Abstract
Objectives
Little to nothing is known about human papillomavirus (HPV) susceptibility to disinfection. HPV is estimated to be among the most common sexually transmitted diseases in humans. HPV is also the causative agent of cervical cancers and other anogenital cancers and is responsible for a significant portion of oropharyngeal cancers. While sexual transmission is well documented, vertical and non-sexual transmission may also be important.
Methods
Using recombinant HPV16 particles (quasivirions) and authentic HPV16 grown in three-dimensional organotypic human epithelial culture, we tested the susceptibility of high-risk HPV to clinical disinfectants. Infectious viral particles were incubated with 11 common clinical disinfectants, appropriate neutralizers were added to inactivate the disinfectant and solutions were filter centrifuged. Changes in the infectivity titres of the disinfectant-treated virus were measured compared with untreated virus.
Results
HPV16 is a highly resistant virus; more so than other non-enveloped viruses previously tested. The HPV16 quasivirions showed similar resistance to native virions, except for being susceptible to isopropanol, the triple phenolic and the lower concentration peracetic acid-silver (PAA-silver)-based disinfectant. Authentic virus and quasivirus were resistant to glutaraldehyde and ortho-phthalaldehyde and susceptible to hypochlorite and the higher concentration PAA-silver-based disinfectant.
Conclusions
We present the first disinfectant susceptibility data on HPV16 native virions, which show that commonly used clinical disinfectants, including those used as sterilants in medical and dental healthcare facilities, have no effect on HPV16 infectivity. Policy changes concerning disinfectant use are needed. The unusually high resistance of HPV16 to disinfection supports other data suggesting the possibility of fomite or non-sexual transmission of HPV16.
Keywords: hospital sterilants, papillomavirus, cancer, glutaraldehydes, ortho-phthalaldehydes
Introduction
Due to the specific life cycle requirements of human papillomavirus (HPV), infectious virus has been difficult to produce in laboratories and an assay for infectious virus has only recently become available. The ability to produce infectious virus outside of host animals is a great benefit to basic research; it often requires less time and is more cost-effective. HPV has a life cycle stringently tied to differentiated epithelial tissue. This has required the development of special systems to make in vitro propagation possible. Because of the historical difficulty in producing high enough titres of infectious HPV particles and the lack of a suitable assay to test for infectivity, little to nothing is known about HPV susceptibility to disinfection. Disinfectants have been tested against many important viruses and these studies are important to public health as they provide information that can be used to reduce the prevalence of infection, transmission and reinfection. Presently, hospitals' and other healthcare institutes' use of disinfectants to inactivate HPV is based on what is used for other viruses or simply on what someone thinks should be effective. Two systems (recombinant based and organotypic) have been developed to produce high amounts of infectious HPV particles in the laboratory. Infectivity can now be measured by using reverse transcription quantitative PCR (RT-qPCR) that detects the viral E1^E4 transcript. Detection of this transcript signals infectious particles that were able to achieve cell entry and start their early viral programmes. HPV16 was used in these initial experiments because it is responsible for up to 60% of all HPV-associated cancers.
The adaptations of virus-like particle (VLP) technologies, quasivirus and organotypic raft culturing systems have made it possible to obtain high concentrations of HPV particles.1,2 Here, we present the first disinfectant susceptibility data on HPV16 native virions, which show that commonly used clinical disinfectants, including those used as sterilants in medical and dental healthcare facilities, have no effect on HPV16 infectivity. HPV16 is a highly resistant virus, more so than other non-enveloped viruses previously tested. The HPV16 quasivirions showed similar resistance to HPV16 native virions, except that they were susceptible to isopropanol, the triple phenolic and the lower concentration peracetic acid-silver (PAA-silver)-based disinfectant. Both authentic virus and quasivirus were resistant to glutaraldehyde (GTA) and ortho-phthalaldehyde (OPA) and susceptible to hypochlorite and the higher concentration PAA-silver-based disinfectant. Policy changes concerning disinfectant use are needed. Given the unusually high resistance of HPV16 to disinfection, our findings support other data suggesting the possibility of fomite or non-sexual transmission of HPV16.
