Abstract
Angiogenesis is a key component of recovery after stroke. Angiotensin II receptor blocker (ARB) treatment improves neurobehavioral outcome and is associated with enhanced angiogenesis after stroke. The purpose of this study is to investigate the temporal pattern of the ARB proangiogenic effect in the ischemic brain and its association with vascular endothelial growth factors VEGF-A and VEGF-B. Wistar rats were exposed to 90-minute middle cerebral artery occlusion and treated with candesartan (1 mg/kg) at reperfusion. The proangiogenic potential of the cerebrospinal fluid was determined at 8, 24, 48, and 72 hours using an in vitro Matrigel tube formation assay. In addition, the expression of VEGF-A and VEGF-B was measured in brain homogenates using Western blotting at the same time points. A single candesartan dose induced a prolonged proangiogenic effect and a prolonged upregulation of VEGF-A and VEGF-B in vivo. In the ischemic hemisphere, candesartan treatment was associated with stabilization of hypoxia-inducible factor-1α and preservation of angiopoietin-1. The effect of ARB treatment on endothelial cells was studied in vitro. Our results identified brain endothelial cells as one target for the action of ARBs and a source of the upregulated VEGF-A and VEGF-B, which exerted an autocrine angiogenic response, in addition to a paracrine neuroprotective effect. Taken together, this study highlights the potential usefulness of augmenting the endogenous restorative capacity of the brain through the administration of ARBs.
Introduction
Angiogenesis has been linked to a better recovery after stroke (Krupinski et al., 1994; Manoonkitiwongsa et al., 2001; Navaratna et al., 2009). The role of angiogenesis, however, is not limited to creating a “conduit” to restore oxygen and nutrient delivery. It is now believed that angiogenesis is coupled to neurorestorative processes, including neurogenesis and synaptogenesis (Teng et al., 2008; Beck and Plate, 2009; Xiong et al., 2010). It has been demonstrated that these vessels provide neurotrophic support to the newly formed neurons, thereby improving functional recovery after an ischemic stroke (Navaratna et al., 2009).
There is extensive preclinical evidence that angiotensin II receptor blocker (ARB) treatment after ischemic stroke improves neurobehavioral outcome and enhances recovery (Engelhorn et al., 2004; Hosomi et al., 2005; Kozak et al., 2008; Guan et al., 2011a,b,c; Ishrat et al., 2013). In contrast with reports from other vascular beds in which inhibition of angiogenesis was documented (Willis et al., 2011), ARB treatment was associated with increased vascular density in the brain (Munzenmaier and Greene, 2006). Long-term pretreatment with losartan (Forder et al., 2005) or valsartan (Li et al., 2008a) increased vascular density and reduced infarct volume after ischemic injury.
The prototypical angiogenic molecule, vascular endothelial growth factor (VEGF)-A, has a well established role in physiologic as well as pathologic angiogenesis, mediated mostly by VEGF receptor (VEGFR)-2 (Ferrara, 1995; Zhang et al., 2000; Shibuya, 2013). In addition to its angiogenic potential, VEGF-A is involved in learning and memory, stimulates neurogenesis, and exerts a neuroprotective effect (Jin et al., 2000, 2002; Matsuzaki et al., 2001; Cao et al., 2004). Stabilization of hypoxia-inducible factor-1 (HIF-1) under ischemic conditions induces VEGF-A expression, possibly by binding to its promoter region (Levy et al., 1995; Huang et al., 1996; Wenger, 2002). Less is known, however, about the relatively new VEGF family member, VEGF-B. Although its proangiogenic effect is controversial, VEGF-B exerts vascular and neuroprotective effects against a wide range of apoptotic stimuli via VEGFR1 (Sun et al., 2006; Li et al., 2008b, 2009; Zhang et al., 2009). In a rodent model of focal cerebral ischemia, VEGF-B knockout animals showed 50% larger infarcts compared with their wild-type counterparts (Li et al., 2008b). Angiopoietins (Angs), another group of vascular-specific growth factors, play a prominent role in angiogenesis as well as in vessel maturation and function (Davis and Yancopoulos, 1999; Thurston et al., 1999, 2000). Ang-1 expression falls dramatically after focal cerebral ischemia, resulting in a leaky blood–brain barrier. Increases in Ang-1 levels, however, preserve vascular integrity after focal ischemia and/or VEGF administration (Thurston et al., 2000; Zhang and Chopp, 2002; Zhang et al., 2002; Tao et al., 2011). The use of the aforementioned angiogenic and vascular protective factors might seem to be a promising treatment of acute ischemic stroke. However, the challenging route of administration limits the benefit of such a treatment. An attempt to augment the endogenous production of these growth factors represents an attractive alternative.
This study was designed to investigate the temporal pattern of candesartan’s proangiogenic effect as well as VEGF-A and VEGF-B upregulation in response to a single treatment. In addition, we show for the first time the involvement of the vascular endothelium in candesartan’s neuroprotective effect.
Materials and Methods
All experimental protocols were approved by the Care of Experimental Animal Committee of Georgia Regents University and the Institutional Animal Care and Use Committee of the Veterans Affairs Medical Center.
Experimental Cerebral Ischemia.
A total of 30 adult male Wistar rats (Charles River Laboratories, Wilmington, MA), weighing 280–300 g, were subjected to 90-minute middle cerebral artery occlusion (MCAO) using a intraluminal suture model, as previously described (Kozak et al., 2009). Successful MCAO was confirmed by the presence of hemiparesis prior to reperfusion. Animals with a Bederson score <3 were excluded from the study together with animals that were obtunded or unable to move. At reperfusion, animals received either saline or 1 mg/kg candesartan (a gift from AstraZeneca, Wilmington, DE) via tail vein injection and were randomized into four different groups (8, 24, 48, and 72 hours). All animals were singly housed before and after surgery, with free access to food and water. At the aforementioned time points, cerebrospinal fluid (CSF) and brain tissues were collected and snap-frozen.
Cell Culture and Treatments.
