Abstract
Aims/Introduction
Patients with diabetic nephropathy (DN) typically show varying degrees of proteinuria and renal impairment. Because these clinical signs are frequently observed in other glomerulopathies, renal biopsy is required to make a definitive diagnosis of DN. We carried out the present study to evaluate the significance of renal biopsy for patients who have been presumptively diagnosed with DN.
Materials and Methods
A total of 55 patients with type 2 diabetes mellitus (DM), and proteinuria, hematuria and/or renal impairment were enrolled in this study.
Results
Renal biopsy showed that just 30 patients (54.5%) were histologically diagnosed with DN. Fasting plasma glucose and glycated hemoglobin levels were associated with the presence of DN, whereas baseline renal function showed no statistically significant relationship to DN. The duration of DM was not associated with the presence of DN. Patients with DN had a higher rate of diabetic retinopathy (DR) than those with non‐DN (DN 18 patients vs non‐DN three patients, P = 0.00029). DN patients with DR showed a more severe renal histology than those without.
Conclusions
These data suggest that, even for patients with long‐term DM, renal biopsy should be carried out in patients with presumed DN. Because treatment options differ between DN and primary glomerulopathies, renal biopsy should especially be considered for presumed DN without DR.
Keywords: Diabetic nephropathy, Diagnostic rate, Renal biopsy
Introduction
Diabetic nephropathy (DN) is a major cause of end‐stage renal disease worldwide. Patients with DN typically show varying degrees of proteinuria and renal impairment. Because these clinical signs frequently present in other glomerulopathies, it is sometimes difficult to make a definitive diagnosis of DN without renal biopsy. Histological confirmation of renal involvement in patients with diabetes mellitus (DM) could prove essential, because DN and primary glomerulopathies require different treatment strategies.
Renal biopsy is necessary for making definite diagnosis of DN. However, it is generally agreed that renal biopsy would not be carried out as a routine diagnostic test in a typical clinical situation of DN. It is important to determine the atypical clinical predictive factors in patients with DM. Previous reports have covered these atypical clinical variables, such as short diabetes duration, absence of retinopathy, presence of hematuria and other indices1. In particular, the absence of diabetic retinopathy (DR) was considered to be a good predictor of non‐DN3.
We carried out the present study to evaluate the significance of renal biopsy for patients who have been presumptively diagnosed with DN.
Materials and Methods
Patients
Patients with DM were enrolled from both Nara Medical University Hospital and Rakuwakai‐Otowa Hospital in Japan. A total of 55 patients with type 2 DM (37 male and 18 female) participated in the present study after giving informed consent. Patients ranged from 32 to 75 years‐of‐age (mean 58.2 ± 12.4 years), while the duration of DM ranged from 1 to 34 years (mean 10.1 ± 8.5 years). We defined the onset of diabetes as when the patient was told that they have DM by the physician for the first time.
Percutaneous renal biopsy was carried out in all patients to elucidate the cause of proteinuria, hematuria, nephrotic syndrome and progressive deterioration of renal function. Two renal pathologists confirmed the histological diagnosis. Although the patients were referred to us for further management of DN by a primary care physician, they were considered viable candidates for renal biopsy by experienced nephrologists, because their clinical history, presentation and laboratory findings including urinalysis differed from the typical features associated with DN. Tissue samples obtained by renal biopsy were separated and allocated for immunofluorescence microscopy, light microscopy and electron microscopy. Immunofluorescence examinations were carried out for immunoglobulin (Ig) G, IgM, IgA, complement component 3 (C3), complement component 1q (C1q) and fibrinogen. For light microscopy, tissue sections were stained with hematoxylin and eosin, periodic acid‐Schiff, methenamine‐silver, and Masson trichrome. Because deposit of IgG along the glomerular basement membrane is reported in DN patients, it sometimes is difficult to diagnose the DN only with light and/or immunofluorescence microscopy. In those cases, we usually carry out electron microscopic evaluation for accurate diagnosis.
Indication for Renal Biopsy
Renal biopsy in patients with DM was carried out for the following indications: two patients for gross hematuria, 21 patients for proteinuria with an unusual clinical course of DN, 14 patients for proteinuria and hematuria, 15 patients for nephrotic syndrome with hematuria, and three patients for rapidly progressive worsening of renal function. All participants had an atypical clinical course.
