Abstract
There is a growing need to understand the underlying mechanisms involved in the progression of cardiovascular disease during obesity and diabetes. Although inhibition of fatty acid oxidation has been proposed as a novel approach to treat ischemic heart disease and heart failure, reduced muscle fatty acid oxidation rates may contribute to the development of obesity-associated insulin resistance. Our aim was to determine whether treatment with the antianginal agent trimetazidine, which inhibits fatty acid oxidation in the heart secondary to inhibition of 3-ketoacyl-CoA thiolase (3-KAT), may have off-target effects on glycemic control in obesity. We fed C57BL/6NCrl mice a high-fat diet (HFD) for 10 weeks before a 22-day treatment with the 3-KAT inhibitor trimetazidine (15 mg/kg per day). Insulin resistance was assessed via glucose/insulin tolerance testing, and lipid metabolite content was assessed in gastrocnemius muscle. Trimetazidine-treatment led to a mild shift in substrate preference toward carbohydrates as an oxidative fuel source in obese mice, evidenced by an increase in the respiratory exchange ratio. This shift in metabolism was accompanied by an accumulation of long-chain acyl-CoA and a trend to an increase in triacylglycerol content in gastrocnemius muscle, but did not exacerbate HFD-induced insulin resistance compared with control-treated mice. It is noteworthy that trimetazidine treatment reduced palmitate oxidation rates in the isolated working mouse heart and neonatal cardiomyocytes but not C2C12 skeletal myotubes. Our findings demonstrate that trimetazidine therapy does not adversely affect HFD-induced insulin resistance, suggesting that treatment with trimetazidine would not worsen glycemic control in obese patients with angina.
Introduction
Ischemic heart disease is a major cause of death and disability in the world today. However, results from numerous epidemiologic studies and randomized, placebo-controlled trials have provided compelling evidence that ischemic heart disease is highly manageable. Current treatment regimens consist of either percutaneous or surgical techniques to restore myocardial blood and oxygen supply, or pharmacotherapy (i.e., β-adrenergic receptor blockers) to reduce myocardial oxygen demand, and have significantly improved the overall prognosis of patients with angina and/or ischemic heart disease (Anderson et al., 2013). Yet, there remains a significant number of patients who are refractory to conventional treatment, and thus, novel therapies to treat ischemic heart disease are necessary. One potential exciting new therapy involves the optimization of cardiac energy metabolism, which can be achieved via reducing myocardial fatty acid oxidation rates (Ussher and Lopaschuk, 2008; Jaswal et al., 2011; Ussher et al., 2012a).
Indeed, preclinical studies demonstrate that reducing fatty acid oxidation rates in the heart, either secondary to limiting the mitochondrial uptake of fatty acids, or directly inhibiting the mitochondrial β-oxidation enzymatic machinery, reduces infarct size and improves cardiac function in experimental models of ischemia/reperfusion injury (Kantor et al., 2000; Dyck et al., 2004; Ussher et al., 2012b). Similar findings have been recapitulated in humans, as treatment with either perhexiline, which restricts mitochondrial fatty acid uptake via inhibition of carnitine palmitoyl transferase-1, or trimetazidine, which directly inhibits fatty acid oxidation via inhibiting the mitochondrial β-oxidation enzyme long-chain 3-ketoacyl-CoA thiolase (3-KAT), improves left ventricular (LV) function in ischemic heart failure patients (Lee et al., 2005; Fragasso et al., 2006; Tuunanen et al., 2008).
Of the metabolic agents available that act via reducing fatty acid oxidation rates, trimetazidine is the best characterized (Kantor et al., 2000; Lopaschuk et al., 2003) and is used clinically in over 80 countries as a treatment for angina (Ciapponi et al., 2005; Ussher and Lopaschuk, 2006). Although reducing fatty acid oxidation rates in the heart may produce beneficial anti-ischemic effects, it has been demonstrated in muscle that decreased fatty acid oxidation rates may promote insulin resistance in the setting of obesity (Choi et al., 2007; Savage et al., 2007). During obesity, excessive fatty acid uptake outpaces mitochondrial oxidative capacity, and as esterified fatty acids are diverted away from carnitine palmitoyl transferase 1 (CPT-1), the rate-limiting enzyme in mitochondrial fatty acid uptake, triacylglycerol (TAG) and other lipid metabolites such as ceramide and diacylglycerol (DAG) accumulate, which may have direct negative effects on muscle insulin sensitivity (Shulman, 2000; Chavez and Summers, 2012; Muoio and Neufer, 2012). Thus, it has been proposed that enhancing muscle fatty acid oxidation can protect against insulin resistance by preventing the accumulation of these lipid metabolites (Choi et al., 2007).
Because of these contrasting views regarding fatty acid oxidation rates in heart and muscle, our objective was to determine whether treatment with trimetazidine would exacerbate insulin resistance induced by a high-fat diet (HFD). As patients with angina and ischemic heart disease are also often obese and at risk of type 2 diabetes (T2D), it is essential to determine whether trimetazidine may have off-target effects on insulin sensitivity that potentially limit its therapeutic utility.
Materials and Methods
Animal Studies.
All animals received care according to the Canadian Council on Animal Care, and all animal procedures were approved by the University of Alberta Health Sciences Animal Welfare Committee. The 26-week-old C57BL/6NCrl mice (Charles River Laboratories, Hollister, CA) received a HFD (60% kcal from lard; Research Diets New Brunswick, NJ) for 10 weeks. At the end of week 10, animals were administered trimetazidine hydrochloride (15 mg/kg per day; Sigma-Aldrich, St. Louis, MO) or saline by intraperitoneal injection for 3 weeks (see Fig. 1 for the study protocol in obese mice). At study completion, ad libitum animals were killed for tissue extraction via intraperitoneal injection of sodium pentobarbital (12 mg) 2 hours into their dark cycle.