The human cerebral microvascular endothelial cell line hCMEC/D3 was a kind gift from Dr. Jason Zastre (University of Georgia, Athens, GA). Endothelial cells were grown in MCDB-131 complete medium (VEC Technologies, Rensselaer, NY). Cells were serum starved overnight before treatment with candesartan (0.1, 1, and 10 μg/ml) or losartan potassium (0.05 μg/ml; Sigma-Aldrich, St. Louis, MO) in serum-free Eagle’s minimum essential medium (EMEM; American Type Culture Collection, Manassas, VA) and were compared with untreated controls. Clinically relevant concentrations of candesartan (0.1 μg/ml) and losartan (0.05 μg/ml) were determined according to the published pharmacokinetic data (Schulz and Schmoldt, 2003). For neutralization experiments, VEGF-A–neutralizing antibody, VEGF-B–neutralizing antibody, or both (2 μg/ml; R&D Systems, Minneapolis, MN) was added to the conditioned media collected from endothelial cells 30 minutes before neuronal treatment. Normal goat IgG (2 μg/ml; R&D Systems) was used as a control. Mouse primary cerebral cortical neuronal cultures were isolated from embryonic day 17 fetuses of CD1 mice (Charles River Laboratories) as previously described (Paxinos and Franklin, 2001; Pillai et al., 2008). The experimental protocol was approved by the Georgia Regents University Committee on Animal Use for Research (Pillai). Isolated neurons were cultured in Neurobasal medium (Life Technologies, Grand Island, NY), supplemented with B27 (Life Technologies), 2 mM l-glutamine (Life Technologies), and antibiotics (CellGro, Manassas, VA). Neurons were used for experiments between days 5 and 7 in vitro.
Oxygen and Glucose Deprivation.
To mimic ischemic conditions that occur during stroke, neuronal and endothelial cells were incubated in a hypoxia chamber (ProOx model C21; BioSpherix, Lacona, NY) at an O2 concentration <1% and 5% CO2 at 37°C. Culture medium was changed to the glucose-free Neurobasal-A medium (Life Technologies). After 2 hours of oxygen and glucose deprivation (OGD), cells were reoxygenated and received serum-free EMEM with or without treatment.
Tube Formation.
The ability of endothelial cells to align into tube-like structures was measured using the Matrigel (BD Biosciences, San Jose, CA) tube formation assay as previously described (Kozak et al., 2009). To measure the proangiogenic potential of CSF, confluent cells were serum starved overnight, harvested, and suspended in a mixture of serum-free EMEM and growth factor–reduced Matrigel in a 70:30 ratio. The mixture was quickly transferred to a 96-well plate (100 μl/well). Approximately 5 × 104 cells in a volume of 100 μl were seeded into each quadruplicate well. CSF (50 μl) was added and the mixture was allowed to solidify. Images were captured for the center of each well after 24 hours using a Zeiss Axiovert microscope (Carl Zeiss, Oberkochen, Germany) at an objective lens magnification of ×10. Tube-like structures were counted in a blinded fashion. Tubes were defined as endothelial cells that had aligned to form >90% closed structures. To measure the proangiogenic effect of candesartan treatment, confluent endothelial cells were exposed to either normoxic or 2-hour OGD conditions as previously explained. The cells were then treated with candesartan or losartan. At 24 hours, cells were harvested and the same procedure was followed. Additional images were captured and analyzed at 24, 48, and 72 hours.
Cell Proliferation.
Endothelial cell proliferation was assessed by a bromodeoxyuridine (BrdU) colorimetric assay kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol. Cells were plated at a density of 5 × 103 cells per well in 96-well plates and left overnight to attach. Cells were serum starved and exposed to normoxic or 2-hour OGD followed by treatment with different doses of candesartan. BrdU-labeling solution was then added. At 24 hours, absorbance was measured at 450 nm.
Cell Migration.
Endothelial cell migration was assessed by the in vitro wound healing assay as previously described (Kochuparambil et al., 2011). Images were taken at 0, 18, and 24 hours using phase-contrast microscopy on an inverted microscope at an objective lens magnification of ×5. The width of the scratch was measured at 20 fixed points in each well and the average was calculated. The percentage of migration was presented as the fold increase relative to the control.
Western Blotting.
Protein expression was measured by Western blotting as previously described (Guan et al., 2011c). Nonspecific binding was blocked by incubating the membranes in 5% milk in Tris-buffered saline/Tween 20 for 60 minutes prior to overnight incubation with primary antibodies against VEGF-A, phospho-VEGFR-1 (Tyr 1213; Millipore, Billerica, MA), VEGF-B (Abcam, Cambridge, MA), HIF-1α, Ang-1 (Santa Cruz Biotechnology, Dallas, TX), total VEGFR-1, phospho-VEGFR-2 (Tyr996), total VEGFR-2, glyceraldehyde-3-phosphate dehydrogenase, and cleaved caspase-3 (Cell Signaling Technology, Danvers, MA). β-Actin (Sigma-Aldrich) was used as an endogenous loading control. Densitometric measurements were performed using ImageJ software (National Institutes of Health, Bethesda, MD), and the results were represented as the fold increase relative to the control group.
Slot Blot.
The detection of nitrotyrosine and 4-hydroxynonenal (4-HNE) was performed using the slot blot technique as previously described (Abdelsaid et al., 2010). In brief, tissue or cell lysate was immobilized on nitrocellulose membrane using a Whatman Minifold slot blot system (GE Healthcare Bio-Sciences, Pittsburgh, PA). Membranes were blocked then incubated with primary anti-nitrotyrosine antibody (Millipore) or anti–4-HNE (Alpha Diagnostic International, San Antonio, TX), followed by peroxidase-labeled goat anti-mouse IgG (EMD Chemicals, San Diego, CA). Densitometric measurements were performed using ImageJ software and the results were represented as the fold increase relative to the control group.
Angiotensin II Determination.
Angiotensin II release into the cell culture media was quantified using an angiotensin II enzyme immunoassay kit (Bertin Pharma, Montigny le Bretonneux, France). The cell culture media were collected 24 hours after treatment and centrifuged at 13,000 rpm for 5 minutes to remove any cells. The supernatant was then used for the assay according to the manufacturer’s instructions.
