Abstract
The aim of the present study was to compare the usefulness of premeal rapid‐acting and regular insulin in type 2 diabetes patients. A total of 56 type 2 diabetic patients were investigated during hospitalization. Premeal rapid‐acting insulin was applied instead of other medications. Premeal insulin was titrated to adjust premeal and bedtime blood glucose levels to 81–120 mg/dL. Premeal rapid‐acting insulin was changed to regular insulin just before a meal at the same dosage if the postmeal blood glucose level was lower than the premeal blood glucose level. A total of 15 patients changed to regular insulin, and 41 patients continued rapid‐acting insulin. The blood glucose level was comparable between these two groups. Body mass index was significantly lower in the patients using regular insulin. According to the multivariate logistic regression analysis, low body mass index was an independent variable accounting for the usefulness of regular insulin. Regular insulin, rather than rapid‐acting insulin, is a suitable choice for premeal insulin in lean type 2 diabetic patients.
Keywords: Hypoglycemia, Insulin action, Insulin therapy
Introduction
The administration of bolus insulin before each meal is important to maintain postprandial blood glucose levels as close to normal as possible. Rapid‐acting insulin analogs have a more rapid onset of action than regular insulin, providing improved postprandial glycemic control in multiple daily insulin injection (MDI) regimens1. Premeal insulin dosage was titrated to control target blood glucose levels before each meal and at bedtime. Even when premeal and bedtime blood glucose levels are within target range, postprandial hypoglycemia is often observed in patients with premeal rapid‐acting insulin. The purpose of the present study was to evaluate suitable premeal bolus insulin for type 2 diabetes.
Materials And Methods
A total of 56 inpatients who met the inclusion and exclusion criteria were consecutively selected in Osaka University Medical Hospital, in Osaka, Japan, from May 2005 to May 2007. We excluded individuals with unstable retinopathy, pregnancy or using steroids. The diabetic meals consisting of 25–30 kcal/ideal bodyweight kg, 50–60% carbohydrate, 15–20% protein and 20–25% fat were prepared by dietitians. All meals were consumed within 20 min without any additional food unless required to treat hypoglycemia.
All patients were switched to premeal MDI using rapid‐acting insulin, either insulin Lispro or Aspart. Other hypoglycemic agents were stopped. Self‐monitoring of blood glucose was carried out before, and 2 h after meals and bedtime throughout the study. Premeal insulin dosage was titrated to adjust premeal and bedtime blood glucose levels to 81–120 mg/dL (Table 1). After each premeal and bedtime, blood glucose levels were equally adjusted; when postmeal blood glucose level was more than 30 mg/dL lower than just before the premeal level for at least three occasions, premeal rapid‐acting insulin was changed to regular insulin just before a meal at the same dosage. Body mass index (BMI), glycated hemoglobin (HbA1c), urinary C‐peptide excretion and diabetic microvascular complications were examined at the beginning of the study. Seven‐point blood glucose testing and the incidence of hypoglycemia were collected for 3 days at the end of the hospitalization. HbA1c was expressed as a National Glycohemoglobin Standardization Program (NGSP) equivalent value; that is, HbA1c (NGSP equivalent value) (%) = HbA1c (Japan Diabetes Society value) (%) + 0.4%2. Microalbuminuria was defined as a urinary albumin/creatinine excretion ratio more than 30 mg/gCr. Autonomic neuropathy was defined as a coefficient of variation of R‐R interval < 2.0%3. Retinopathy was diagnosed by ophthalmologists. Urinary C‐peptide excretion was tested with radioimmunoassay.
Table 1. Insulin titration.
| Blood glucose levels before each meal and at bedtime | Titration of bolus insulin dosage (U) before each meal and basal insulin dosage (U) at bedtimea |
|---|---|
| >270 mg/dL | +4 |
| 221–270 mg/dL | +3 |
| 171–220 mg/dL | +2 |
| 121–170 mg/dL | +1 |
| 81–120 mg/dL | Maintain dose |
| 60–80 mg/dL | −2 |
| <60 mg/dL | −4 |
When blood glucose level before lunch, supper or at bedtime was elevated, bolus insulin dosage before breakfast, lunch or supper was titrated, respectively; and when fasting blood glucose level was elevated, basal insulin dosage at bedtime was titrated.
The present study was carried out in accordance with the Declaration of Helsinki and with approval from the ethical committee for Human Studies at Osaka University Graduate School of Medicine. The written informed consent was obtained from each patient.
All values are presented as means ± standard deviation. Between‐group differences of the average were compared using unpaired t‐test for parametric data. Categorical variables were compared using χ2‐test. Multivariate logistic regression analysis was used with StatView 5.0 software (SAS Institute, Cary, NC, USA). For all tests, P < 0.05 was considered statistically significant.