Renal Histology Classification
Diabetic glomerular changes were diagnosed according to generally accepted criteria10. Other glomerulopathies were categorized following approved pathological criteria as described previously11. After histological diagnosis was made, DM patients with other glomerulopathies were classified as having non‐DN. Furthermore, we also classified the patients as follows according to the histological findings: (i) the pure DN group had only diabetic lesions; (ii) the complicated group had histological changes of other glomerulopathies superimposed on DN; and (iii) the non‐DN group had other glomerulopathies without diabetic lesions.
The severity of diffuse and nodular lesions of DN was graded according to a five‐point scale developed by Gellman et al.12, and the severity of vascular lesions was classified on a scale of 0–III, according to the criteria described by Takazakura13. Furthermore, the percentage of sclerotic glomeruli was calculated by counting the number of sclerotic glomeruli and total number of glomeruli in the renal biopsy tissues. The extent of interstitial damage and chronic inflammation was evaluated semiquantitatively using a three‐grade system as follows: (i) Grade 0 represented tissues with damaged tubules and the presence of infiltrating cells (lymphocyte) limited to <25% of the total tubulo‐interstitial area; (ii) grade 1 represented those with tubulo‐interstitial injury limited to ≥25% to <50% of the total tubulo‐interstitial area; and (iii) grade 2 represented severe tubulo‐interstitial injury exceeding 50% of the total tubulo‐interstitial area.
Diagnosis of DR
Diabetic retinopathy was classified by ophthalmologists using standardized fundoscopic examination14 into three classes as follows:
no retinopathy;
non‐proliferative retinopathy; and
proliferative retinopathy.
Values of Glycated Hemoglobin
Glycated hemoglobin (HbA1c) values were expressed as National Glycohemoglobin Standardization Program (NGSP) values in the present study15.
Statistical Analysis
Statistical calculations were made using the Statview 5.0 (SAS Institute, Cary, NC, USA) and JMP 5.1 (SAS Institute) software packages. Results were expressed as mean ± standard deviation and the statistical analysis was carried out using non‐parametric tests. The Mann–Whitney U‐test and the Wilcoxon signed rank test were used for paired and unpaired subjects, respectively. Differences in the parameters between the three groups were analyzed using the Kruskal–Wallis and Scheffe's multiple comparison test. Odds ratio (OR) of DR and DN were assessed using Fisher's exact test. Spearman's rank correlation analysis was used to assess the correlation between clinical parameters and several renal histological parameters. P < 0.05 was considered significant in all analyses.
Results
Clinical Characteristics of Patients
The clinical characteristics of patients with DN and non‐DN at the time of renal biopsy are shown in Table 1. The duration of DM was not associated with the presence of DN, although a correlation between patients with a longer duration of DM and DN was observed. Fasting plasma glucose (FPG) and HbA1c are associated with the presence of DN (FPG: 183 ± 61.9 mg/mL vs 143 ± 62.1 mg/mL, P = 0.0128; HbA1c: 8.6 ± 2.4% vs 6.6 ± 1.1%, P = 0.0004), whereas baseline renal function showed no statistically significant relationship to DN.
Table 1. Clinical parameters of diabetes mellitus patients undergoing renal biopsy.