Statistical Analysis.
All statistical analyses were carried out using NCSS 8 software (NCSS, LLC, Kaysville, UT). Results were expressed as the mean ± S.E.M. Data were statistically analyzed using the two-sample unpaired t test for single comparisons. One-way analysis of variance was used for multiple comparisons and was followed by Dunnett’s two-sided multiple comparison test. Neutralizing antibody experiments were analyzed by two-way analysis of variance. Data from the CSF proangiogenic effect experiment were analyzed using linear regression. Results were considered statistically significant at P < 0.05.
Results
Candesartan Treatment Induces a Prolonged Proangiogenic State.
CSF collected from candesartan-treated animals induced a prolonged proangiogenic response as evident by profound increases in tube formation in brain endothelial cells. Candesartan treatment enhanced the proangiogenic potential of CSF compared with saline treatment, an effect that was maintained throughout the measured time points. The mean slope for the candesartan group was 10-fold higher than the saline group (0.24 ± 0.03 versus 0.02 ± 0.01; P < 0.05) (Fig. 1).
Fig. 1.
CSF from candesartan-treated animals induces a prolonged proangiogenic effect. (A and B) Representative micrographs of tubes formed after treatment with CSF collected at 72 hours from saline-treated and candesartan-treated (1 mg/kg) animals, respectively. CSF was collected 72 hours after reperfusion. (C) Quantification of tubes after treatment with CSF collected from saline-treated and candesartan-treated (1 mg/kg) animals 8, 24, 48, and 72 hours after reperfusion (n = 2–3 per group). *P < 0.05. Cand, candesartan.
A Single Candesartan Dose Induces a Prolonged Upregulation of VEGF-A and VEGF-B Expression In Vivo.
In the contralateral hemisphere, candesartan enhanced VEGF-A and VEGF-B expression at all the studied time points. Compared with saline treatment, VEGF-A expression increased by 40–50% as early as 8 hours and continued until 48 hours. At 72 hours, candesartan increased VEGF-A expression 2-fold. In the same hemisphere, VEGF-B expression was elevated by 20–40% in the first 48 hours and by >50% at 72 hours compared with the saline group (Fig. 2, A and C). In the ipsilateral hemisphere; however, candesartan treatment induced an early increase in the expression of both VEGF-A and VEGF-B, an effect that was blunted at later time points. At 8 and 24 hours, VEGF-A expression was approximately 20% higher in the candesartan group than in the saline group. VEGF-B elevation, however, was more dramatic. Candesartan resulted in an >85% increase in VEGF-B levels at 8 hours that decreased to 30% at 24 hours compared with the saline levels (Fig. 2, B and D).
Fig. 2.
A single candesartan dose induces a prolonged enhancement of VEGF-A and VEGF-B expression in both hemispheres. (A and B) Quantification of VEGF-A expression in the contralateral and ipsilateral hemispheres, respectively, at 8, 24, 48, and 72 hours after reperfusion with saline or candesartan (1 mg/kg). (C and D) Quantification of VEGF-B expression in the contralateral and ipsilateral hemispheres at the same time points (n = 3 per group). *P < 0.05 (significantly different from the saline group at the same time point). Cand, candesartan.
Candesartan Treatment Stabilizes HIF-1α, Exerts an Antinitrative Effect, and Preserves Ang-1 Expression in the Ischemic Hemisphere.
In saline-treated animals, HIF-1α stabilization was observed as early as 8 hours after focal cerebral ischemia. The ipsilateral hemisphere had 60% higher HIF-1α levels than the contralateral hemisphere. Such stabilization was blunted at 24 hours after stroke. Candesartan treatment enhanced both the extent and duration of HIF-1α stabilization. HIF-1α levels were 2.3-fold higher in the ipsilateral hemisphere compared with the contralateral hemisphere at 8 hours. This stabilization continued to be observed at 24 hours in which HIF-1α levels were 30% higher in ipsilateral versus contralateral hemispheres (Fig. 3A).
Fig. 3.
Candesartan treatment stabilizes HIF-1α, exerts an antinitrative effect, and preserves Ang-1 in the ischemic hemisphere. (A) Quantification of HIF-1α in both ipsilateral and contralateral hemispheres at 8 and 24 hours after reperfusion with saline or 1 mg/kg candesartan (n = 3 per group for the 8-hour time point; n = 5–6 per group for the 24-hour time point). (B and C) Quantification of nitrotyrosine and Ang-1, respectively, in both hemispheres 24 hours after reperfusion with either saline or 1 mg/kg candesartan (n = 4 per group). *P < 0.05. Cand, candesartan; NY, nitrotyrosine.
In saline-treated animals, a 67% elevation in the nitrative stress marker nitrotyrosine was detected in the ipsilateral side compared with the contralateral side. Candesartan treatment reduced nitrotyrosine levels back to control levels (Fig. 3B). Likewise, direct treatment of endothelial cells with peroxynitrite (ONOO−) increased the nitrotyrosine level by 84%. Treatment with candesartan reduced the nitrotyrosine level to 25% (Supplemental Fig. 1A). Moreover, candesartan exerted an antioxidant effect after treatment with either H2O2 or ONOO−. Either treatment increased the level of 4-HNE, an oxidative stress marker, by 40%. Candesartan treatment (0.1 μg/ml) reduced its level back to control levels (Supplemental Fig. 1, B and C).
We further investigated whether candesartan treatment affects Ang-1 levels after stroke. In the saline-treated group, we observed a 40% reduction of Ang-1 levels in the ipsilateral hemisphere compared with the contralateral hemisphere. Candesartan preserved Ang-1 levels in the ipsilateral hemisphere at 24 hours, resulting in an only 8% reduction in Ang-1 compared with the contralateral hemisphere (Fig. 3C).
Candesartan Enhances Endothelial VEGF-A and VEGF-B Expression as well as Their Receptor Activation In Vitro.
