 to 7.3 ± 0.3%) and in the group that switched from insulin (from 10.0 ± 0.6 to 7.8 ± 0.5%). A significant improvement was observed in both groups, and the extent of improvement was comparable between the two without significance. With respect to bodyweight, a 6.6 ± 1.8% reduction was observed in the drug‐naïve/previous OHA group, and a 6.2 ± 1.3% reduction was observed in the group that switched from insulin; a significant improvement was observed in both, but not significant between the two (Figure 2c, upper). Meanwhile, in the outpatient group, the improvement in HbA1c levels was observed in the drug‐naïve/previous OHA group (from 8.2 ± 0.2 to 7.1 ± 0.2%), and in the group that switched from insulin (from 8.2 ± 0.2 to 7.8 ± 0.2%). A significant improvement was observed in both groups, and the extent was significant in the former. With respect to bodyweight, a 6.6 ± 1.3% reduction was observed in the drug‐naïve/previous OHA group, with a 6.2 ± 1.3% reduction in the group that switched from insulin; a significant improvement was observed in both groups, but was not significant between the two (Figure 2c, lower).
Table 2. Characteristics of responders that confirmed improvement in blood glucose levels and non‐responders that discontinued liraglutide on account of its side‐effects or no improvement of glycemic control (divided with respect to pretreatment status before liraglutide administration).
| Drug‐naïve/previous OHAs | Switched from insulin | |||
|---|---|---|---|---|
| Responders (n = 65) | Non‐responders (n = 8) | Responders (n = 57) | Non‐responders (n = 25) | |
| Male:female (n) | 32:33 | 7:1 | 26:31 | 9:16 |
| Inpatients (%) | 35/65 (53.8) | 4/8 (50.0) | 12/57 (21.1) | 6/25 (24.0) |
| Age (years) | 61.3 ± 1.5 | 67.9 ± 2.6 | 60.3 ± 1.8 | 69.4 ± 2.1‡ |
| DM duration (years) | 11.6 ± 1.0 | 14.9 ± 3.2 | 13.5 ± 1.2 | 20.2 ± 2.0‡ |
| BMI (kg/m2) | 29.8 ± 0.7 | 26.7 ± 0.9 | 25.8 ± 0.7** | 27.2 ± 1.1 |
| HbA1c (%) | 9.1 ± 0.2 | 9.7 ± 0.5 | 8.6 ± 0.2 | 8.7 ± 0.3 |
| Gly‐A (%) | 24.1 ± 0.9 | 28.5 ± 2.7 | 24.3 ± 0.9 | 24.6 ± 1.0 |
| Fasting CPR (ng/mL) | 2.5 ± 0.2 | 2.2 ± 0.3 | 1.8 ± 0.1** | 1.2 ± 0.2‡ |
| Maximum CPR (ng/mL) | 5.1 ± 0.3 | 4.8 ± 0.4 | 3.8 ± 0.2** | 3.0 ± 0.3‡ |
| Delta CPR (ng/mL) | 2.5 ± 0.3 | 2.2 ± 0.5 | 1.9 ± 0.2* | 1.1 ± 0.1‡ |
| Urine CPR (μg/day) | 93.2 ± 8.0 | 112.4 ± 22.4 | 71.7 ± 6.4* | 39.6 ± 6.0‡ |
| Insulin dose (U/day) | – | – | 22.0 ± 1.7 | 32.6 ± 4.4† |
All values are expressed as mean ± SEM.
*P < 0.05, **P < 0.01 versus responders of drug naïve/previous oral hypoglycemic agents (OHAs); †P < 0.05, ‡P < 0.01 versus responders that switched from insulin.
BMI, body mass index; CPR, C‐peptide immunoreactivity; DM, diabetes mellitus; Gly‐A, glycosylated albumin; HbA1c, glycated hemoglobin.
Figure 2.