| DN | Non‐DN | P | |
|---|---|---|---|
| Patients | 30 (54.5%) | 25 (45.5%) | |
| Age (years) | 57.6 ± 12.4 | 59.0 ± 12.7 | NS |
| Sex (male/female) | 21/9 | 16/9 | NS |
| Duration of diabetes (years) | 12.4 ± 9.5 | 7.4 ± 6.1 | NS |
| BMI (kg/cm2) | 24.6 ± 3.3 | 27.6 ± 4.6 | 0.0225 |
| SBP (mmHg) | 146 ± 20.6 | 136 ± 22.4 | NS |
| DBP (mmHg) | 77.9 ± 14.2 | 80.0 ± 15.4 | NS |
| Urinary excretion protein (g/day) | 3.3 ± 4.0 | 2.1 ± 2.8 | NS |
| Degree of urinary occult blood | 1.3 ± 1.3 | 1.8 ± 1.1 | NS |
| FPG (mg/dL) | 183 ± 61.9 | 143 ± 62.1 | 0.0128 |
| HbA1c (%) | 8.6 ± 2.4 | 6.6 ± 1.1 | 0.0004 |
| Blood urea nitrogen (mg/dL) | 21.4 ± 9.5 | 19.7 ± 15.5 | NS |
| Serum creatinine (mg/dL) | 1.2 ± 0.9 | 1.4 ± 1.5 | NS |
| Estimated GFR (mL/min/1.73 m2) | 50.2 ± 23.2 | 49.4 ± 26.1 | NS |
| Total protein (g/dL) | 6.5 ± 1.1 | 6.6 ± 1.3 | NS |
| Albumin (g/dL) | 3.7 ± 0.9 | 3.7 ± 1.0 | NS |
| Total cholesterol (mg/dL) | 233 ± 79.1 | 221 ± 75.7 | NS |
| Triglyceride (mg/dL) | 188 ± 97.1 | 202 ± 121 | NS |
| HDL‐cholesterol (mg/dL) | 48.9 ± 12.5 | 48.6 ± 10.1 | NS |
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetic nephropathy; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; GFR, glomerular filtration rate; NS, not significant; SBP, systolic blood pressure.
Renal Biopsy Findings
Renal biopsy showed that just 30 patients (54.5%) were histologically diagnosed with DN, whereas 25 (45.5%) were diagnosed with non‐DN. Furthermore, patients were classified into three groups: (i) the pure DN group (n = 30, 54.5%) had only diabetic lesions; (ii) the complicated group (n = 6, 10.9%) had histological changes of other glomerulopathies superimposed on DN; and (iii) the non‐DN group (n = 19, 34.5%) had other glomerulopathies without diabetic lesions.
Non‐DN patients were diagnosed with IgA nephropathy, focal segmental glomerulosclerosis, membranous nephropathy, membranoproliferative glomerulonephritis, minimal change nephritic syndrome and crescentic glomerulonephritis. The histological findings in patients with non‐DN are summarized in Table 2.
Table 2. Histological diagnosis of patients with presumed diabetic nephropathy.
| n (%) | |
|---|---|
| DN | 30 (54.5%) |
| Non‐DN | 25 (45.5%) |
| Minor glomerular abnormalities | 1 |
| Arteriosclerosis | 3 |
| Chronic glomerulonephritis | 21 |
| IgA nephropathy | 13 |
| Focal segmental glomerulosclerosis | 3 |
| Membranous nephropathy | 1 |
| Membranoproliferative glomerulonephritis type I | 1 |
| Crescentic glomerulonephritis | 1 |
| Non‐IgA nephropathy | 1 |
| Minimal change nephrotic syndrome | 1 |
DN, diabetic nephropathy; IgA, immunoglobulin A.
Association Between DN and DR
Patients with DN showed a higher incidence of DR than those with non‐DN (18 patients with DM also had retinopathy, whereas three patients with non‐DN also had retinopathy; OR 11.0, 95% confidence interval 3.03–39.9; P = 0.00029). None of the patients with non‐DN had proliferative DR. Furthermore, it was shown that none of the patients without diabetic lesions had DR in all three groups. The relationship between DR and renal histological findings is summarized in Table 3.
Table 3. Relationship between diabetic retinopathy and renal histological finding.
| DN group | Complicated group | Non‐DN group | |
|---|---|---|---|
| DR | 18 | 3 | 0 |
| NDR | 12 | 3 | 19 |
DN, diabetic nephropathy; DR, diabetic retinopathy; NDR, no diabetic retinopathy.
Histological Characteristics of DN and Non‐DN
The histological features of the two groups at the time of renal biopsy are shown in Table 4. Patients with DN had significantly more severe tubulo‐interstitial and vascular damage than those with other renal diseases; however, there was no significant difference in the percentage of sclerotic glomeruli (%GS) between the two groups.
Table 4. Histological findings of diabetic nephropathy and non‐diabetic nephropathy.
| Histological findings | DN | Non‐DN | P |
|---|---|---|---|
| Percentage of glomeruli showing global or segmental sclerosis | 20.5 ± 20.4 | 17.0 ± 15.2 | NS |
| Extent of interstitial damage and chronic inflammation | 0.93 ± 0.47 | 0.36 ± 0.49 | <0.0001 |
| Severity of arteriolosclerosis or arteriolar hyalinosis | 1.79 ± 0.88 | 1.00 ± 0.76 | 0.0013 |
DN, diabetic nephropathy; NS, not significant.
