Abstract
A 72‐year‐old woman presented with repeated hypoglycemic and hyperglycemic episodes because of an insulin allergy. On admission, she was diagnosed with type B insulin resistance syndrome. She was also found to have anti‐insulin antibodies. After steroid therapy, glycemic control improved dramatically accompanied by the disappearance of the insulin allergy. We then introduced liraglutide, which successfully stabilized her glycemic episodes without allergic reactions. Liraglutide might be useful to treat patients with severe insulin allergy.
Keywords: Insulin allergy, Liraglutide, Type B insulin resistance syndrome
Introduction
Insulin allergy is an important clinical problem, and causes insulin resistance and instability of glycemic control1. To date, definitive options for treating insulin allergy have not been established. Glucagon‐like peptide‐1 (GLP‐1) analogs promote endogenous insulin secretion and suppress secretion of glucagon from the pancreatic islets. Hence, GLP‐1 analogs are considered as a therapeutic option for patients with severe insulin allergy. In the present case report, we describe a patient with severe insulin allergy complicated with type B insulin resistance syndrome, whose condition was successfully controlled by liraglutide.
Case Report
A 72‐year‐old woman (body mass index 21.7 kg/m2) was diagnosed with type 2 diabetes at 60 years‐of‐age, and received glibenclamide. She had begun insulin therapy at 66 years‐of‐age. After the initiation of insulin therapy, she noticed local itchy wheal‐flare reactions at the injection sites within 1 min of injection, which lasted for a couple of hours. These skin lesions subsequently developed large subcutaneous indurations. She had been undergoing treatment with four daily insulin injections: three injections of insulin aspart before breakfast (62 units), lunch (64 units) and dinner (54 units), respectively, and one of neutral protamine Hagedorn insulin (50 units) before breakfast. Despite this, control of her blood glucose remained difficult, and she had been experiencing frequent repeated hypoglycemic and hyperglycemic episodes. On admission, her glycohemoglobin (HbA1c) was 11.1%. A skin biopsy was taken from a large plaque of injection sites on the abdominal wall, and histological examination revealed a remarkable accumulation of inflammatory cells around blood vessels and massive deposition of adjacent connective tissue in deeper dermal areas, symptomatic of severe insulin allergy (Figure 1). As anti‐insulin receptor antibody was detected in her serum, she was unexpectedly diagnosed with type B insulin resistance syndrome. Furthermore, she was found to have a high titer of circulating polyclonal anti‐insulin antibodies with a low affinity constant and high binding capacity, as evaluated by Scatchard analysis (Figure 2). Gliclazide (40 mg/day), acarbose (300 mg/day), metformin (750 mg/day) and pioglitazone (30 mg/day) were introduced in combination with insulin therapy, all of which were ineffective. Finally, we decided to initiate intravenous methylprednisolone therapy (500 mg/day for 3 days) followed by oral prednisolone therapy (30 mg/day). After introduction of steroid therapy, the allergic skin reaction disappeared immediately accompanied by reduced subcutaneous induration. Serum levels of insulin‐specific immunoglobulin E (IgE) decreased from 1.27 to 0.44 UA/mL and HbA1c level fell progressively to 7.1% after 18 months (Figure 3). At this point, the patient wished to cease insulin injection because of repeated hypoglycemia. As her endogenous insulin secretion was still preserved (urinary C‐peptide: 63.7 μg/day), we introduced liraglutide after 18 months from starting steroid therapy while tapering prednisolone to 2 mg/day. Liraglutide was initiated at 0.3 mg/day, and was increased to a maintenance dose of 0.9 mg/day in conjunction with gliclazide (20 mg/day). After the introduction of liraglutide, she did not show any allergic reactions, hence, the use of prednisolone was terminated. Continuous glucose monitoring clearly showed a dose‐dependent striking improvement of glycemic control by liraglutide (Figure 4). Thereafter, HbA1c was maintained at approximately 7.0% with liraglutide (0.9 mg/day) and gliclazide (20 mg/day).
Figure 1.
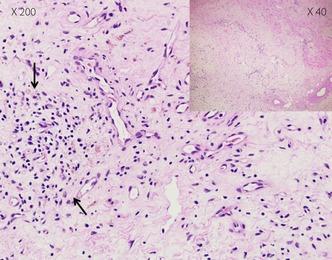
Histological slide of a skin biopsy obtained from an allergic skin reaction on the injection site. Congestion of different inflammatory cells in blood vessels with emission in the adjacent connective tissue of deeper dermal parts was observed (indicated by arrows; hematoxylin–eosin staining).
Figure 2.