Results
The characteristics of the study participants were as follows: sex (male/female) 41/15, age 60.5 ± 12.8 years, BMI 25.2 ± 5.2 kg/m2, duration of diabetes 14.4 ± 7.3 years, HbA1c 9.3 ± 2.0%, urinary C‐peptide excretion 48.7 ± 38.0 μg/day.
Among 56 patients, premeal rapid‐acting insulin was used throughout the period in 41 patients (rapid group), and it was changed to regular insulin in 15 patients (regular group). The prevalence of basal insulin (Table 2) and the averages of blood glucose levels (Figure 1) were comparable between the rapid group and the regular group. The prevalence rate of premeal hypoglycemia was comparable in these groups (regular 3.0 ± 6.6 vs 0.8 ± 2.9%, not significant). The prevalence rate of postmeal hypoglycemia was significantly higher in the regular group than in the rapid group (5.5 ± 8.4 vs 0.5 ± 2.4%, P < 0.005). BMI in the regular group was significantly lower than that in the rapid group. The numbers of patients in the regular group were two out of two for BMI ≤18, four out of seven for BMI >18 and ≤20, three out of eight for BMI >20 and ≦22, four out of nine for BMI >22 and ≦24, and one out of 30 for BMI >24. In the regular group, age tended to be higher (P = 0.07) and urinary C‐peptide immunoreactivity tended to be lower (P = 0.08) than the rapid group. There were no significant differences in the prevalence of microalbuminuria, autonomic neuropathy and retinopathy between these two groups.
Table 2. Characteristics of rapid‐acting insulin using group (rapid group) and regular insulin using group (regular group).
| Rapid group (range) | Regular group (range) | P‐value | |
|---|---|---|---|
| Age (years) | 58.7 ± 12.6 (25–75) | 65.5 ± 12.3 (33–81) | P = 0.07 |
| Sex (male/female) | 30/12 | 11/3 | NS |
| Duration (years) | 14.4 ± 7.9 (1–40) | 14.6 ± 5.2 (8–26) | NS |
| Total bolus insulin (U/day) | 23.5 ± 8.1 (12–52) | 22.5 ± 10.8 (11–55) | NS |
| BMI (kg/m2) | 26.9 ± 4.9 (18.3–39.4) | 20.6 ± 2.9 (14.5–25.9) | P < 0.0001 |
| HbA1c (%) | 9.2 ± 1.8 (5.5–13.8) | 9.5 ± 2.4 (7.3–15.8) | NS |
| Urinary CPR (μg/day) | 54.4 ± 42.5 (14.2–182.0) | 34.2 ± 17.3 (12.7–68.6) | P = 0.08 |
| Microalbuminuria | 18/41 | 6/15 | NS |
| Autonomic neuropathy | 12/41 | 8/15 | NS |
| Retinopathy | 20/41 | 7/15 | NS |
| Use of basal insulin | 21/41 | 4/15 | NS |
Data shown as mean ± SD (range). Unpaired t‐test for parametric data χ2‐test for between‐group differences in numbers. BMI, body mass index; CPR, C‐peptide immunoreactivity; NS, not significant.
Figure 1.
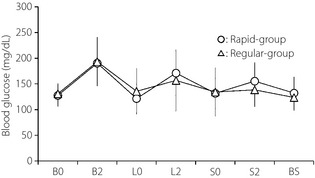
Seven‐point blood glucose profiles in the rapid‐acting insulin group (rapid group) and regular insulin group (regular group). Data presented as mean ± SD. B0, before breakfast; B2, 2 h after breakfast; BS, before sleep; L0, before lunch; L2, 2 h after lunch; S0, before supper; S2, 2 h after supper.
Multivariate logistic regression analysis was carried out with regular insulin use as a dependent variable, and age, BMI, the prevalence of autonomic neuropathy and urinary C‐peptide excretion as independent variables (Table 3). BMI was only an independent predictor of regular insulin use (standardized odds ratio 0.649, P < 0.005), showing that regular insulin is suitable in lean type 2 diabetic patients.
Table 3. Odds ratios of clinical factors for the use of regular insulin determined by multivariate logistic regression analysis.
| Variable | Standardized odds ratio | 95% Confidence interval | P‐value |
|---|---|---|---|
| BMI (kg/m2) | 0.649 | 0.491–0.858 | <0.005 |
| Age (years) | 1.026 | 0.962–1.094 | NS |
| Urinary CPR (μg/day) | 0.983 | 0.940–1.029 | NS |
Confounding variables are listed in the panel. Variables were considered for the multivariable models when their univariable P‐value was < 0.10. Values are odds ratios with 95% confidence intervals. BMI, body mass index; CPR, C‐peptide immunoreactivity.