Histological Grade of DN in Relation to DR and Clinical Parameters
Patients with both DN and DR showed a more severe renal histology than those without DR (Table 5). Furthermore, there was no significant difference in the extent of tubulo‐interstitial and vascular damage between patients with DN without DR and non‐DN (Figure 1). To evaluate the significance of clinical parameters in the progression of DN, we examined the correlation between histological findings and clinical parameters. The severity of tubulo‐interstitial damage and diffuse glomerular lesions were closely correlated with the duration of DM (tubulo: Spearman's rank correlation r s = 0.437; P = 0.0258, diffuse: r s = 0.512; P = 0.0135); however, %GS, and the severity of nodular and vascular lesions were not significantly correlated with the duration of DM. Among the laboratory findings, the severity of all histological parameters was positively correlated with urinary protein excretion (tubulo: r s = 0.544, P = 0.0055; diffuse: r s = 0.671, P = 0.0009; nodular: r s = 0.689, P = 0.0007; vascular: r s = 0.567, P = 0.0054) and was inversely correlated with serum albumin level (tubulo: r s = −0.385, P = 0.0496; diffuse: r s = −0.446, P = 0.0073, nodular: r s = −0.587, P = 0.0002; vascular: r s = −0.460, P = 0.0063). Among the renal histology, only the extent of interstitial damage and chronic inflammation was significantly correlated with estimated glomerular filtration rate (r s = −0.473, P = 0.0158; Figure 2).
Table 5. Diabetic retinopathy in relation to the histological grade of diabetes nephropathy.
| Histological findings | DR | NDR | P |
|---|---|---|---|
| Percentage of glomeruli showing global or segmental sclerosis | 20.7 ± 18.7 | 20.4 ± 23.6 | NS |
| Extent of interstitial damage and chronic inflammation | 1.13 ± 0.34 | 0.64 ± 0.51 | 0.0080 |
| Severity of diffuse lesion | 3.13 ± 0.72 | 2.18 ± 0.87 | 0.0075 |
| Severity of nodular lesion | 1.69 ± 1.25 | 0.36 ± 0.92 | 0.0114 |
| Severity of arteriolosclerosis or arteriolar hyalinosis | 2.06 ± 0.85 | 1.18 ± 0.98 | 0.0227 |
DR, diabetic retinopathy; NDR, no diabetic retinopathy; NS, not significant.
Figure 1.
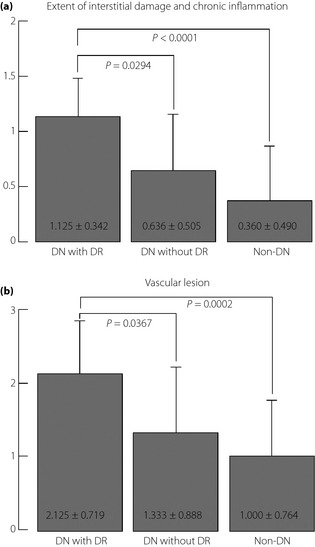
Diabetic retinopathy (DR) in relation to the histological grade of diabetic nephropathy (DN). DN patients with DR had a worse renal histology than those without DR. (a) Extent of interstitial damage and chronic inflammation. (b) Vascular lesion.
Figure 2.
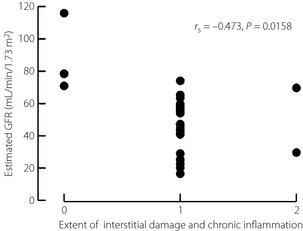
Correlation between interstitial changes and estimated glomerular filtration rate (GFR). The extent of interstitial damage and chronic inflammation was significantly correlated with estimated GFR.