Under normoxic conditions, VEGF-A expression was enhanced by 31, 36, and 25% in groups treated with 0.1, 1, and 10 μg/ml candesartan, respectively (Fig. 4A). The induction of VEGF-A expression was more pronounced under OGD conditions, resulting in 36, 57, and 60% increases in the same treatment groups, respectively (Fig. 4B). VEGF-B followed the same pattern, with an increase of 30–35% under normoxic conditions in all treatment groups (Fig. 4C). However, in OGD, VEGF-B increased by 30, 85, and 90% in the same treatment groups, respectively (Fig. 4D). We studied whether the upregulated VEGF-A and VEGF-B could exert an autocrine effect on endothelial cells. Therefore, we measured the phosphorylation of VEGFR1 and VEGFR2 as an indicator of VEGF-B and VEGF-A functions, respectively. Candesartan enhanced phosphorylation of VEGFR1 by 25 and 65% and VEGFR2 by 30 and 65% in normoxic and OGD conditions, respectively (Fig. 4, E–H). Taken together, candesartan enhances expression of endothelial VEGF-A and VEGF-B as well as their receptor phosphorylation irrespective of the oxygenation status of the cell.
Fig. 4.
Candesartan enhances the expression of VEGF-A and VEGF-B as well the phosphorylation of their receptors in vitro. (A and C) Quantification of VEGF-A and VEGF-B, respectively, in endothelial cell lysate after 24-hour treatment with candesartan 0.1, 1, and 10 μg/ml under normoxic conditions (n = 4–6 per group). (B and D) Quantification of VEGF-A and VEGF-B in endothelial cell lysate, respectively, after 2-hour OGD and 24-hour treatment with candesartan 0.1, 1, and 10 μg/ml, respectively (n = 4 per group). (E and G) Determination of VEGFR1 and VEGFR2 activities, as measured by their phosphorylation levels, after 24 hours of treatment with candesartan (1 μg/ml). (F and H) Determination of VEGFR1 and VEGFR2 phosphorylation after 2 hours of OGD and 24-hour treatment with 1 μg/ml candesartan (n = 3–4 per group). *P < 0.05. Cand, candesartan; Ctrl, control; pVR1, phosphorylated VEGFR1; pVR2, phosphorylated VEGFR2.
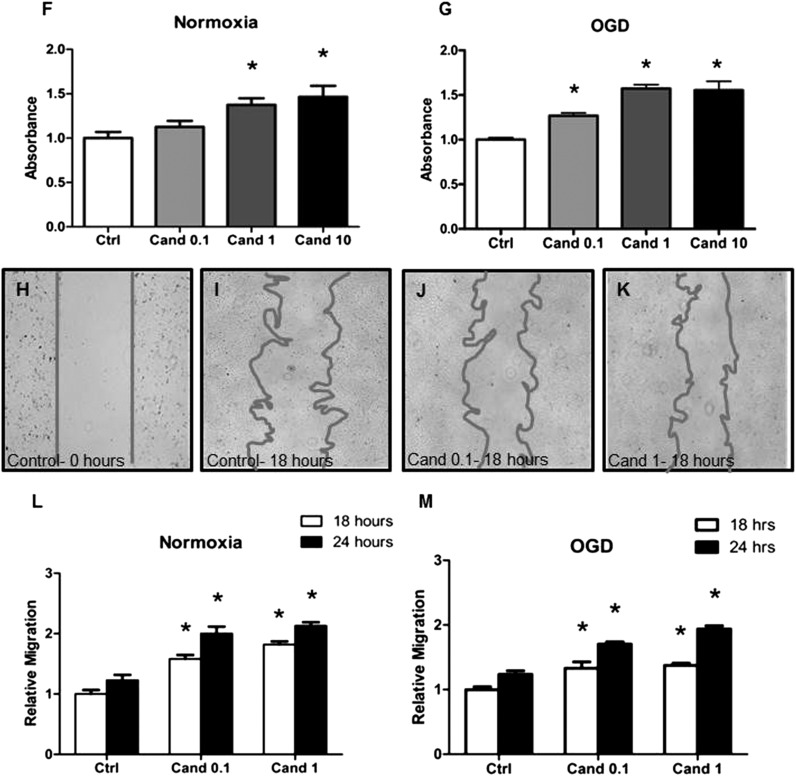
Candesartan Enhances Endothelial Cell Proliferation, Migration, and Alignment into Tube-Like Structures in a Dose-Dependent Manner.
At 24 hours, candesartan increased tube formation by 3-, 3.5-, and 4.5-fold in groups treated with 0.1, 1, and 10 μg/ml candesartan, respectively, displaying a dose-dependent effect (Fig. 5A). We conducted a 3-day time course under both normoxic and OGD conditions, using one candesartan dose (1 μg/ml). Candesartan treatment resulted in 5- to 11-fold and 3- to 4-fold increases in tube-like structures under normoxic and OGD conditions, respectively (Fig. 5, B–E). Likewise, endothelial cell proliferation increased under normoxic conditions by 13, 37, and 46% in the groups treated with 0.1, 1, and 10 μg/ml candesartan, respectively, displaying a dose-response relationship (Fig. 5F). Candesartan treatment under OGD conditions resulted in 27, 57, and 55% increases in BrdU incorporation in the aforementioned candesartan concentrations, respectively (Fig. 5G). In agreement with the proliferation and tube formation data, 0.1 and 1 μg/ml candesartan enhanced cell migration by 58 and 82%, respectively, at 18 hours and by 63 and 73%, respectively, at 24 hours under normoxic conditions (Fig. 5, H–L). Under OGD conditions, candesartan enhanced cell migration by 33 and 37% at 18 hours and by 38 and 57% at 24 hours in the tested concentrations, respectively (Fig. 5M).
Fig. 5.