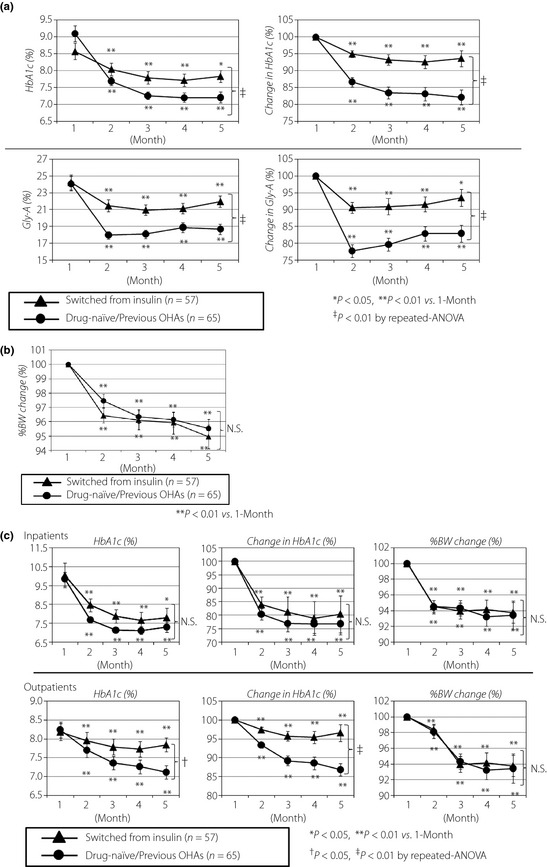
(a) The improvement in glycated hemoglobin (HbA1c) levels in the group of drug‐naïve/previous oral hypoglycemic agents (OHAs; round) and in the group that switched from insulin (triangle) in the 4‐month observation period (upper panel). The improvement in glycosylated albumin (Gly‐A) levels in the group of drug‐naïve/previous OHAs (round) and in the group that switched from insulin (triangle) in the 4‐month observation period (lower panel). (b) The improvement in percent bodyweight in the group of drug‐naïve/previous OHAs (round) and in the group that switched from insulin (triangle) in the 4‐month observation period. (c) The improvement in HbA1c levels and percent bodyweight in the group of drug‐naïve/previous OHAs (round) and in the group that switched from insulin (triangle) in the 4‐month observation period in hospitalized patients (upper panel). The improvement in HbA1c levels and percent bodyweight in the group of drug‐naïve/previous OHAs (round) and in the group that switched from insulin (triangle) in the 4‐month observation period in outpatients (lower panel). The comparison was carried out by paired two‐tailed Student's t‐test and repeated anova tests. All values are expressed as mean ± SEM. BW, bodyweight; N.S., not significant.
In the group that changed from OHAs to liraglutide, eight of 64 patients (12.5%) found liraglutide to be ineffective (Figure 1). We carefully carried out a detailed comparison of background factors between the responders and non‐responders of the drug‐naïve/previous OHA group; however, no significant differences were observed between the two (Table 2, left column).
Next, we carried out a study on the group that switched to liraglutide from insulin. As shown in (Figure 1), 57 of 82 patients (70%) showed improved glycemic control in the group that switched from insulin; in the remaining 25 patients (30%), no improvement was observed or liraglutide was discontinued because of side‐effects. Compared with the non‐responders, the responders were younger (60.3 ± 1.8 vs 69.4 ± 2.1 years; P < 0.01), had a shorter history of diabetes (13.5 ± 1.2 vs 20.2 ± 2.0 years; P < 0.01), had higher fasting CPR levels (1.8 ± 0.1 vs 1.2 ± 0.2 ng/mL; P < 0.01), higher maximum CPR levels (3.8 ± 0.2 vs 3.0 ± 0.3 ng/mL; P < 0.01), higher delta CPR levels before and after breakfast (1.9 ± 0.2 vs 1.1 ± 0.1 ng/mL; P < 0.01), higher urine CPR levels (71.7 ± 6.4 vs 39.6 ± 6.0 μg/day; P < 0.01), and used fewer insulin units per day (22.0 ± 1.7 vs 32.6 ± 4.4 U/day; P < 0.05), respectively (Table 2, right column). We focused on individual CPR levels in blood and urine for both responders and non‐responders (Figure 3a). From this viewpoint, switching to liraglutide from insulin might succeed if fasting CPR was >2.2 ng/mL, if delta CPR before and after breakfast was >1.6 ng/mL, and if urine CPR was >70 μg/day. We also focused on the daily insulin dosage (U/day) and age with respect to switching to liraglutide from insulin (Figure 3b). In elderly patients, liraglutide was discontinued because of side‐effects in most cases. For patients aged 30–70 years, switching to liraglutide from insulin was effective for those using insulin approximately 20 U/day, although some cases were unsuccessful despite using smaller doses (approximately 10 U/day); most cases were unsuccessful in switching if the amount of insulin per day exceeded 40 U/day, except in some younger patients. Therefore, when switching to liraglutide from insulin, although several cases were observed with some exceptions, a cautious decision with confirming endogenous insulin secretion capacity, age and daily insulin usage should be obtained.