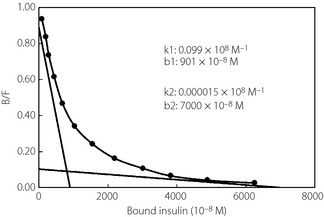
Scatchard plot analysis of insulin antibody. The insulin antibody showed a low affinity constant and high binding capacity. B/F: bound/free insulin.
Figure 3.
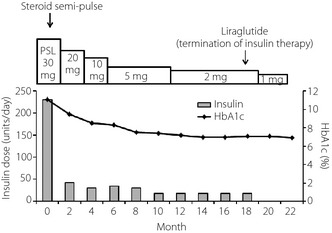
Changes of insulin dose and glycohemoglobin (HbA1c) during the time course. The amount of steroid is shown at the top. PSL, prednisolone.
Figure 4.
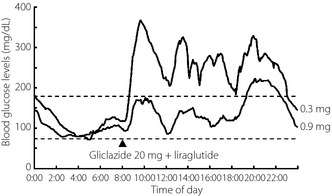
Representative daily profile of blood glucose levels after withdrawal of insulin therapy (continuous glucose monitoring). Liraglutide improved glycemic control in a dose‐dependent manner.
Discussion
Many factors were considered to be involved in the insulin resistance and glycemic instability of the present case. Type B insulin resistance syndrome is sometimes accompanied by other autoimmune disorders, and the present patient had a history of rheumatoid arthritis that might correspond to type B insulin resistance syndrome. However, evidence of hyperinsulinemia, such as acanthosis nigricance, had not been previously confirmed.
Adverse immune reaction leads to instability of glycemic control through two different mechanisms in patients treated with insulin therapy, namely, anti‐insulin antibodies and insulin allergy. Recent studies have shown that anti‐insulin antibodies raised in response to repeated insulin injection can induce hypoglycemia through a mechanism similar to that of insulin autoimmune syndrome (IAS)2. Scatchard analysis of anti‐insulin antibodies showed that the present patient possessed polyclonal antibodies, which have similar characteristics to IAS (Figure 2). Generally, an allergic skin reaction at an insulin injection site is classified as an IgE‐dependent‐immediate type of allergy, impairing the action of exogenous insulin and promoting degradation and/or disrupting absorption of insulin5.
After initiation of steroid therapy, skin lesions immediately improved, accompanied by a gradual reduction in blood glucose levels, suggesting that skin lesions were associated with impaired insulin absorption. Along with improvement in glycemic control, serum levels of insulin‐specific IgE decreased, indicating that insulin allergy was a major contributor to glycemic instability in this patient. Steroid therapy did not alter binding of anti‐insulin antibodies to insulin (data not shown). Investigation of the distinctive binding specificity of the anti‐insulin antibodies against endogenous and exogenous insulin will be required to elucidate the role of anti‐insulin antibodies. The best option for treatment of insulin allergy is to discontinue insulin injection. As long as endogenous insulin secretion is preserved, GLP‐1 analogs might be effective in patients with insulin allergy. As anticipated, liraglutide effected marked improvement in hyperglycemia and stabilized glycemic episodes without hypoglycemia (Figure 4).
To our knowledge, this is the first report showing the usefulness of liraglutide in patients with type 2 diabetes whose glycemic control is affected by severe insulin allergy. Our observations raise the possibility of a novel therapeutic strategy against insulin allergy.
Acknowledgment
There is no conflict of interest.
(J Diabetes Invest, doi: 10.1111/j.2040-1124.2012.00239.x, 2013)
HbA1c levels are expressed by NGSP values.
References
- 1.Ghazavi MK, Johnston GA. Insulin allergy. Clin Dermatol 2011; 3: 300–305 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Hirayama S, Ito S. A case of a non‐insulin dependent diabetic patient with regular spontaneous hypoglycemic attacks, which were due to insulin‐binding antibodies induced by human insulin therapy. Tohoku J Exp Med 1997; 2: 163–173 [DOI] [PubMed] [Google Scholar]
- 3.Koyama R, Nakanishi K, Kato M, et al Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin: treatment with double filtration plasmapheresis and prednisolone. Am J Med Sci 2005; 5: 259–264 [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Oida K, Miyamori I. A 75‐year‐old type 2 diabetes mellitus case responding strikingly troglitazone: possible mechanism of insulin resistance induced by insulin antibody. Nihon Ronen Igakkai Zasshi 2000; 37: 344–348 [DOI] [PubMed] [Google Scholar]
- 5.Fineberg SE, Kawabata TT, Finco‐Kent D, et al Immunological responses to exogenous insulin. Endocr Rev 2007; 6: 625–652 [DOI] [PubMed] [Google Scholar]