Candesartan enhances endothelial cell tube formation, proliferation, and migration in a dose-dependent fashion. (A) Quantification of the number of tubes formed after 2-hour OGD and 24-hour treatment with 0.1, 1, and 10 μg/ml candesartan (n = 4–10 per group). (B and C) Representative micrographs of tubes formed at 72 hours under normoxic conditions in control and candesartan-treated (1 μg/ml) groups, respectively. (D and E) Quantification of the tubes formed under normoxic and OGD conditions, respectively, 24, 48, and 72 hours after treatment with 1 μg/ml candesartan (n = 4–9 per group). (F and G) Quantification of endothelial cell proliferation 24 hours after treatment with 0.1, 1, and 10 μg/ml candesartan under normoxic conditions and OGD conditions, respectively (n = 4 per group). (H–K) Representative micrographs of endothelial cell migration in the wound healing assay at 0 hours (H), untreated controls at 18 hours (I), and groups treated with 0.1 μg/ml candesartan (J) and 1 μg/ml candesartan (K) at 18 hours. (L and M) Quantification of endothelial cell migration after 18 and 24 hours of treatment under normoxic and OGD conditions, respectively (n = 5–8 per group). *P < 0.05 (significantly different from control at the same time point). Cand, candesartan; Ctrl, control.
Losartan Treatment Promotes a Proangiogenic State and Increases VEGF-A and VEGF-B Expression In Vitro.
We conducted in vitro experiments to test whether a candesartan proangiogenic effect can be exerted by other ARBs. Losartan treatment increased VEGF-A and VEGF-B expression by 1.8- and 2.5-fold 24 hours after treatment (Fig. 6A). Phosphorylation of VEGFR1 and VEGFR2 was increased by 2.5- and 3-fold at the same time point (Fig. 6, B and C). We quantified tube formation and migration after losartan treatment. Losartan enhanced endothelial tube formation by 2.5-fold (Fig. 6, D–F). Likewise, endothelial cell migration increased by 45 and 70% at 18 and 24 hours after treatment, respectively (Fig. 6G).
Fig. 6.
Losartan treatment promotes a proangiogenic state and increases VEGF-A and VEGF-B expression in vitro. (A) Determination of VEGF-A and VEGF-B expression in the endothelial cell lysate after 2 hours of OGD and 24 hours of treatment with 0.05 μg/ml losartan (n = 3–5). (B and C) Determination of VEGFR1 and VEGFR2 phosphorylation in endothelial cell lysate after 2 hours of OGD and 24 hours of treatment with 0.05 μg/ml losartan (n = 3). (D and E) Representative micrographs of tubes formed after 2-hour OGD and 24-hour losartan (0.05 μg/ml) treatment. (F) Quantification of tubes formed after 2-hour OGD and 24-hour treatment with the same losartan concentration (n = 5 and n = 4 for control and losartan groups, respectively). (G) Quantification of endothelial cell migration after 18 and 24 hours of treatment with the same concentration of losartan (n = 4 and n = 3 for control and losartan groups, respectively). *P < 0.05 (significantly different from control at the same time point). Ctrl, untreated control; Los, losartan-treated cell.
Both VEGF-A and VEGF-B Are Required for the Proangiogenic Effect of Candesartan.
Consistent with the controversial angiogenic role of VEGF-B, we observed a modest reduction in endothelial cell migration after its neutralization. A more pronounced reduction was observed after VEGF neutralization, consistent with its well documented angiogenic role. Neutralization of both growth factors exerted a synergistic, rather than an additive, inhibitory effect on endothelial cell migration at 24 hours (Fig. 7).
Fig. 7.
The candesartan-induced proangiogenic effect is mediated through VEGF-A and VEGF-B. (A–F) Representative micrographs of endothelial cell migration in wound healing assay with or without candesartan treatment and VEGF-A– and VEGF-B–neutralizing antibodies. (G) Quantification of endothelial cell migration after 18 and 24 hours of treatment with candesartan with or without neutralizing antibodies (n = 3–6 per group). *P < 0.05 (significantly different from control at the corresponding time point); #P < 0.05 (significantly different from candesartan 0.1- at the corresponding time point). Cand 0.1, candesartan 0.1 μg/ml; Ctrl, control; VAnAB, VEGF-A–neutralizing antibody; VBnAB, VEGF-B–neutralizing antibody.
Candesartan Induces a Paracrine Neuroprotective Effect via Endothelial VEGF-A and VEGF-B.
Conditioned media collected from candesartan-treated endothelial cells decreased neuronal death by 40%, as documented by decreased cleaved caspase-3 expression. There was a trend of increasing levels of cleaved caspase-3 with the blockade of either growth factor. The increase in cell death was not significant, however, until both growth factors were blocked simultaneously (Fig. 8A).
Fig. 8.
(A) Candesartan-induced VEGF-A and VEGF-B upregulation exerts a paracrine neuroprotective effect. Determination of cleaved caspase-3 in mouse primary cortical neurons after 2 hours of hypoxia and 24 hours of treatment with conditioned media. Conditioned media were collected from endothelial cells after 2 hours of OGD and 24 hours of reoxygenation with or without candesartan (0.1 μg/ml) treatment. VEGF-A– and VEGF-B–neutralizing antibodies were added to the collected media prior to neuronal reoxygenation (n = 3–6 per group). *P < 0.05 (significantly different from control); #P < 0.05 (significantly different from E-Cand 0.1). (B) Schematic representation of the mechanisms involved in candesartan proangiogenic, vascular, and neuroprotective effects. Candesartan treatment induces neuroprotective and proangiogenic effects via an integrated action of VEGF-A and VEGF-B. In addition, candesartan’s antioxidant and antinitrative actions suggest improved VEGF-A signaling. Preservation of Ang-1 might contribute to the synergistic angiogenic response, while exerting a simultaneous antipermeabilizing effect, hence the preservation of barrier function after candesartan treatment. E-Cand, conditioned media collected from candesartan-treated endothelial cells; E-Ctrl, conditioned media collected from untreated endothelial cells; E-Cand+VAnAb, conditioned media from candesartan-treated endothelial cells plus VEGF-A–neutralizing antibody; E-Cand+VBnAb, conditioned media from candesartan-treated endothelial cells plus VEGF-B–neutralizing antibody; E-Cand+both: conditioned media from both candesartan-treated endothelial cells plus VEGF-A–neutralizing antibody and VEGF-B–neutralizing antibody.