Discussion
The prevalence of non‐DN in the patients with DM who underwent renal biopsy varies from 10% to 85% in different reports1. In the reports with Japanese diabetic patients, Suzuki et al.1 reported that 73.4% of 109 Japanese type 2 diabetic patients with proteinuria were diagnosed as DN, whereas Tone et al.2 reported just 36.1% of 97 patients were DN. Even in the studies with Japanese patients, the prevalence of DN in diabetic patients varies widely. Huang et al. suggested that the large variation between these reports could be a result of selection bias, because renal biopsies were carried out in DM patients with various backgrounds4. This might be because there are no standardized criteria for renal biopsy in diabetic patients. Generally, renal biopsy is commonly carried out in patients with DM who show the following features1: (i) macroalbuminuria occurring within 5 years of onset of DM; (ii) macroalbuminuria without diabetic retinopathy; (iii) severe microhematuria; (iv) rapid deterioration of renal function; (v) rapid worsening of proteinuria; or (vi) suspected other renal diseases in addition to diabetic glomerulosclerosis. The indication of renal biopsy in the present study was essentially the same as the ‘general’ indication. In the present study, all participants who underwent renal biopsy had not only the aforementioned indications, but also an atypical clinical course. In the present study, 45.5% of patients with DM were histologically diagnosed with non‐DN; furthermore, 52% of the patients with non‐DN had IgA nephropathy, consistent with other reports from Asian countries1.
Renal function and degree of proteinuria did not correlate with the presence of DN. However, poor glycemic control was associated with DN, which is compatible with previous reports16.
Histological analysis showed that patients with DN had significantly more severe tubulo‐interstitial and vascular damage than those with other renal diseases. Therefore, because DN patients had more severe tubulo‐interstitial and vascular damage, if the patients had a similar clinical profile, such as the degree of proteinuria and renal function, the patients with DN would have a worse renal prognosis than those with other renal diseases. It is well known that glomerulosclerosis is correlated with interstitial change, and thought to be important for renal survival. However, there was no significant difference in %GS between DN and non‐DN in the present study. Because we showed that DN had not only more severe tubulo‐interstitial damage, but also vascular damage, than non‐DN, we assume that interstitial injury of DN was affected by vascular damage as well as glomerular damage.
The present study showed that the duration of DM was not associated with the presence of DN, although a higher prevalence of DN was observed in patients with a longer duration of DM. Although some reports showed that the duration of DM was an important indicator of DN3, others reported no significant impact of the duration of DM in the development of DN8. Generally, it is thought that patients with DM demonstrate microalbuminuria at least 5–10 years after the onset of diabetes. Many DM patients with glomerular lesions, particularly nodular lesions, reportedly had a long duration of DM19; however, no significant relationship between nodular lesions and duration of DM was shown in the present study. The lack of association between the duration of DM and the presence of DN, especially nodular DN observed in the present study, could be explained by the onset of DM in our patients possibly being much earlier than they stated. We defined the onset of diabetes as the patient was told that they have DM by the physician for the first time. Because the Japanese often have an annual check‐up in the workplace or in the local clinic/hospital, the diabetic duration might be more accurate than previous studies; however, diabetic duration did not correlate with prevalence of DN in the present study. Because most type 2 diabetic patients don't have the exact information of diabetic duration, and diabetic patients who have undergone renal biopsy have an atypical clinical course, we considered that diabetic duration does not correlate with DN.
Diabetic retinopathy has good concordance with the presence of DN1, whereas lack of DR is a poor predictor of non‐DN3. The present data also suggested that although the positive predictive value for DN with DR was 85.7%, the negative predictive value was 64.7%, which is consistent with previous reports1. Parving et al.9 stated that the diagnostic specificity of retinopathy was 100%. We classified the patients into three groups to more precisely clarify the relationship between DN and DR. It is notable that no patient in the non‐DN group had DR, this result showed that DR has good concordance with the presence of diabetic lesions. Furthermore, we showed that patients with both DN and DR showed more severe renal histology than those without DR. Because patients without DR had not only worse diabetic histological change, but also similar tubulo‐interstitial and vascular damage to non‐DN, renal biopsy should especially be considered to evaluate the degree of renal damage, and to differentiate between DN and primary glomerulopathies. We define this category of patients as atypical clinical presentation of renal disease with DM without DR. Examples of these patients were those with short duration of DM history, more progressive deterioration of renal function and those who were considered to have unusual presentation by a nephrology specialist. As we described in the present study, various primary glomerulopathies were found especially in patients without DR, therefore we could treat the patients accordingly.