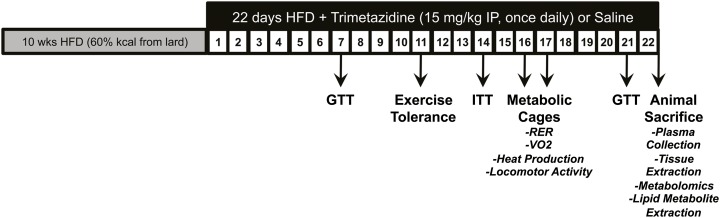
Fig. 1.
Schematic outlining the trimetazidine treatment protocol and physiological parameters tested in HFD-induced obese mice. We fed 26-week-old C57BL/6NCrl mice a HFD for 10 weeks. At the end of week 10, animals were administered trimetazidine hydrochloride (15 mg/kg per day) or saline by intraperitoneal injection for 22 days. A glucose tolerance test (GTT) was performed at 7 and 21 days after treatment, whereas an insulin tolerance test (ITT) was performed at 14 days after treatment. At 16–17 days after treatment, mice underwent indirect calorimetry in metabolic cages and were subjected to an exercise tolerance study at 11 days after treatment. At study completion, ad libitum animals were killed for tissue extraction and plasma collection.
Glucose and Insulin Tolerance.
Intraperitoneal glucose and insulin tolerance tests were performed 6 hours after food withdrawal using glucose and insulin doses of 2 g/kg and 0.7 U/kg, respectively. Blood glucose levels were determined at 0, 20, 30, 60, and 90 minutes after glucose/insulin administration via tail bleed with the Accu Check Advantage system (Roche Applied Science, Indianapolis, IN).
Plasma Insulin Levels.
Plasma insulin concentrations were determined via use of a commercially available enzymatic assay kit (Alpco Diagnostics, Salem, NH) as previously described elsewhere (Bates et al., 2012). In brief, 5 μl of sample was added per well with 75 μl of a provided enzyme conjugate, and the 96-well plate was then incubated for 2 hours at room temperature on an orbital microplate shaker. After incubation, the plate was washed six times with wash buffer, and then 100 μl of a provided substrate was added to each well to start the reaction, which was terminated after 30 minutes via addition of 100 μl of stop solution. Air bubbles were removed, and the plasma insulin levels (ng/ml) were determined via reading the absorbance of the plate at a 450 nm wavelength.
Isolated Working Heart Perfusions.
Mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.), and the hearts were subsequently excised for perfusion in the isolated working heart mode, as previously described elsewhere (Ussher et al., 2012b). In brief, hearts were perfused with oxygenated Krebs-Henseleit solution consisting of 5.0 mM glucose, 0.4 mM palmitate bound to 3% fatty acid-free bovine serum albumin, and 100 μU/ml insulin. The perfusate was labeled with [U-14C]glucose and [9,10-3H]palmitate, and 3H2O and 14CO2 production were assessed for the measurement of glucose and palmitate oxidation as previously described elsewhere (Ussher et al., 2012b).
Muscle Metabolic Profiling.
Gastrocnemius muscle and liver extracts for quantification of acylcarnitine (mass spectrometry/gas chromatography), long-chain acyl-CoA (high-performance liquid chromatography), TAG (chloroform/methanol extraction), DAG (DAG kinase thin-layer chromatography assay), and ceramide (DAG kinase thin-layer chromatography assay) content were determined as previously described elsewhere (Ussher et al., 2010).
Indirect Calorimetry.
In vivo whole-body metabolic assessment was performed using an Oxymax Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH) to determine the respiratory exchange ratio, oxygen consumption rates, heat production, locomotor activity, and 24-hour food intake, as previously described elsewhere (Ussher et al., 2010). Animals were initially acclimatized in the system for a 24-hour period; the subsequent 24-hour period was used for data collection.
Exercise Capacity.
Exercise capacity was performed by running mice on a calibrated, motor-driven treadmill (Columbus Instruments) at a speed of 3 m/min for 1 minute, followed by increasing speeds of 4 m/min for 1 minute, 5 m/min for 1 minute, 6 m/min for 3 minutes, 8 m/min for 14 minutes, 9 m/min for 10 minutes, 10 m/min for 7 minutes, 12 m/min for 7 minutes, and 14 m/min until exhaustion. The first 6 minutes were used as an acclimatization period for the animals to become familiar with the treadmill and were not used for data collection. Exhaustion was determined as the animal spending >5 consecutive seconds on the shock grid, or the animal running off the shock grid and immediately falling back onto the shock grid three consecutive times.
Cell Culture.
Primary rat cardiomyocytes were isolated from the hearts of 1- to 3-day-old neonatal rat pups (Biosciences) and cultured in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s Nutrient Mixture F-12 containing 5% fetal bovine serum, 10% horse serum, and 1% penicillin-streptomycin, as previously described elsewhere (Samokhvalov et al., 2012). The C2C12 myotubes [CRL-1772; American Type Culture Collection (ATCC), Manassas, VA] were cultured as myoblasts on Primeria six-well plates (Falcon, Corning Life Sciences, Tewksbury, MA) with DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin, whereas myotube differentiation was induced via culturing in DMEM containing 2% horse serum and 1% penicillin-streptomycin, as previously described (Ussher et al., 2009). Muscle biopsies from vastus lateralis of lean women were extracted via the percutaneous needle biopsy technique, and cultured myoblasts from the biopsy samples were subsequently differentiated into skeletal myocytes, as previously described elsewhere (Kovalik et al., 2011).
For the glucose and palmitate oxidation experiments, the medium was switched to Krebs-Henseleit solution consisting of 11.0 mM glucose and 0.8 mM palmitate bound to 4% fatty-acid-free bovine serum albumin. The Krebs-Henseleit solution was labeled with either [U-14C]glucose or [1-14C]palmitate, and 14CO2 was captured in hyamine hydroxide–soaked filter paper for the measurement of glucose and palmitate oxidation in separate experiments, as previously described elsewhere (Samokhvalov et al., 2012). Oleate oxidation in human skeletal myocytes also was measured, as previously described elsewhere (Kovalik et al., 2011).
Statistical Analysis.