Discussion
The main findings of this study include a prolonged angiogenic effect, associated with enhanced VEGF-A and VEGF-B expression in vivo and in vitro, in response to a single dose of candesartan treatment. These effects were associated with the stabilization of HIF-1α, the preservation of Ang-1, and the reduction in tyrosine nitration at 24 hours. In addition, we identified endothelial cells as one of the cellular sources of the enhanced production of VEGF-A and VEGF-B that induced an autocrine angiogenic response as well as a paracrine neuroprotective effect.
Although several studies have demonstrated the proangiogenic effect of ARBs, the temporal pattern and the molecular mechanisms involved in the angiogenic response remain to be fully elucidated. Here, we show that the CSF collected after a single poststroke candesartan administration stimulated a proangiogenic response as early as 8 hours and lasted for up to 72 hours. This effect was evident by the ability of the CSF, collected at different time points, to transform brain endothelial cells into tube-like structures resembling blood vessels in vitro. Our previous work linked candesartan’s beneficial effects to enhanced production of VEGF-A, the prototypical angiogenic molecule, as well as the relatively new VEGF isoform, VEGF-B (Guan et al., 2011c). The exact function of VEGF-B is still controversial. Although its neuroprotective and antiapoptotic functions are proven in several models (Li et al., 2008b), there is conflicting evidence regarding its angiogenic potential (Wright, 2002; Bhardwaj et al., 2003; Rissanen et al., 2003; Silvestre et al., 2003; Mould et al., 2005). In a recent study, VEGF-B was upregulated in the ischemic border zone, after temporary MCAO (Xie et al., 2013). We sought to examine the temporal pattern of VEGF-A and VEGF-B upregulation in both hemispheres after a single candesartan administration. Our findings show an increased expression of both isoforms in the ipsilateral and the contralateral hemispheres. Nevertheless, VEGF-A upregulation was more pronounced and lasted longer in the contralateral hemisphere, suggesting a role of this hemisphere in the process of recovery (Guan et al., 2011c). The increase in VEGF-B, on the other hand, was most pronounced at 8 hours in the ipsilateral hemisphere, consistent with its main role as a prosurvival factor.
We sought to investigate the upstream signaling molecules that lead to the upregulation of VEGF-A in both hemispheres. HIF-1 regulates the expression of multiple genes involved in hypoxia-related adaptations, including VEGF-A (Wenger, 2002; Youn et al., 2011). We report an enhanced HIF-1α stabilization by the treatment, which might explain, at least partly, the upregulation of VEGF-A expression. In the contralateral hemisphere, however, other mechanisms could be involved in VEGF-A upregulation in response to treatment. In a retinopathy of prematurity model, candesartan treatment upregulated the expression of hemeoxygenase-1 and VEGF-A under normoxic conditions (El-Remessy et al., 2013). Further studies are warranted, however, to extrapolate these findings to the contralateral hemisphere in our stroke model.
Previous studies demonstrated the inactivation of VEGF-A signaling by oxidative stress via phosphoinositide 3-kinase tyrosine nitration (el-Remessy et al., 2005; Abdelsaid et al., 2010). In this study, we show an antioxidant and antinitrative effect of candesartan treatment. In vitro, candesartan decreased the 4-HNE level, a marker of lipid peroxidation, after exposure to oxidizing conditions. Likewise, candesartan reduced nitrotyrosine levels in response to ONOO− treatment in the same model. We thereby postulate that candesartan, by the virtue of its antioxidant and antinitrative effects, reduces peroxynitrite production and protein nitration, respectively. In vivo, we report decreased protein nitration in the ipsilateral hemisphere 24 hours after ischemia/reperfusion, a condition characterized by massive production of oxidizing and nitrating species. This finding suggests the possibility of improved VEGF-A signaling due to reduced protein nitration.
VEGF-A is a known vascular permeability factor and high-serum VEGF levels have been associated with cerebral microbleeds after acute ischemic stroke (Dassan et al., 2012). We previously showed a preservation of barrier function by candesartan treatment after an ischemic insult. We sought to explain the dilemma of the improved barrier function in spite of the elevated VEGF-A levels. In this study, we report a preservation of Ang-1 expression in the stroke hemisphere 24 hours after treatment. Ang-1 exerts a barrier protective function as well as a synergistic angiogenic effect with VEGF-A after stroke (Valable et al., 2005). Figure 8B provides a diagram that depicts the proposed mechanisms of action of candesartan.
To study the contribution of endothelial cells to the observed effect of candesartan, we measured VEGF-A and VEGF-B expression in human brain microvascular endothelial cells. Our results have shown increased VEGF-A and VEGF-B production in a dose-dependent fashion irrespective of the oxygenation status. Yet the increase was more pronounced under OGD than normoxic conditions, suggesting a role of hypoxia-induced adaptations in the function of candesartan. Indeed, candesartan treatment enhanced different steps of angiogenesis in vitro in microvascular endothelial cells, including cell proliferation, migration, and tube formation in a dose-dependent manner. Interestingly, losartan showed a proangiogenic action in vitro similar to that of candesartan treatment (Fig. 6), in spite of the different functional inhibitory characteristics as well as different lipophilicities, among other differences between the two ARBs. However, studies using other ARBs are needed to confirm the existence of a drug class effect.
To assess the contribution of VEGF-A and VEGF-B to the proangiogenic effects of candesartan, we neutralized either or both growth factors in vitro. Neutralization of either VEGF-A or VEGF-B reduced the angiogenic potential of candesartan as evident by reduced endothelial cell migration. Nevertheless, a sharp reduction of the candesartan angiogenic effect was observed after neutralization of both growth factors simultaneously. This supports the role of VEGF-B as an angiogenic factor, either directly or indirectly via increasing cell survival. We previously showed an enhanced proangiogenic state in vitro by candesartan treatment with or without exogenous angiotensin II treatment. Our recent work identified brain-derived neurotrophic factor as a mediator of this effect secondary to unopposed angiotensin II type 2 receptor stimulation (Alhusban et al., 2013). This study further supports our previous findings, because VEGF-A and brain-derived neurotrophic factor expression is interrelated (Chen et al., 2005; Li et al., 2006). In this study, because no exogenous angiotensin II was added, we tested whether brain endothelial cells can produce and secrete angiotensin II locally. Angiotensin II was detectable in cell culture supernatant under normoxic and OGD conditions (Supplemental Fig. 2A). There was a trend of increasing angiotensin II with OGD conditions that did not reach significance in the tested sample size. Candesartan treatment enhanced endothelial cell secretion of angiotensin II in a dose-dependent fashion (Supplemental Fig. 2B), possibly because of the interrupted negative feedback system secondary to angiotensin receptor blockade. It was previously shown that locally produced angiotensin II is regulated by an autocrine negative feedback mechanism, operating independently of the systemic renin angiotensin system (Gigante et al., 1997), lending further support to our findings.
