The topic of increased risk of bone fracture associated with thiazolidinediones (TZD) treatment first drew international attention when a higher rate of fractures in women with diabetes treated with rosiglitazone was reported in the A Diabetes Outcome Program Trial (ADOPT)1. Subsequently, a number of clinical trials and observational studies addressing this issue have been reported and most of them have confirmed the increased risk of fracture in elderly women with type 2 diabetes mellitus treated with both rosiglitazone and pioglitazone. A recent report by Bilik et al. provided additional insight into the association between TZD treatment and bone fractures in patients with type 2 diabetes mellitus, showing that postmenopausal women taking TZD and the subset of men taking both loop diuretics and TZD were at increased risk for fractures2. Nevertheless, the results of currently available reports have still been inconclusive. For example, rosiglitazone and pioglitazone were reported to show comparable risk of fractures in some studies, whereas others have found a difference in the risk of fracture between these two drugs. Does the risk for fracture with TZD extend to men and to younger patients? Is it also applicable in Asian populations?
TZD exert many metabolic actions by interaction with peroxisome proliferator‐activated receptor (PPAR)γ, which is expressed in a number of tissues and directly regulates gene expression involved in adipogenesis, glucose homeostasis and inflammation responses. Most importantly, PPARγ plays an important role in adipocyte differentiation, as shown by studies in which overexpression of PPARγ in fibroblast cell lines initiates adipogenesis, and embryonic stem (ES) cells and embryonic fibroblasts from PPARγ deficient mice could not differentiate into adipocytes.
A series of experiments, in which TZD were given to rodents, showed adverse effects of TZD against bone metabolism. Both rosiglitazone and pioglitazone have been shown to consistently cause bone loss accompanied by decreased osteoblast activity and bone formation, and often by an increase in bone marrow adiposity. Although the effects of TZD administration on bone resorption were somewhat inconsistent and less compelling, increased bone resorption was often concomitant with decreased bone mass, particularly in aged animals given rosiglitazone. TZD are likely to have suppressive effect on bone formation and their stimulatory effects on bone resorption might be enhanced when given to aged animals (Figure 1).
Figure 1.
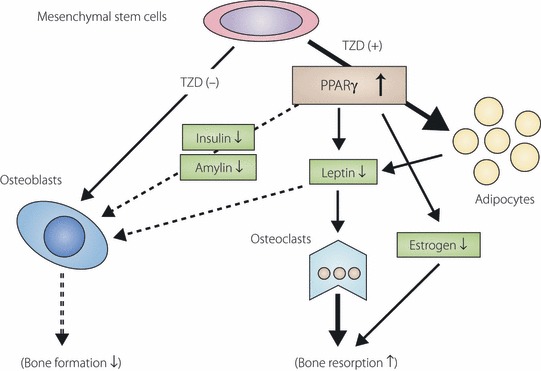
Effect of thiazolidinedione on bone metabolism. The activation of peroxisome proliferator‐activated receptor (PPAR)γ by thiazolidinedione (TZD) stimulates adipogenesis, thereby regulating a number of cellular signaling pathways involved in bone metabolism. Differentiation of mesenchymal stem cells into osteoblasts is affected by preferential differentiation of mesenchymal stem cells into adipocytes. Reduced osteoblastogenesis likely indirectly modulates osteoclastogenesis. Altered maturation of mesenchymal stem cells, in concert with humoral factors, shifts the balance between bone formation and resorption.
At a cellular level, a number of signaling pathways mediated by TZD might indirectly influence bone metabolism (Figure 1). Amelioration of insulin resistance by TZD lowers circulating levels of insulin and amylin, each of which is anabolic to osteoblasts. Activation of PPARγ in adipocytes influences expression and production of an array of adipocytokines, many of which have been suggested in the regulation of bone metabolism. Rosiglitazone is reported to decrease circulating insulin‐like growth factor (IGF)‐1 level, which is one of the crucial factors in the regulation of osteoblast differentiation and proliferation. Decrease in IGF‐1 in bone might be detrimental to bone mass through the inhibition of osteoblast formation. Thus, many humoral factors, under the regulation of TZD, are likely to modulate bone metabolism concurrently with improvement of insulin resistance.
A report of increased risk of fracture with TZD treatment in the ADOPT study1 was subsequently followed by clinical studies addressing the effects of TZD on bone mineral density (BMD). In the Health, Aging, and Body Composition (ABC) prospective observational study, each year of TZD (troglitazone, pioglitazone and/or rosiglitazone) use by older (age range 70–79 years) diabetic women was associated with a statistically significant increase in the annualized rate of whole‐body bone loss (0.6–1.2% per year)3. Another observational study examining BMD of 32 men with type 2 diabetes treated with rosiglitazone for 16 months showed a three to sixfold increase in the rate of bone loss in the spine, hip and proximal femur, compared with 128 matched men with type 2 diabetes mellitus not taking rosiglitazone4, despite the fact that no effect was observed on BMD in the male diabetes group (n = 32) taking TZD in the Health ABC study.