Renal biopsy should be considered in DM patients with unusual clinical presentations, even those with long‐term DM. Furthermore, the present study showed that poor glycemic control and the existence of DR were significantly predictive makers. As the treatment options differ between DN and primary glomerulopathies, renal biopsy should especially be considered in DM patients with presumed DN without either poor glycemic control or DR.
Acknowledgement
There is no conflict of interest statement for all authors.
References
- 1.Suzuki D, Takano H, Toyoda M, et al Evaluation of renal biopsy samples of patients with diabetic nephropathy. Intern Med 2001; 40: 1077–1084 [DOI] [PubMed] [Google Scholar]
- 2.Tone A, Shikata K, Matsuda M, et al Clinical features of non‐diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract 2005; 69: 237–242 [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Chen X, Xie Y, et al A differential diagnostic model of diabetic nephropathy and non‐diabetic renal diseases. Nephrol Dial Transplant 2008; 23: 1940–1945 [DOI] [PubMed] [Google Scholar]
- 4.Huang F, Yang Q, Chen L, et al Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clin Nephrol 2007; 67: 293–297 [DOI] [PubMed] [Google Scholar]
- 5.Prakash J, Sen D, Kumar NS. Non‐diabetic renal disease in patients with type 2 diabetes mellitus. J Assoc Physicians India 2001; 49: 415–420 [PubMed] [Google Scholar]
- 6.Lee EY, Chung CH, Choi SO. Non‐diabetic renal disease in patients with non‐insulin dependent diabetes mellitus. Yonsei Med J 1999; 40: 321–326 [DOI] [PubMed] [Google Scholar]
- 7.Olsen S, Mogensen CE. How often is NIDDM complicated with nondiabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia 1996; 39: 1638–1645 [DOI] [PubMed] [Google Scholar]
- 8.Christensen PK, Larsen S, Horn T, et al Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 2000; 58: 1719–1731 [DOI] [PubMed] [Google Scholar]
- 9.Parving HH, Gall MA, Skøtt P, et al Prevalence and causes of albuminuria in non‐insulin‐dependent diabetic patients. Kidney Int 1992; 41: 758–762 [DOI] [PubMed] [Google Scholar]
- 10.Olsen S. Light microscopy of diabetic glomerulopathy: the classic lesion In: Mogesen C (ed). The Kidney and Hypertension in Diabetes Mellitus, 2nd edn Kluwer Academic Publishers, Boston, 1994: 141–150 [Google Scholar]
- 11.Churg J, Bernstein J, Glassock RJ. Renal Disease: Classification and Atlas of Glomerular Diseases. Igaku‐Shoin, New York, NY, 1995 [Google Scholar]
- 12.Gellman DD, Pirani CL, Soothill JF, et al Diabetic nephropathy: a clinical and pathologic study based on renal biopsies. Medicine 1959; 38: 321–327 [PubMed] [Google Scholar]
- 13.Takazakura E, Nakamoto Y, Hayakawa H, et al Onset and progression of diabetic glomerulosclerosis; a prospective study based on serial renal biopsies. Diabetes 1975; 24: 1–9 [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment Diabetic Retinopathy Study Research Group . Grading diabetic retinopathy from stereoscopic color fundus photographs – an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98: 786–806 [PubMed] [Google Scholar]
- 15.Seino Y, Nanjo K, Tajima N, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DCCT Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in IDDM. N Engl J Med 1993; 329: 977–983 [DOI] [PubMed] [Google Scholar]
- 17.Service FJ, O'Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetologia 2001; 44: 1215–1220 [DOI] [PubMed] [Google Scholar]
- 18.Gambara V, Mecca G, Remuzzi G, et al Heterogeneous nature of renal lesions in Type 2 diabetes. J Am Soc Nephrol 1993; 3: 1458–1466 [DOI] [PubMed] [Google Scholar]
- 19.Hong D, Zheng T, Jia‐qing S, et al Nodular glomerular lesion: a later stage of diabetic nephropathy? Diabetes Res Clin Pract 2007; 78: 189–195 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MM, Lewis EJ, Leonard‐Martin TC, et al Renal pathology patterns in Type 2 diabetes mellitus: relationship with retinopathy. Nephrol Dial Transplant 1998; 13: 2547–2552 [DOI] [PubMed] [Google Scholar]