Because in vivo studies are limited in being correlative and are hard to prove the causal relationship between VEGF-A and VEGF-B upregulation and neurovascular protection, we attempted to examine that concept in vitro. We neutralized either or both growth factors in the conditioned media collected from endothelial cells. Neutralization of either isoform reduced the protective effect of the conditioned media on primary neurons, as evident by the higher expression of cleaved caspase-3, a marker of apoptosis. However, neuroprotection was significantly minimized when both isoforms were blocked simultaneously. This key figure demonstrates, for the first time, that neuroprotection attributed to candesartan is mediated, at least partly, through augmenting the endothelial cell–secreted growth factors VEGF-A and VEGF-B. The failure of direct neuroprotection in stroke therapy has promoted a more holistic approach to treatment, taking into consideration the communication between different brain cell types and especially considering endothelial cells as a “neuroprotective organ” (Guo et al., 2008). In agreement, a recent study demonstrated the involvement of VEGF-A in endothelium-mediated neuroprotection after stroke (Ishikawa et al., 2013).
In summary, our findings provide new insights on the benefits of candesartan and show that these benefits are mediated through orchestrated actions of multiple players, including endothelial VEGF-A and VEGF-B. This study further points to the potential usefulness of augmenting the endogenous reparative capacity of the brain for the management of acute ischemic stroke.
Supplementary Material
Acknowledgments
The authors thank Dr. Jason Zastre for providing the human cerebral microvascular endothelial cells, Dr. Lauren Willis and Lydia Newsom for their assistance with the endothelial cell culture experiments, Dr. Chirayu Pandya and Bindu Pillai for the excellent technical assistance, Maribeth Johnson for reviewing data analysis, and Abdelrahman Fouda for reviewing the manuscript.
Abbreviations
- 4-HNE
4-hydroxynonenal
- Ang
angiopoietin
- ARB
angiotensin II type 1 receptor blocker
- BrdU
bromodeoxyuridine
- CSF
cerebrospinal fluid
- EMEM
Eagle’s minimum essential medium
- HIF-1
hypoxia-inducible factor-1
- MCAO
middle cerebral artery occlusion
- OGD
oxygen and glucose deprivation
- ONOO−
peroxynitrite
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Authorship Contributions
Participated in research design: Soliman, Pillai, Somanath, Ergul, El-Remessy, Fagan.
Conducted experiments: Soliman, Ishrat.
Contributed new reagents or analytic tools: Pillai.
Performed data analysis: Soliman.
Wrote or contributed to the writing of the manuscript: Soliman, El-Remessy, Fagan.
Footnotes
This research was supported in part by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS063965 (to S.C.F.) and R21-NS063965 (to A.E.)]; the National Institutes of Health National Eye Institute [Grant R01-EY022408 (to A.B.E.)]; the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL103952 (to P.R.S.)]; the U.S. Department of Veterans Affairs Merit Review [Grants BX000891 (to S.C.F.) and BX000347 (to A.E.)]; and the American Heart Association [Grant 12PRE12030197 (to S.S.)]. The contents do not represent the views of the funding agencies, the Department of Veterans Affairs, or the U.S. government.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. (2010) Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther 332:125–134 [DOI] [PubMed] [Google Scholar]
- Alhusban A, Kozak A, Ergul A, Fagan SC. (2013) AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther 344:348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Plate KH. (2009) Angiogenesis after cerebral ischemia. Acta Neuropathol 117:481–496 [DOI] [PubMed] [Google Scholar]
- Bhardwaj S, Roy H, Gruchala M, Viita H, Kholova I, Kokina I, Achen MG, Stacker SA, Hedman M, Alitalo K, et al. (2003) Angiogenic responses of vascular endothelial growth factors in periadventitial tissue. Hum Gene Ther 14:1451–1462 [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. (2005) Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab 25:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassan P, Brown MM, Gregoire SM, Keir G, Werring DJ. (2012) Association of cerebral microbleeds in acute ischemic stroke with high serum levels of vascular endothelial growth factor. Arch Neurol 69:1186–1189 [DOI] [PubMed] [Google Scholar]
- Davis S, Yancopoulos GD. (1999) The angiopoietins: Yin and Yang in angiogenesis. Curr Top Microbiol Immunol 237:173–185 [DOI] [PubMed] [Google Scholar]
- El-Remessy A, Shanab A, Matragoon S, Fagan S. and Retinopathy (2013) Vascular protective effects of candesartan in ischemic retinopathy (Abstract). Invest Ophthalmol Vis Sci 54:453823744997 [Google Scholar]
- el-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. (2005) Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci 118:243–252 [DOI] [PubMed] [Google Scholar]
- Engelhorn T, Goerike S, Doerfler A, Okorn C, Forsting M, Heusch G, Schulz R. (2004) The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab 24:467–474 [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1995) The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 36:127–137 [DOI] [PubMed] [Google Scholar]
- Forder JP, Munzenmaier DH, Greene AS. (2005) Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am J Physiol Heart Circ Physiol 288:H1989–H1996 [DOI] [PubMed] [Google Scholar]
- Gigante B, Rubattu S, Russo R, Porcellini A, Enea I, De Paolis P, Savoia C, Natale A, Piras O, Volpe M. (1997) Opposite feedback control of renin and aldosterone biosynthesis in the adrenal cortex by angiotensin II AT1-subtype receptors. Hypertension 30:563–568 [DOI] [PubMed] [Google Scholar]
- Guan W, Kozak A, El-Remessy AB, Johnson MH, Pillai BA, Fagan SC. (2011a) Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl Stroke Res 2:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W, Kozak A, Fagan SC. (2011b) Drug repurposing for vascular protection after acute ischemic stroke. Acta Neurochir Suppl (Wien) 111:295–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, Johnson MH, Alhusban A, Soliman S, Fagan SC. (2011c) Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS ONE 6:e24551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. (2008) Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci USA 105:7582–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomi N, Nishiyama A, Ban CR, Naya T, Takahashi T, Kohno M, Koziol JA. (2005) Angiotensin type 1 receptor blockage improves ischemic injury following transient focal cerebral ischemia. Neuroscience 134:225–231 [DOI] [PubMed] [Google Scholar]