Figure 3.
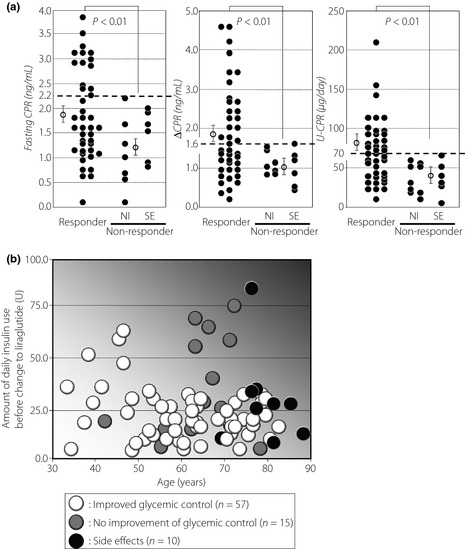
(a) Comparisons of fasting C‐peptide immunoreactivity (CPR; left panel), delta CPR before and after breakfast (middle panel), and urine CPR (right panel) levels between responders and non‐responders. The comparison was carried out by unpaired two‐tailed Student's t‐test. Bars indicate mean ± standard error of the mean. NI, no improvement for glycemic control; SE, side‐effects. (b) Investigation and comparison of background factors between responders (white circles) and non‐responders (gray circles, no improvement of glycemic conrol; black circles, side‐effects) after switching to liraglutide from insulin. The vertical axis represents the amount of daily insulin use before switching to liraglutide, and the horizontal axis represents the age.
Discussion
Type 2 diabetes is a progressive disease, and its pathogenesis includes insulin resistance and insulin secretory dysfunction, which is recognized before disease onset. A decrease by half in pancreatic β‐cell function has been reported on diagnosis20, and a 20% decrease at the stage of impaired glucose tolerance21. Furthermore, compared with the Western population, the Japanese population is more prone to insulin secretion failure caused by pancreatic β‐cell dysfunction22. DPP‐4 inhibitors and GLP‐1 receptor agonists, which are incretin‐related drugs, have a protective effect on pancreatic β‐cells3. Given the long‐term perspective, pancreatic β‐cell dysfunction in diabetic patients can be prevented by relatively early intensive therapy with these agents when postprandial hyperglycemia becomes detectable. In phase III liraglutide clinical trials in Japan, greater improvement in glycemic control was observed in drug‐naïve patients compared with those who switched from oral agents15. However, the difference between the liraglutide effects with respect to pretreatment status, including insulin and disease stage, has not been previously reported. Here, we examined and compared the liraglutide effects in type 2 diabetic patients having a relatively shorter diabetic history and were drug‐naïve or previously treated with OHAs, and patients who switched from insulin. Compared with the group that switched from insulin, the drug‐naïve/previous OHA group showed no differences in age, but their diabetes history tended to be shorter, with higher BMI values, higher CPR levels in blood and urine, and particularly, a more pronounced improvement in blood glucose levels with a higher percentage of responders. From point of this view, liraglutide administration in early diabetic patients who have retained some endogenous insulin secretory capacity are more effective. We propose that liraglutide is more effective in earlier diabetic stages. Randomized clinical trials of incretin‐related drugs including liraglutide should be carried out, and by accumulating evidence on optimal hyperglycemia management, a diabetes treatment algorithm should be proposed in the future.