The significance of differences between two groups was determined by the use of an unpaired, two-tailed Student’s t test. The significance of differences for multiple comparisons was estimated by two-way analysis of variance (ANOVA). When ANOVA revealed differences, multiple t tests with a Bonferroni correction were performed on the data sets. P < 0.05 was considered statistically significant.
Results
Trimetazidine Treatment Mildly Affects Substrate Preference In Vivo without Affecting Body Weight, Adiposity, or Glycemia in HFD-Induced Obese Mice.
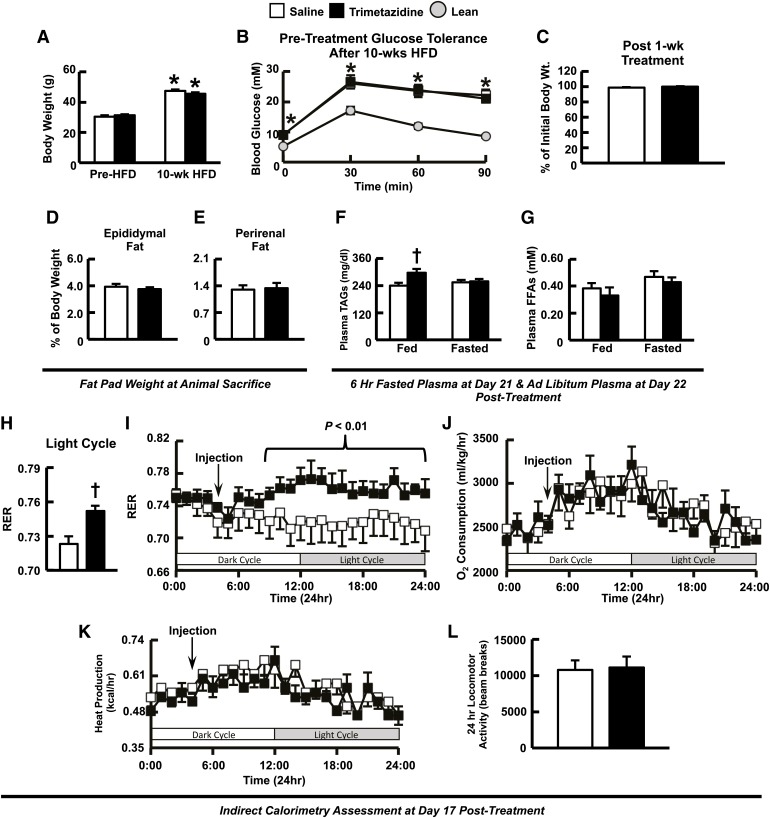
As expected, mice fed a HFD for 10 weeks experienced a significant increase in weight gain (Fig. 2A) and became glucose intolerant (Fig. 2B). Starting at week 11, animals received daily injections of either trimetazidine (15 mg/kg) or saline for 22 days. Body weight was not altered in trimetazidine-treated HFD-induced obese mice after 1 week (Fig. 2C) or after study completion in either obese (44.85 ± 1.31 g versus 43.77 ± 1.16 g) or lean mice (28.68 ± 0.99 g versus 27.64 ± 0.35 g). Likewise, overall adiposity as determined by measurement of epididymal and perirenal adipose depot weights was unaffected in trimetazidine-treated HFD-induced obese mice after animal sacrifice at 22 days after treatment (Fig. 2, D and E). Furthermore, plasma TAG and free fatty acid levels were similar in trimetazidine-treated HFD-induced obese mice after a 6-hour fast at day 21 after treatment, whereas plasma TAG levels were elevated with no change in free fatty acid levels ad libitum at day 22 after treatment (Fig. 2, F and G; Table 1).
Fig. 2.
Treatment with trimetazidine has no effect on body weight in HFD-induced obese mice but induces a shift in energy substrate metabolism away from fatty acids and toward carbohydrates as an oxidative energy source in vivo. Body weight (A) and glucose intolerance (B) in mice after 10 weeks of high-fat feeding. (C) Body weight of HFD-induced obese mice at 7 days after treatment. Epididymal (D) and perirenal (E) fat pad weight (normalized to body weight) at the time the animals were killed in saline- and trimetazidine-treated HFD-induced obese mice. Plasma TAGs (F) and free fatty acids (FFAs) (G) in the ad libitum state (day 22 after treatment) and fasted state (day 21 after treatment) from HFD-induced obese mice treated with saline or trimetazidine. Respiratory exchange ratio (H—I), whole-body oxygen consumption rates (J), whole-body heat production (K), and locomotor activity (L) in HFD-induced obese mice at days 16–17 after treatment. Values represent mean ± S.E. (n = 5–6). The statistical significance of differences between the two groups was determined by the use of an unpaired, two-tailed Student’s t test. The significance of differences for multiple comparisons was estimated by two-way analysis of variance (ANOVA). When ANOVA revealed differences, multiple t tests with a Bonferroni correction were performed on the data sets. *P < 0.05, statistically significantly different from lean/pre–HFD mice. †P < 0.05, statistically significantly different from saline-treated HFD-induced obese mice.
TABLE 1.
Plasma parameters after trimetazidine treatment of HFD-induced obese mice
Plasma parameters in trimetazidine-treated HFD-induced obese mice. Ad libitum plasma was collected on day 22 after treatment, 2 hours into the dark cycle during animal euthanization, whereas fasted plasma was collected after a 6-hour fast on day 21 after treatment (n = 5–6). Values represent mean ± S.E.M.
| Ad Libitum Saline | 6-h Fast Saline | Ad Libitum Trimetazidine | 6-h Fast Trimetazidine | |
|---|---|---|---|---|
| Glucose (mM) | 7.95 ± 0.70 | 7.13 ± 0.29 | 7.83 ± 0.43 | 7.52 ± 0.56 |
| Insulin (ng/ml) | 4.41 ± 0.62 | 0.92 ± 0.11 | 4.21 ± 0.34 | 0.95 ± 0.08 |
| TAG (mg/dl) | 240.65 ± 12.52 | 254.89 ± 11.65 | 298.05 ± 16.24* | 258.33 ± 12.26 |
| FFA (mM) | 0.38 ± 0.04 | 0.47 ± 0.04 | 0.33 ± 0.06 | 0.43 ± 0.03 |
FFA, free fatty acid.