- Huang LE, Arany Z, Livingston DM, Bunn HF. (1996) Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem 271:32253–32259 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, Mimura O, Dezawa M, Kim SU, Borlongan CV. (2013) Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke 44:3473–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Pillai B, Ergul A, Hafez S, Fagan SC. (2013) Candesartan reduces the hemorrhage associated with delayed tissue plasminogen activator treatment in rat embolic stroke. Neurochem Res 38:2668–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99:11946–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. (2000) Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA 97:10242–10247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochuparambil ST, Al-Husein B, Goc A, Soliman S, Somanath PR. (2011) Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J Pharmacol Exp Ther 336:496–505 [DOI] [PubMed] [Google Scholar]
- Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, Abdelsaid M, Wiley DC, Fagan SC. (2009) Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke 40:1870–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Kozak A, Johnson MH, Elewa HF, Fagan SC. (2008) Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. J Pharmacol Exp Ther 326:773–782 [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. (1994) Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25:1794–1798 [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. (1995) Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 270:13333–13340 [DOI] [PubMed] [Google Scholar]
- Li JM, Mogi M, Iwanami J, Min LJ, Tsukuda K, Sakata A, Fujita T, Iwai M, Horiuchi M. (2008a) Temporary pretreatment with the angiotensin II type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke 39:2029–2036 [DOI] [PubMed] [Google Scholar]
- Li Q, Ford MC, Lavik EB, Madri JA. (2006) Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res 84:1656–1668 [DOI] [PubMed] [Google Scholar]
- Li X, Lee C, Tang Z, Zhang F, Arjunan P, Li Y, Hou X, Kumar A, Dong L. (2009) VEGF-B: a survival, or an angiogenic factor? Cell Adhes Migr 3:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, Lennartsson J, Zhu C, Qu Y, Fang C, et al. (2008b) VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest 118:913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. (2001) Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab 21:1223–1231 [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. (2001) Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J 15:1218–1220 [PubMed] [Google Scholar]
- Mould AW, Greco SA, Cahill MM, Tonks ID, Bellomo D, Patterson C, Zournazi A, Nash A, Scotney P, Hayward NK, et al. (2005) Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res 97:e60–e70 [DOI] [PubMed] [Google Scholar]
- Munzenmaier DH, Greene AS. (2006) Chronic angiotensin II AT1 receptor blockade increases cerebral cortical microvessel density. Am J Physiol Heart Circ Physiol 290:H512–H516 [DOI] [PubMed] [Google Scholar]
- Navaratna D, Guo S, Arai K, Lo EH. (2009) Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes Migr 3:216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2001) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego [Google Scholar]
- Pillai A, Dhandapani KM, Pillai BA, Terry AV, Jr, Mahadik SP. (2008) Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology 33:1942–1951 [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholová I, Kauppinen RA, Achen MG, Stacker SA, et al. (2003) VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res 92:1098–1106 [DOI] [PubMed] [Google Scholar]
- Schulz M, Schmoldt A. (2003) Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 58:447–474 [PubMed] [Google Scholar]
- Shibuya M. (2013) Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, Lévy BI. (2003) Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res 93:114–123 [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. (2006) Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol 289:329–335 [DOI] [PubMed] [Google Scholar]
- Tao Z, Chen B, Tan X, Zhao Y, Wang L, Zhu T, Cao K, Yang Z, Kan YW, Su H. (2011) Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci USA 108:2064–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, et al. (2008) Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab 28:764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. (2000) Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6:460–463 [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. (1999) Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286:2511–2514 [DOI] [PubMed] [Google Scholar]
- Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, et al. (2005) VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab 25:1491–1504 [DOI] [PubMed] [Google Scholar]
- Wenger RH. (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16:1151–1162 [DOI] [PubMed] [Google Scholar]
- Willis LM, El-Remessy AB, Somanath PR, Deremer DL, Fagan SC. (2011) Angiotensin receptor blockers and angiogenesis: clinical and experimental evidence. Clin Sci (Lond) 120:307–319 [DOI] [PubMed] [Google Scholar]
- Wright CE. (2002) Effects of vascular endothelial growth factor (VEGF)A and VEGFB gene transfer on vascular reserve in a conscious rabbit hindlimb ischaemia model. Clin Exp Pharmacol Physiol 29:1035–1039 [DOI] [PubMed] [Google Scholar]
- Xie L, Mao X, Jin K, Greenberg DA. (2013) Vascular endothelial growth factor-B expression in postischemic rat brain. Vasc Cell 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. (2010) Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 11:298–308 [PMC free article] [PubMed] [Google Scholar]
- Youn SW, Lee SW, Lee J, Jeong HK, Suh JW, Yoon CH, Kang HJ, Kim HZ, Koh GY, Oh BH, et al. (2011) COMP-Ang1 stimulates HIF-1α-mediated SDF-1 overexpression and recovers ischemic injury through BM-derived progenitor cell recruitment. Blood 117:4376–4386 [DOI] [PubMed] [Google Scholar]
- Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, Scotney P, Lee C, Arjunan P, Dong L, et al. (2009) VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA 106:6152–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. (2002) Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med 12:62–66 [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. (2002) Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience 113:683–687 [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, van Bruggen N, Chopp M. (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.