Because insulin exerts a potent hypoglycemic action, a significant risk for hypoglycemia is often observed. Insulin induces fat accumulation in the peripheral adipose tissue by inhibiting lipolysis through hormone‐sensitive lipases23, leading to weight gain19. Insulin exerts an anorexic effect on the central nervous system24; however, insulin‐treated diabetic patients often eat too much for the fear or experience of hypoglycemia attack, which can also lead to weight gain25. Diabetic patients are often obese, which is frequently complicated by metabolic syndrome with hypertension or dyslipidemia26. Therefore, by using insulin for glycemic control, blood glucose levels per se improve, but weight gain could increase accompanied with hyperlipidemia and hypertension. For example, higher insulin doses in the Veteran Affairs Diabetes Trial were associated with weight gain as well as hypoglycemia, a predictor of cardiovascular mortality and macrovascular outcomes27. Liraglutide treatment has been shown to be associated with reduced hypoglycemia, and it promotes weight loss6 by reducing appetite through the suppression of eliminating gastric contents28 or by acting on the central nervous system29. Therefore, switching to liraglutide from insulin reduces hypoglycemia risk, and alleviates dyslipidemia and hypertension because of weight loss. Furthermore, except for basal supported oral therapy, insulin is often injected twice (in morning and evening before meals) or four times in basal‐bolus therapy. Because liraglutide has a half‐life of 13 h, only one injection per day is required, thus relieving patients from multiple daily insulin injections. Therefore, the disadvantages of insulin, such as the risk of hypoglycemia, weight gain and the necessity of multiple injections might be overcome by switching to liraglutide in certain cases. However, liraglutide is not a substitute for insulin. In Japan, during the 4 months (11 June 2010 to 7 October 2010) since the approval of liraglutide, four cases of diabetic ketoacidosis and 16 cases of hyperglycemia have been reported. In 17 out of 20 hyperglycemic cases, the condition resulted after switching to liraglutide from insulin. Therefore, liraglutide should not be considered a substitute for insulin, and the insulin‐dependent status should be determined to gauge whether liraglutide treatment is appropriate before administration. Some facilities carry out glucagon load testing to evaluate endogenous insulin secretion; however, the criterion has not yet been clearly defined. To determine the standard criterion for switching to liraglutide from insulin, we carried out a study on the group that switched to liraglutide from insulin, and carried out a detailed comparison of background factors between the responders and non‐responders. Consequently, switching to liraglutide from insulin might succeed if fasting CPR is >2.2 ng/mL, delta CPR before and after breakfast is >1.6 ng/mL, and urine CPR is >70 μg/day. Several cases were successfully treated even with low CPR levels; however, in such cases, insulin was used to avoid glucose toxicity, which was believed to lower CPR levels. Conversely, the unsuccessful cases were found to show relatively high fasting CPR levels that were complicated by reduced renal function caused by diabetic nephropathy, as decreased CPR clearance might increase apparent CPR levels. By carefully omitting these cases, CPR values might be more accurately delineated. In type 2 diabetic patients, the elderly frequently experience progressive exhaustion of pancreatic β‐cells, but younger people have relatively functional pancreatic β‐cells30. The capacity of liraglutide to assist with pancreatic β‐cell proliferation has also been reported to decline with age31. We then focused on the age and daily insulin dosage with respect to switching to liraglutide from insulin. Most elderly patients discontinued liraglutide mainly on account of its side‐effects. In patients aged 30–70 years, most found that switching to liraglutide was effective if their daily insulin dosage was approximately 20 U/day; most cases were unsuccessful in switching if the amount of insulin per day exceeded 40 U/day, except in some younger patients. Therefore, switching to liraglutide from insulin might succeed if the patient is not elderly, and if a smaller insulin dose is used. No other reports have described liraglutide use in patients who switched from insulin, and only one report described switching to exenatide from insulin32. In that study, 29 diabetic patients switched to exenatide from insulin. Consequently, 18 patients (62%) successfully achieved glycemic control, whereas the remaining 11 (38%) were unsuccessful. However, responders were defined if a HbA1c increase was <0.5%, and the content of that paper has also received critical comments33.
Several exceptions were observed in the present study. In switching from insulin, some patients found liraglutide to be ineffective despite using less insulin (approximately 10 U/day). Liraglutide was ineffective in some drug‐naïve/previous OHA patients, although their CPR levels were high, suggesting that endogenous insulin secretory capacity was well preserved. We could not clearly explain the reason, but can only speculate that the patients' habits (exercise, TV viewing, smoking and eating habits) during the present study might have directly worsened their glycemic control.