P < 0.05, indicates a statistically significant difference from saline-treated counterpart.
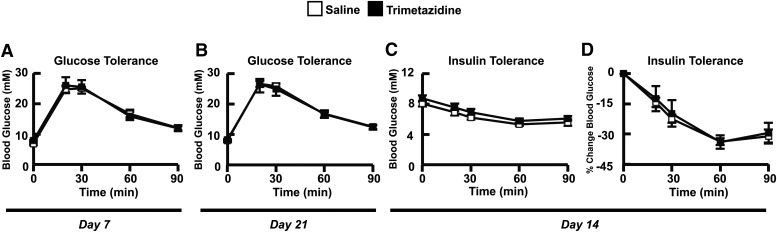
At days 16 and 17 after treatment, indirect calorimetry was assessed through use of metabolic cages, whereby saline-treated HFD-induced obese mice exhibited a respiratory exchange ratio (RER) approaching 0.7, indicative of fatty acid oxidation as their primary metabolic substrate. Interestingly, treatment of HFD-induced obese mice with trimetazidine induced a mild shift in metabolic substrate preference, as seen by an increase in RER during the light cycle (Fig. 2, H and I), illustrating a greater reliance on carbohydrates for oxidative energy metabolism. This shift in metabolism in trimetazidine-treated HFD-induced obese mice was not associated with alterations in whole-body oxygen consumption rates, heat production, locomotor activity (Fig. 2, J–L), or exercise capacity (14.09 ± 2.86 minutes versus 14.70 ± 3.11 minutes during treadmill running) and food intake (2.42 ± 0.17 g versus 2.35 ± 0.19 g over 24 hours). Moreover, this mild effect on substrate preference after trimetazidine treatment did not worsen insulin resistance, as glucose tolerance at both 7 and 21 days after treatment was similar between saline- and trimetazidine-treated HFD-induced obese mice (Fig. 3, A and B), as were plasma insulin levels during the glucose tolerance test (data not shown). Likewise, insulin tolerance at 14-days after treatment was also comparable between saline and trimetazidine-treated HFD-induced obese mice (Fig. 3, C and D).
Fig. 3.
Treatment with trimetazidine does not affect glucose or insulin tolerance in HFD-induced obese mice. Day-7 (A) and day-21 (B) glucose tolerance in trimetazidine-treated HFD-induced obese mice. (C) Absolute change in blood glucose levels (millimolars) during an insulin tolerance test in trimetazidine-treated HFD-induced obese mice 14 days after treatment. (D) Percentage change in blood glucose levels during the insulin tolerance test 14 days after treatment. Values represent mean ± S.E. (n = 6).
Trimetazidine Inhibits Fatty Acid Oxidation in Cardiac but Not Skeletal Muscle.
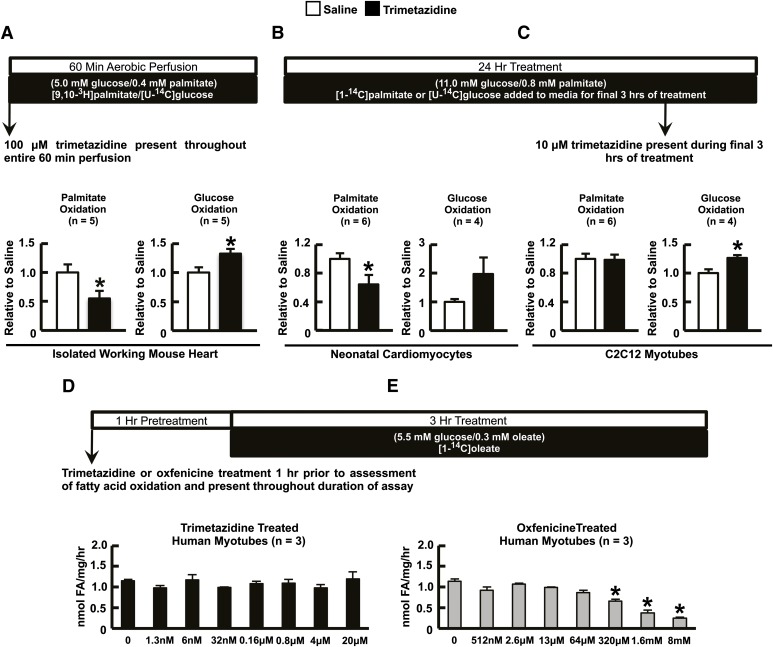
Palmitate oxidation was significantly inhibited in trimetazidine-treated (100 μM) isolated working mouse hearts, which was associated with a corresponding increase in glucose oxidation (Fig. 4A). Similar findings were observed in cultured neonatal rat cardiomyocytes (10 μM trimetazidine, Fig. 4B). In contrast, trimetazidine (10 μM) did not inhibit palmitate oxidation in C2C12 skeletal muscle myotubes (Fig. 4C). It is noteworthy, however, that trimetazidine did stimulate glucose oxidation in C2C12 myotubes (Fig. 4C), similar to its effect in the heart (Fig. 4A). In addition, we observed a significant decrease in oleate oxidation in primary cultured human myocytes treated with the CPT-1 inhibitor oxfenicine, but not in response to treatment with trimetazidine (Fig. 4, D and E).
Fig. 4.
Trimetazidine selectively inhibits fatty acid oxidation in vitro in a cell-specific manner. Oxidative metabolism (glucose and palmitate oxidation) in the isolated working mouse heart (A), neonatal cardiomyocytes (B), and C2C12 myotubes treated with trimetazidine (100 μM in the isolated working heart and 10 μM in the cultured cells) (C). Oleate oxidation rates in primary cultures of human skeletal muscle myocytes treated with increasing concentrations of either trimetazidine (D) or oxfenicine (E). Values represent mean ± S.E. (n = 3–6). The statistical significance of differences between two groups was determined by the use of an unpaired, two-tailed Student’s t test. The significance of differences for multiple comparisons was estimated by two-way analysis of variance (ANOVA). When ANOVA revealed differences, multiple t tests with a Bonferroni correction were performed on the data sets. *P < 0.05, significantly different from saline-treated counterpart.