Discussion
Diabetes is increasing dramatically in Asia, and Asian people develop diabetes with a lesser degree of obesity4. Time‐action profiles of currently available subcutaneous insulin products are attenuated and delayed in obese patients5. Although time‐action profiles of rapid‐acting insulin in lean type 2 diabetes has not been determined, skinfold thickness was reported to cause insulin malabsorption7. In addition, thinner subcutaneous fat tissue at the injection site was associated with enhanced subcutaneous insulin absorption8. Rapid‐acting insulin enables us to achieve tighter postprandial glycemic control in the MDI regimen9; however, we showed that 15 out of 56 Japanese type 2 diabetic patients changed from premeal rapid‐acting to regular insulin to avoid postprandial hypoglycemia. Regular insulin is usually injected 30 min before a meal; however, it was injected just before meals in the regular group in the present study, and the seven‐point blood glucose profile was comparable with that of the rapid group. If regular insulin was injected 30 min before a meal, the peak of insulin effect might be close to rapid‐acting insulin. A slower glucose lowering effect was preferable to achieve a good glycemic profile for patients with postprandial hypoglycemia. In addition, insulin injection just before meals promotes better adherence in diabetic patients.
The multivariate logistic regression analysis showed that low BMI was an independent factor accounting for the use of regular insulin. Therefore, we assume that rapid absorption of insulin in lean patients explains, at least in part, the reason why regular insulin is suitable for lean diabetic patients.
Aging and autonomic neuropathy have been reported to be inversely related with gastric emptying10. Age tended to be higher in the regular group than in the rapid group (P = 0.07), but was not an independent factor accounting for the use of regular insulin. The prevalence of autonomic neuropathy was similar between these two groups. Therefore, slow gastric emptying was not a determinant for the usefulness of regular insulin in the present study.
Postmeal hypoglycemia was not frequent in both groups, but was significantly higher in the regular group than in the rapid group. Insulin secretion capacity is important for blood glucose stability. Urinary C‐peptide secretion tended to be higher in the rapid group than in the regular group; however, it did not account for postmeal hypoglycemia according to the multivariate analysis (data not shown). In the regular group, 2‐h postmeal blood glucose declined by an average of 65.9 ± 37.8 mg/dL from the premeal blood glucose in 3 days before switching the insulin from rapid to regular insulin, whereas it increased by 1.2 ± 64.0 mg/dL in the final 3 days with regular insulin.
Therefore, if we continued rapid‐acting insulin in the regular group, the incidence of hypoglycemia could become more frequent to achieve the target level of premeal glucose.
In conclusion, it is likely that regular insulin, rather than rapid‐acting insulin, is a suitable choice for mealtime bolus insulin in lean type 2 diabetic patients.
Acknowledgments
We acknowledge Kazumi Hata, Tomomi Hirano and Chikayo Yokogawa for secretarial work. The authors have no conflict of interest.
References
- 1.Noble SL, Johnston E, Walton B. Insulin lispro: a fast‐acting insulin analog. Am Fam Physician 1998; 57: 279–286 [PubMed] [Google Scholar]
- 2.Seino Y, Nanjo K, Tajima N, et al The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus . Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothschild AH, Weinberg CR, Halter JB, et al Sensitivity of R‐R variation and valsalva ratio in assessment of cardiovascular diabetic autonomic neuropathy. Diabetes Care 1987; 10: 735–741 [DOI] [PubMed] [Google Scholar]
- 4.Yoon KH, Lee JH, Kim JW, et al Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688 [DOI] [PubMed] [Google Scholar]
- 5.Vora JP, Burch A, Peters JR, et al Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care 1992; 15: 1484–1493 [DOI] [PubMed] [Google Scholar]
- 6.Vora JP, Burch A, Peters JR, et al Absorption of radiolabelled soluble insulin in type 1 (insulin‐dependent) diabetes: influence of subcutaneous blood flow and anthropometry. Diabet Med 1993; 10: 736–743 [DOI] [PubMed] [Google Scholar]
- 7.Hildebrandt P. Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients. Acta Physiol Scand Suppl 1991; 603: 41–45 [PubMed] [Google Scholar]
- 8.Sindelka G, Heinemann L, Berger M, et al Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia 1994; 37: 377–380 [DOI] [PubMed] [Google Scholar]
- 9.Rodbard HW, Jellinger PS, Davidson JA, et al Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15: 540–559 [DOI] [PubMed] [Google Scholar]
- 10.Salles N. Basic mechanisms of the aging gastrointestinal tract. Dig Dis 2007; 25: 112–117 [DOI] [PubMed] [Google Scholar]
- 11.Iida M, Ikeda M, Kishimoto M, et al Evaluation of gut motility in type II diabetes by the radiopaque marker method. J Gastroenterol Hepatol 2000; 15: 381–385 [DOI] [PubMed] [Google Scholar]