The present study had a limitation on liraglutide dose. In the LEAD test, daily liraglutide usage was 1.2 or 1.8 mg, and dose‐dependent effects were confirmed8. In Japan, dose‐dependent improvement in glycemic control using liraglutide was also confirmed17. The magnitude of HbA1c reduction with liraglutide in the present study (a 1.3% reduction in HbA1c using 0.6 mg of liraglutide in 4 months) was comparable with that reported in Japanese patients34 (a 1.6% reduction in HbA1c using 0.6 mg of liraglutide over 14 weeks) and in non‐Japanese patients35 (a 0.7% reduction in HbA1c using 0.6 mg of liraglutide over 12 weeks). In the present study, we used a fixed amount of daily liraglutide; that is, 0.6 mg, during the observation period. Improvement in glycemic control was not observed in 20 of 155 patients, and achievement of HbA1c < 7.0% was not observed in approximately half of the patients. The maximum dose of liraglutide is 0.9 mg in Japan, and some patients believed that the drug effect would increase if 0.9 mg was used, which should be investigated in the future.
In conclusion, the present study showed that liraglutide improved blood glucose levels, which was more pronounced in the drug‐naïve/previous OHA group compared with the group that switched from insulin. In addition, CPR levels, age and the daily insulin dosage were considered important factors when deciding to switch to liraglutide from insulin. Based on these findings, we can establish standards for liraglutide treatment that are appropriate for type 2 diabetic patients.
Acknowledgements
The authors have nothing to disclose. No potential conflicts of interest relevant to this article were reported. The authors thank the nursing staff of the Osaka Red Cross Hospital, and all of the participants in this study.
(J Diabetes Invest, doi: 10.1111/j.2040-1124.2012.00242.x, 2013)
References
- 1.deHeer J, Rasmussen C, Coy DH, et al Glucagon‐like peptide‐1, but not glucose‐dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 2008; 51: 2263–2270 [DOI] [PubMed] [Google Scholar]
- 2.Balas B, Baig MR, Watson C, et al The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single‐dose administration in type 2 diabetic patients. J Clin Endocrinol Metab 2007; 92: 1249–1255 [DOI] [PubMed] [Google Scholar]
- 3.Shimoda M, Kanda Y, Hamamoto S, et al The human glucagon‐like peptide‐1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia 2011; 54: 1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoda K, Okitsu T, Yamane S, et al GLP‐1 receptor signaling protects pancreatic beta cells in intraportal islet transplant by inhibiting apoptosis. Biochem Biophys Res Commun 2008; 367: 793–798 [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Man CD, Charbonnel B, et al Effect of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, on beta‐cell function in patients with type 2 diabetes: a model‐based approach. Diabetes Obes Metab 2008; 10: 1212–1220 [DOI] [PubMed] [Google Scholar]
- 6.Astrup A, Carraro R, Finer N, et al Safety, tolerability and sustained weight loss over 2 years with the once‐daily human GLP‐1 analog, liraglutide. Int J Obes (Lond) 2012; 36: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonaka K, Kakikawa T, Sato A, et al Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 79: 291–298 [DOI] [PubMed] [Google Scholar]
- 8.Marre M, Shaw J, Brändle M, et al Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck M, Frid A, Hermansen K, et al Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber A, Henry R, Ratner R, et al Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481 [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Gerich J, Buse JB, et al Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009; 32: 1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell‐Jones D, Vaag A, Schmitz O, et al Liraglutidevs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buse JB, Rosenstock J, Sesti G, et al Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47 [DOI] [PubMed] [Google Scholar]
- 14.Monami M, Marchionni N, Mannucci E. Glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a meta‐analysis of randomized clinical trials. Eur J Endocrinol 2009; 160: 909–917 [DOI] [PubMed] [Google Scholar]
- 15.Seino Y, Rasmussen MF, Nishida T, et al Efficacy and safety of the once‐daily human GLP‐1 analogue, liraglutide, vsglibenclamidemonotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin 2010; 26: 1013–1022 [DOI] [PubMed] [Google Scholar]