Muscle and Hepatic Lipid Metabolite Content in HFD-Induced Obese Mice after 22 Days of Treatment with Trimetazidine.
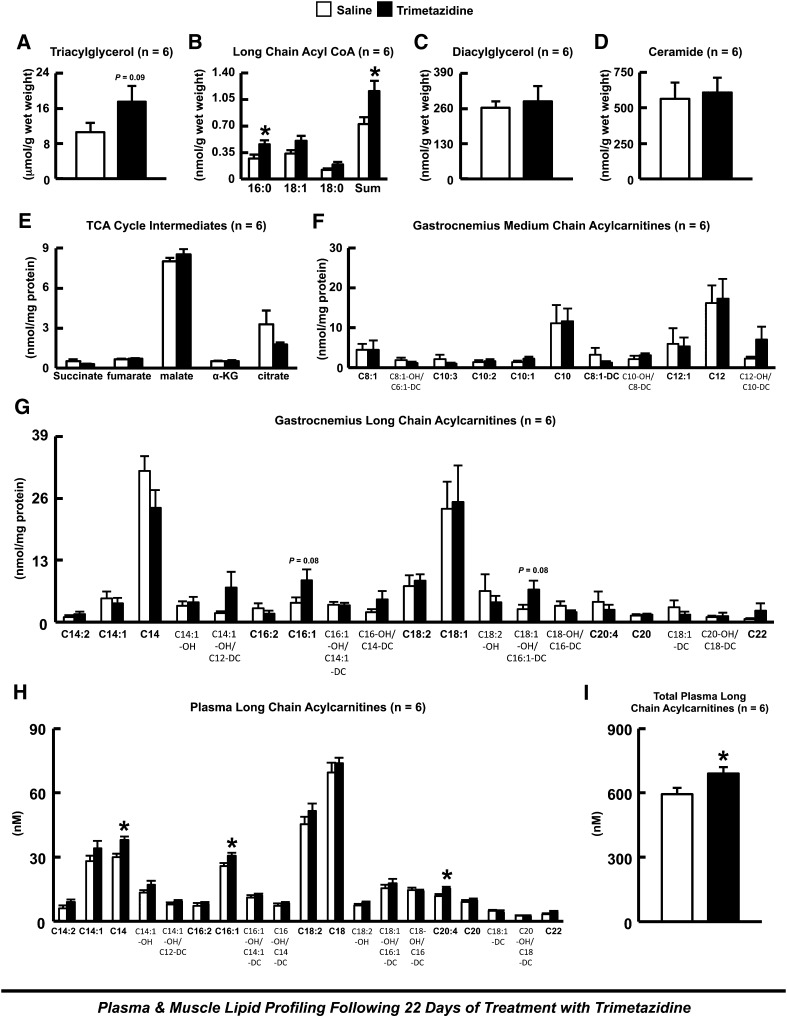
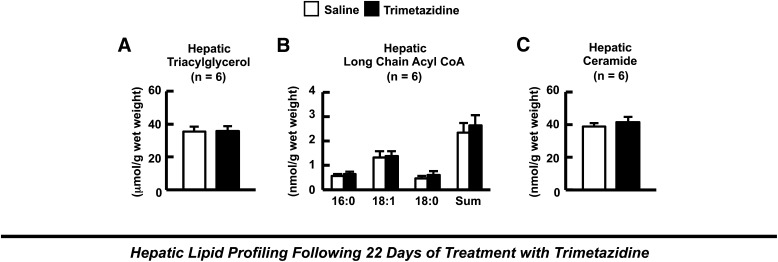
Treatment with trimetazidine for 22 days resulted in a trend to an increase in gastrocnemius TAG content, which was accompanied by a significant increase in gastrocnemius long-chain acyl-CoA content (Fig. 5, A and B). In contrast, trimetazidine treatment had no effect on gastrocnemius DAG or ceramide content (Fig. 5, C and D). Gas chromatography/mass spectrometry metabolic profiling of gastrocnemius muscle from control and trimetazidine-treated HFD-induced obese mice demonstrated no change in any of the intermediates of the tricarboxylic acid cycle (Fig. 5E). Although the majority of long-chain acylcarnitine species such as palmitoyl- and steroylcarnitine did not accumulate further in gastrocnemius muscle of HFD-induced obese mice treated with trimetazidine (data not shown), there was a trend to a further increase in other long-chain acylcarnitine species such as palmitoleoylcarnitine (Fig. 5, F and G). Furthermore, a number of plasma long-chain acylcarnitine species were increased in trimetazidine-treated HFD-induced obese mice, consistent with trimetazidine inhibiting muscle fatty acid oxidation in vivo (Fig. 5, H and I). On the contrary, we observed no differences in hepatic TAG, long-chain acyl-CoA, or ceramide content in HFD-induced obese mice treated with trimetazidine for 22 days (Fig. 6).
Fig. 5.
Muscle lipid metabolite accumulation and levels of tricarboxylic acid cycle intermediates in HFD-induced obese mice after 22 days of treatment with trimetazidine. (A) Gastrocnemius triacylglycerol content. (B) Long-chain acyl-CoA content. (C) Diacylglycerol content. (D) Ceramide content. (E) Tricarboxylic acid (TCA) cycle intermediate levels. Medium-chain acylcarnitine content (F) and long-chain acylcarnitine content (G) in saline and trimetazidine-treated HFD-induced obese mice. (H–I) Plasma long-chain acylcarnitine content in saline and trimetazidine-treated HFD-induced insulin-resistant mice. Values represent mean ± S.E. (n = 6). Differences were determined by the use of an unpaired, two-tailed Student’s t test. *P < 0.05, significantly different from HFD-induced insulin-resistant saline-treated mice.