According to the results from ADOPT, a 4‐year study of glycemic durability of monotherapy, the rate of fracture in diabetic patients randomized to receive rosiglitazone was higher in women, but not in men, than that in patients randomized to receive either metformin or glyburide1. A pooled analysis of fracture incidence from randomized trials of pioglitazone, with the maximum duration of exposure to pioglitazone of 3.5 years collectively including 15,000 subjects (8100 with pioglitazone and 7400 with comparator), also showed an increase of limb fractures in women, but not in men, receiving pioglitazone. In contrast, a prospective, randomized, controlled study in Japan with the duration of 2.5–4 years that included 587 Japanese type 2 diabetes patients did not show any difference in the risk of fracture between groups receiving or not receiving pioglitazone, even in the older women5. More recently, a meta‐analysis, in which 10 randomized controlled trials involving 13,715 participants and two observational studies involving 31,679 participants were used to determine the risk of fractures associated with TZD therapy, was reported. Rosiglitazone and pioglitazone were associated with a significantly increased risk of fractures overall in the 10 randomized controlled trials (OR 1.45, 95% CI 1.18–1.79, P < 0.001). A significant increase in the risk of fractures among women (OR 2.23, 95% CI 1.65–3.01; P < 0.001), but not among men (OR 1.00, 95% CI 0.73–1.39, P = 0.98), was shown in five randomized controlled trials. The two observational studies showed an increased risk of fractures associated with rosiglitazone and pioglitazone. It was thus concluded in that study that long‐term TZD use doubles the risk of fractures among women with type 2 diabetes mellitus, without a significant increase in the risk of fractures among men with type 2 diabetes mellitus6. Although it is important to acknowledge the fact that many of the studies described here are observational and were carried out in European and American populations, and that fractures were not a prespecified end‐point, menopausal status was not considered and patients studied were relatively young (age range 55–60 years); an increased risk of fractures, at least in older women, is regarded as a class effect of TZD in a limited ethnic population at this time.
Generally, type 2 diabetes mellitus is associated with an increased risk of fractures, with the risk increasing with longer duration of disease. Recent meta‐analysis of observational studies has confirmed an increase in fracture risk in patients with type 2 diabetes mellitus, particularly of the hip, proximal humerus and foot. Risk factors that also contribute to increased fracture in diabetic patients include number of falls, insulin use, functional disability and impaired vision. The Health ABC study showed the increase in fracture risk not only in type 2 diabetes patients, but also in patients with elevated fasting plasma glucose3. Thus, it is not always simple to clinically discriminate risk of fracture induced by TZD use and to set up a prospective study to investigate the relationship between risk of fractures and TZD in patients with type 2 diabetes mellitus. Considering the undisputed reward provided by the use of TZD, particularly in patients with higher risk for cardiovascular diseases, tentativeness for TZD use in such patients might be excessive and lack an appropriate balance between usefulness and disadvantage. Nevertheless, it should be required to assess risk factors of fracture, such as age, sex, bodyweight, menopausal status and family history of fracture, particularly for patients being considered for treatment with TZD. In particular, it would be prudent, in the light of a fact that female patients are generally more sensitive to this class of drugs, to discreetly prescribe lower doses of TZD in younger women who are expected to take them over long periods.
References
- 1.Kahn SE, Zinman B, Lachin JM, et al. for the A Diabetes Outcome Progression Trial (ADOPT) Study Group . Rosiglitazone‐associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 2008; 31: 845–851 [DOI] [PubMed] [Google Scholar]
- 2.Bilik D, McEwen LN, Brown MB, et al. Thiazolidinediones and fractures: evidence from translating research into action for diabetes. J Clin Endocrinol Metab 2010; 95: 4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 2005; 165: 1612–1617 [DOI] [PubMed] [Google Scholar]
- 4.Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases BMD in type 2 diabetic men. Diabetes Care 2007; 30: 1574–1576 [DOI] [PubMed] [Google Scholar]
- 5.Kaku K, Daida H, Kashiwagi A, et al. Long‐term effects of pioglitazone in Japanese patients with type 2 diabetes without a recent history of macrovascular morbidity. Curr Med Res Opin 2009; 25: 2925–2932 [DOI] [PubMed] [Google Scholar]
- 6.Loke YK, Singh S, Furberg CD. Long‐term use of thiazolidinediones and fractures in type 2 diabetes: a meta‐analysis. CMAJ 2009; 180: 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]