- 16.Kaku K, Rasmussen MF, Nishida T, et al Fifty‐two‐week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon‐like peptide‐1 analog liraglutidevsglibenclamide in patients with type 2 diabetes. J Diabetes Invest 2011; 2: 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaku K, Rasmussen MF, Clauson P, et al Improved glycaemic control with minimal hypoglycaemia and no weight change with the once‐daily human glucagon‐like peptide‐1 analogue liraglutide as add‐on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 341–347 [DOI] [PubMed] [Google Scholar]
- 18.Seino Y, Rasmussen MF, Nishida T, et al Glucagon‐like peptide‐1 analog liraglutide in combination with sulfonylurea safely improves blood glucose measures vs sulfonylurea monotherapy in Japanese patients with type 2 diabetes: results of a 52‐week, randomized, multicenter trial. J Diabetes Invest 2011; 2: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migdalis IN. Insulin analogs versus human insulin in type 2 diabetes. Diabetes Res Clin Pract 2011; 93: S102–S104 [DOI] [PubMed] [Google Scholar]
- 20.Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin‐based therapy: therapeutic potential, patient selection and clinical use. Am J Med 2009; 122: S37–S50 [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Gastaldelli A, Miyazaki Y, et al Predominant role of reduced beta‐cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia 2003; 46: 1211–1219 [DOI] [PubMed] [Google Scholar]
- 22.Kuroe A, Fukushima M, Usami M, et al Impaired beta‐cell function and insulin sensitivity in Japanese subjects with normal glucose tolerance. Diabetes Res Clin Pract 2003; 59: 71–77 [DOI] [PubMed] [Google Scholar]
- 23.Strålfors P, Björgell P, Belfrage P. Hormonal regulation of hormone‐sensitive lipase in intact adipocytes: identification of phosphorylated sites and effects on the phosphorylation by lipolytic hormones and insulin. Proc Natl Acad Sci USA 1984; 81: 3317–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods SC, D'Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab 2008; 93: S37–S50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes – causes, effects and coping strategies. Diabetes Obes Metab 2007; 9: 799–812 [DOI] [PubMed] [Google Scholar]
- 26.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world – a growing challenge. N Engl J Med 2007; 356: 213–215 [DOI] [PubMed] [Google Scholar]
- 27.Kirkman MS, McCarren M, Shah J, et al VADT Study Group. The association between metabolic control and prevalent macrovascular disease in Type 2 diabetes: the VA Cooperative Study in diabetes. J Diabetes Complications 2006; 20: 75–80 [DOI] [PubMed] [Google Scholar]
- 28.Juhl CB, Hollingdal M, Sturis J, et al Bedtime administration of NN2211, a long‐acting GLP‐1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 2002; 51: 424–429 [DOI] [PubMed] [Google Scholar]
- 29.Kanoski SE, Fortin SM, Arnold M, et al Peripheral and central glp‐1 receptor populations mediate the anorectic effects of peripherally administered glp‐1 receptor agonists, liraglutide and exendin‐4. Endocrinology 2011; 152: 3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zangeneh F, Arora PS, Dyck PJ, et al Effects of duration of type 2 diabetes mellitus on insulin secretion. Endocr Pract 2006; 12: 388–393 [DOI] [PubMed] [Google Scholar]
- 31.Parnaud G, Bosco D, Berney T, et al Proliferation of sorted human and rat beta cells. Diabetologia 2008; 51: 91–100 [DOI] [PubMed] [Google Scholar]
- 32.Davis SN, Johns D, Maggs D, et al Exploring the substitution of exenatide for insulin in patients with type 2 diabetes treated with insulin in combination with oral antidiabetes agents. Diabetes Care 2007; 30: 2767–2772 [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock J, Fonseca V. Missing the point: substituting exenatide for nonoptimized insulin: going from bad to worse! Diabetes Care 2007; 30: 2972–2973 [DOI] [PubMed] [Google Scholar]
- 34.Seino Y, Rasmussen MF, Zdravkovic M, et al Dose‐dependent improvement in glycemia with once‐daily liraglutide without hypoglycemia or weight gain: a double‐blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81: 161–168 [DOI] [PubMed] [Google Scholar]
- 35.Madsbad S, Schmitz O, Ranstam J, et al Improved glycemic control with no weight increase in patients with type 2 diabetes after once‐daily treatment with the long‐acting glucagon‐like peptide 1 analog liraglutide (NN2211): a 12‐week, double‐blind, randomized, controlled trial. Diabetes Care 2004; 27: 1335–1342 [DOI] [PubMed] [Google Scholar]