Fig. 6.
Hepatic lipid metabolite accumulation in HFD-induced obese mice after 22 days of treatment with trimetazidine. (A) Hepatic triacylglycerol content. Long-chain acyl-CoA content (B) and ceramide content (C) in saline and trimetazidine-treated HFD-induced insulin-resistant mice. Values represent mean ± S.E. (n = 6).
Discussion
This study demonstrates that treatment with the 3-KAT inhibitor trimetazidine does not exacerbate HFD-induced insulin resistance, even though trimetazidine treatment resulted in a greater accumulation of intramuscular TAG and long-chain acyl-CoA. As previous findings have suggested that a reduction in skeletal muscle fatty acid oxidation can cause insulin resistance and T2D (Choi et al., 2007; Savage et al., 2007), our goal was to determine whether treatment with trimetazidine might have off-target adverse effects on muscle insulin resistance and glycemic control. Surprisingly, although trimetazidine inhibited fatty acid oxidation rates in the isolated working mouse heart and cultured neonatal cardiomyocytes, trimetazidine did not inhibit fatty acid oxidation rates in cultured muscle myotubes, which may explain why trimetazidine did not worsen HFD-induced insulin resistance. However, our in vivo indirect calorimetry findings suggest a mild inhibition of whole-body fatty acid oxidation rates, evidenced by the small increase in RER after treatment with trimetazidine, consistent with the increase in intramuscular lipid accumulation. Although indirect calorimetry reflects whole-body metabolism, a significant fraction of the RER is accounted for by skeletal muscle oxidative metabolism (Zurlo et al., 1990; Sleigh et al., 2011).
A potential limitation that may account for the discrepancy between our in vivo and in vitro findings involves the inherent low oxidative rates measured in the in vitro cell culture systems. Indeed, nanomoles per minute rates for fatty acid oxidation are often reported in vivo/ex vivo (Ussher et al., 2012b) versus picomoles per minute rates in vitro (An et al., 2006; Watt et al., 2006). This is probably a reflection of the low work performed by these isolated cell systems, which is an important determinant of mitochondrial oxidative rates (Neely et al., 1967). Indeed, our palmitate oxidation rates measured in both neonatal rat cardiomyocytes and C2C12 myotubes are substantially lower than the rates obtained in our isolated working mouse hearts, which may increase the difficulty of capturing an actual inhibition of fatty acid oxidation. Furthermore, we have previously shown that the ability to measure inhibition of fatty acid oxidation via trimetazidine in vitro is complicated by the accumulation of substrate for 3-KAT over time, which overcomes trimetazidine-mediated inhibition of 3-KAT (Lopaschuk et al., 2003). Whether the kinetics for trimetazidine-mediated inhibition of 3-KAT are different in neonatal cardiomyocytes and C2C12 myotubes is of interest but is beyond the scope of this study’s objectives.
To support our indirect calorimetry observations, we used targeted metabolomics to quantify gastrocnemius and plasma acylcarnitines as an index of potential alterations in fatty acid oxidation. With trimetazidine-mediated inhibition of 3-KAT, the last enzyme involved in mitochondrial fatty acid oxidation, fatty acids would still have free access into the mitochondria through CPT-1, thereby allowing partial oxidation. This is evidenced by similar levels of long-chain acylcarnitines in gastrocnemius muscle from both vehicle control and trimetazidine-treated animals. As skeletal muscle has been demonstrated to be a major source of circulating acylcarnitines (Noland et al., 2009), measurement of plasma acylcarnitines should also provide an index of intramuscular acylcarnitine accumulation and perturbations in fatty acid flux. Indeed, plasma long-chain acylcarnitines were elevated in mice after treatment with trimetazidine, consistent with 3-KAT inhibition not impeding CPT-1-mediated mitochondrial fatty acid uptake, enhancing the accumulation of intramuscular long-chain acylcarnitines and their subsequent export into the circulation.
Therefore, on the basis of our in vivo observations, we do believe that treatment with trimetazidine results in a very mild inhibition of muscle fatty acid oxidation, which is not captured in our in vitro studies likely due to lack of sensitivity. To our surprise, however, this does not result in a worsening of HFD-induced insulin resistance. Although current dogma demonstrates that an acceleration of muscle fatty acid oxidation alleviates insulin resistance via reducing lipid metabolite accumulation (Steinberg et al., 2006; Watt et al., 2006; Choi et al., 2007; Bruce et al., 2009), this is an extremely controversial area of active debate with numerous studies reporting conflicting findings. As a matter of fact, the original work of Randle et al. (1963) demonstrated that an increase in fatty acid oxidation reduces glucose oxidation and subsequent glucose uptake in the isolated perfused heart and diaphragm, although these conclusions were simply extrapolated to skeletal muscle. In contrast, meticulous work from the Shulman laboratory suggests that fatty acid oxidation rates are impaired in muscle in the setting of obesity, and that accelerating fatty acid oxidation may improve glucose homeostasis in obesity via reducing the accumulation of lipid metabolites and subsequent inhibition of insulin signaling (Choi et al., 2007; Savage et al., 2007; Zhang et al., 2007).
Likewise, muscle overexpression of CPT-1 via electroporation of an adenovirus increases fatty acid oxidation rates and reduces membrane accumulation of DAG/ceramide, which results in a significant improvement in insulin signaling and glucose uptake in rats fed a HFD (Bruce et al., 2009). Indeed, long-term inhibition of CPT-1 in rats with etomoxir increases muscle lipid accumulation, which is associated with a worsening of obesity-induced insulin resistance (Dobbins et al., 2001). On the other hand, we have shown in a mouse model of diet-induced obesity that a 4-week treatment with the CPT-1 inhibitor oxfenicine reverses insulin resistance and glucose intolerance (Keung et al., 2013). Reasons for this discrepancy are not clear but may be attributed to species-related differences or CPT-1 inhibition being provided at the onset of high-fat feeding in the etomoxir study (Dobbins et al., 2001), whereas we allowed mice to become obese and insulin resistant before treating with oxfenicine (Keung et al., 2013).
In support of our studies, Finck et al. (2005) have shown that elevated fatty acid oxidation rates in mice overexpressing muscle-specific peroxisome proliferator–activated receptor α induce insulin resistance, and that this can also be improved via treatment with the CPT-1 inhibitor oxfenicine. Studies in humans consuming a HFD for 3 days have recapitulated these effects, as treatment with etomoxir (five doses totaling 600 mg spread over 36 hours) inhibited fatty acid oxidation, which was associated with a corresponding increase in glucose oxidation, sarcolemmal glucose transporter type 4 (GLUT4) content in muscle fibers, and a lowering in the homeostatic model assessment (HOMA) index scores (Timmers et al., 2012).
A key difference between our observations and the aforementioned studies where inhibiting fatty acid oxidation has beneficial effects on muscle insulin sensitivity involves the method of inhibition. Indeed, we used a 3-KAT inhibitor (trimetazidine) in this particular study, whereas the other studies inhibited fatty acid oxidation secondary to an inhibition of CPT-1 and subsequent mitochondrial fatty acid uptake (Finck et al., 2005; Timmers et al., 2012; Keung et al., 2013). It has been suggested that lipid overload specifically in the mitochondria elicits mitochondrial dysfunction, increases oxidative stress, and impairs insulin sensitivity in muscle (Koves et al., 2008; Anderson et al., 2009). Thus, restricting mitochondrial fatty acid uptake versus directly inhibiting mitochondrial β-oxidation should not yield equivalent biologic outcomes, as lipids may still enter the mitochondria if the inhibition takes place at the level of β-oxidation. Nevertheless, the discordant results of these multiple studies collectively illustrates the need for further work to elucidate exactly how alterations in fatty acid oxidation affect the development and progression of muscle insulin resistance.
The fact that intramuscular ceramide and DAG content did not increase further after 3-KAT inhibition may also explain why insulin resistance was not exacerbated in our model. At the same time, it may also be possible that an additional accumulation of muscle lipid metabolites (i.e., 10–20% further increase) would not impart any further damage on glucose/insulin tolerance beyond that already attributed to the muscle lipid metabolites accumulated after HFD-induced obesity. Indeed, our model of HFD-induced obesity causes a doubling in the levels of muscle long-chain acyl-CoA compared with standard chow-fed mice (Ussher et al., 2010), and hence the additional increase after trimetazidine treatment may not yield any further consequences on glucose tolerance and insulin sensitivity.
Although inhibition of 3-KAT-regulated fatty acid oxidation may not be harmful in our study, it is important to note that the inhibition of fatty acid oxidation in liver may have detrimental effects, potentially accelerating steatosis development (Savage et al., 2007). Nonetheless, we did not observe any further increase in hepatic TAG, long-chain acyl-CoA, or ceramide content in trimetazidine-treated HFD-induced obese mice. Adiposity was also similar in our study, as treatment of obese mice with trimetazidine did not increase epididymal or perirenal fat pad weight.
Conversely, it is also possible that life-long modest reductions in fatty acid oxidation, such as which may occur in obese patients with ischemic heart disease who may be treated with trimetazidine over years or decades, may ultimately result in impaired insulin sensitivity. However, the answer to that question cannot be determined from this study, which only investigated the effects of a 3-week treatment regimen with trimetazidine on HFD-induced metabolic dysfunction. This treatment duration was chosen as we were concurrently investigating a separate cardiovascular study, determining whether trimetazidine therapy could alleviate obesity-induced cardiac dysfunction.
Ultrasound echocardiography analyses at day 20 after treatment revealed a significant amelioration of diet-induced cardiac hypertrophy (6.43 ± 0.50 g/mm versus 4.98 ± 0.46 g/mm LV mass/tibia length) and contractile dysfunction in HFD-induced obese mice receiving trimetazidine (data not shown). These results are consistent with findings from Tuunanen et al. (2008), whereby treatment of idiopathic dilated cardiomyopathy patients with trimetazidine caused a modest 10% decrease in myocardial fatty acid oxidation rates but a significant improvement in LV function. These authors also showed that trimetazidine inhibits endogenous TAG-derived fatty acid oxidation, consistent with the trend to increased gastrocnemius TAG content that we observed in HFD-induced obese mice treated with trimetazidine (Bucci et al., 2011). Therefore, it appears that the antianginal agent trimetazidine elicits cardioprotection in the setting of obesity without adversely affecting muscle insulin sensitivity, though a more prolonged treatment may yield different results.
Overall, our results reveal important insights regarding the contribution of fatty acid oxidation to insulin resistance. It has been postulated that impaired skeletal muscle fatty acid oxidation can cause insulin resistance (Savage et al., 2007). Thus, treatment with trimetazidine in obese mice would be anticipated to exacerbate skeletal muscle insulin resistance, which we did not observe, suggesting that trimetazidine would not impair insulin sensitivity in obese patients with angina. Furthermore, our observations in vitro demonstrate that, unlike in the heart, trimetazidine’s effect on muscle fatty acid oxidation is extremely mild. In spite of the ongoing debate concerning reduced fatty acid oxidation rates and insulin resistance, our findings do not raise caution regarding the use of trimetazidine to treat obese patients with angina.
Acknowledgments
The authors thank the dedicated staff of the Metabolomics and Biomarker Core of the Sarah W. Stedman Nutrition and Metabolism Center for measurement of acylcarnitines and tricarboxylic acid intermediates, and the Cardiovascular Translational Research Centre HPLC Core Facility for the measurement of long-chain acyl-CoAs and ceramides.
Abbreviations
- ANOVA
analysis of variance
- CPT-1
carnitine palmitoyl transferase 1
- DAG
diacylglycerol
- DMEM
Dulbecco’s modified Eagle’s medium
- HFD
high-fat diet
- 3-KAT
3-ketoacyl-CoA thiolase
- LV
left ventricular
- RER
respiratory exchange ratio
- T2D
type 2 diabetes
- TAG
triacylglycerol
Authorship Contributions
Participated in research design: Ussher, G.D. Lopaschuk.
Conducted experiments: Ussher, Keung, Fillmore, Koves, Mori, Zhang, D.G. Lopaschuk, Ilkayeva, Wagg.
Performed data analysis: Ussher, Jaswal, Muoio, G.D. Lopaschuk.
Wrote or contributed to the writing of the manuscript: Ussher, Keung, Jaswal, Muoio, G.D. Lopaschuk.
Footnotes
This study was funded by a grant from the Heart and Stroke Foundation of Alberta (to G.D.L.); and the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL101189] (to D.M.M.). J.R.U. is a trainee of the Alberta Heritage Foundation for Medical Research.
References
- An D, Kewalramani G, Chan JK, Qi D, Ghosh S, Pulinilkunnil T, Abrahani A, Innis SM, Rodrigues B. (2006) Metformin influences cardiomyocyte cell death by pathways that are dependent and independent of caspase-3. Diabetologia 49:2174–2184 [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, et al. (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines (2013) 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127:e663–e828 [DOI] [PubMed] [Google Scholar]
- Bates HE, Campbell JE, Ussher JR, Baggio LL, Maida A, Seino Y, Drucker DJ. (2012) Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr−/− mice. Diabetes 61:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. (2009) Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 58:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Borra R, Nagren K, Parkka JP, Del Ry S, Maggio R, Tuunanen H, Viljanen T, Cabiati M, Rigazio S, et al. (2011) Trimetazidine reduces endogenous free fatty acid oxidation and improves myocardial efficiency in obese humans. Cardiovasc Ther 30:333–341 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Summers SA. (2012) A ceramide-centric view of insulin resistance. Cell Metab 15:585–594 [DOI] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. (2007) Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci USA 104:16480–16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapponi A, Pizarro R, Harrison J. (2005) Trimetazidine for stable angina. Cochrane Database Syst Rev (4):CD003614. [DOI] [PubMed] [Google Scholar]
- Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. (2001) Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 50:123–130 [DOI] [PubMed] [Google Scholar]
- Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G, et al. (2004) Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res 94:e78–e84 [DOI] [PubMed] [Google Scholar]
- Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP. (2005) A potential link between muscle peroxisome proliferator- activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 1:133–144 [DOI] [PubMed] [Google Scholar]
- Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, Margonato A. (2006) A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 48:992–998 [DOI] [PubMed] [Google Scholar]
- Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. (2011) Targeting fatty acid and carbohydrate oxidation—a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 1813:1333–1350 [DOI] [PubMed] [Google Scholar]
- Kantor PF, Lucien A, Kozak R, Lopaschuk GD. (2000) The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 86:580–588 [DOI] [PubMed] [Google Scholar]
- Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS, Lopaschuk GD. (2013) Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 62:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalik JP, Slentz D, Stevens RD, Kraus WE, Houmard JA, Nicoll JB, Lea-Currie YR, Everingham K, Kien CL, Buehrer BM, et al. (2011) Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes 60:1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56 [DOI] [PubMed] [Google Scholar]
- Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, et al. (2005) Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 112:3280–3288 [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Barr R, Thomas PD, Dyck JR. (2003) Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ Res 93:e33–e37 [DOI] [PubMed] [Google Scholar]
- Muoio DM, Neufer PD. (2012) Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely JR, Liebermeister H, Battersby EJ, Morgan HE. (1967) Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 212:804–814 [DOI] [PubMed] [Google Scholar]
- Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. (2009) Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284:22840–22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789 [DOI] [PubMed] [Google Scholar]
- Samokhvalov V, Ussher JR, Fillmore N, Armstrong IK, Keung W, Moroz D, Lopaschuk DG, Seubert J, Lopaschuk GD. (2012) Inhibition of malonyl-CoA decarboxylase reduces the inflammatory response associated with insulin resistance. Am J Physiol Endocrinol Metab 303:E1459–E1468 [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. (2007) Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh A, Raymond-Barker P, Thackray K, Porter D, Hatunic M, Vottero A, Burren C, Mitchell C, McIntyre M, Brage S, et al. (2011) Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest 121:2457–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Görgün CZ, Carling D, et al. (2006) Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4:465–474 [DOI] [PubMed] [Google Scholar]
- Timmers S, Nabben M, Bosma M, van Bree B, Lenaers E, van Beurden D, Schaart G, Westerterp-Plantenga MS, Langhans W, Hesselink MK, et al. (2012) Augmenting muscle diacylglycerol and triacylglycerol content by blocking fatty acid oxidation does not impede insulin sensitivity. Proc Natl Acad Sci USA 109:11711–11716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuunanen H, Engblom E, Naum A, Någren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, et al. (2008) Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 118:1250–1258 [DOI] [PubMed] [Google Scholar]
- Ussher JR, Jaswal JS, Lopaschuk GD. (2012a) Pyridine nucleotide regulation of cardiac intermediary metabolism. Circ Res 111:628–641 [DOI] [PubMed] [Google Scholar]
- Ussher JR, Jaswal JS, Wagg CS, Armstrong HE, Lopaschuk DG, Keung W, Lopaschuk GD. (2009) Role of the atypical protein kinase Czeta in regulation of 5′-AMP-activated protein kinase in cardiac and skeletal muscle. Am J Physiol Endocrinol Metab 297:E349–E357 [DOI] [PubMed] [Google Scholar]
- Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, et al. (2010) Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher JR, Lopaschuk GD. (2006) Clinical implications of energetic problems in cardiovascular disease. Heart and Metabolism 32:9–17 [Google Scholar]
- Ussher JR, Lopaschuk GD. (2008) The malonyl CoA axis as a potential target for treating ischaemic heart disease. Cardiovasc Res 79:259–268 [DOI] [PubMed] [Google Scholar]
- Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, et al. (2012b) Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 94:359–369 [DOI] [PubMed] [Google Scholar]
- Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. (2006) CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 12:541–548 [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. (2007) Mitochondrial dysfunction due to long-chain acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104:17075–17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo F, Larson K, Bogardus C, Ravussin E. (1990) Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86:1423–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]