Methods
Organotypic cultures
Human foreskin keratinocytes (HFKs) were isolated and maintained from newborn circumcision tissue specimens as previously described.3 The use of HFK tissues to develop cell lines for these studies was approved by the Institutional Review Board at the Pennsylvania State University College of Medicine and by the Institutional Review Board at Pinnacle Health Hospitals. Discarded, de-identified tissues were exempt from needing informed patient consent. Informed consent was waived by both Institutional Review Boards. Full-length HPV16 was electroporated into low-passage HFK monolayer cultures and keratinocyte lines shown to stably maintain HPV16 genomes were selected for organotypic cultures. Organotypic ‘raft’ epithelial cultures were grown as previously described.4 Briefly, HPV16-infected HFK cells were seeded onto rat-tail type-1 collagen matrices containing J2 3T3 feeder cells. After epithelial cells were grown to confluence, these matrices were lifted onto stainless steel grids to establish a liquid–air interface. Raft cultures were then fed by diffusion using E medium. Raft culture epithelia were allowed to differentiate and then harvested. Tissue was collected and stored for particle isolation.
Quasivirus production
Quasivirus was produced as described by Buck et al.1 with minor modifications. Briefly, 293TT cell lines, known to express high levels of SV40 large T antigen, were co-transfected with codon-modified HPV16 capsid genes L1 and L2 and with full-length HPV16. The HPV16 capsid gene plasmid contained the SV40 origin of replication for expression. After 48 h, cells were harvested for particle isolation.
Particle isolation
Raft culture tissue and 293TT cells were homogenized in 0.6 mL of ice-cold 1 M NaCl/0.05 M Na-phosphate buffer with a 7.5 mL homogenizer. The homogenizer was washed twice with 200 μL of 1 M NaCl/0.05 M Na-phosphate buffer, pH 8. The solution was centrifuged at 10 500 rpm for 10 min at 4°C and the supernatant was transferred to a 1.8 mL Nalgene cryovial. The crude viral stocks were stored at −20°C.
Disinfection testing
The disinfectants used in the study were ethanol (Fisher Scientific), isopropanol (Sigma–Aldrich), GTA (Cidex and Cidexplus; Advanced Sterilization Products), OPA (Cidex OPA; Advanced Sterilization Products), phenol (CiDecon; Decon Labs), PAA-silver (STERIPLEX SD and STERIPLEX SD Plus; sBioMed) and hypochlorite (Activate; Deardorff Fitzsimmons Corp.).
Each disinfectant was prepared according to manufacturer's recommendation and stated dilution (see Table 1) immediately before testing. Virus and disinfectant were mixed thoroughly and incubated for 45 min at room temperature. After incubation, appropriate neutralizers were added to the virus/disinfectant solutions. The neutralizer used for the three aldehyde disinfectants was 7% (w/v) glycine. The neutralizer used for the PAA-silver-based disinfectants contained 0.1% Tween 80, 1% peptone, 1% cysteine and 0.5 M Tris buffer, pH 7.5. This second general neutralizer was also used for all the other disinfectants tested. The solution was then centrifuged in Amicon Ultra centrifugal filters 100 000 molecular weight cut-off (MWCO) (Millipore) at 4000 rpm for 10 min. More neutralizer was added to the columns and they were recentrifuged at the same speed and duration. Two more washes were performed with HaCaT culture medium prepared as previously described.5 The resulting liquid containing the treated virus was collected and assayed for infectivity.
Table 1.
Effectiveness of clinical disinfectants on HPV virions
| Disinfectant | Native virion (log10 reduction) | ±SD | Quasivirion (log10 reduction) | ±SD |
|---|---|---|---|---|
| 70% Ethanol | −0.789 | 0.106 | 0.197 | 0.530 |
| 95% Ethanol | −0.076 | 0.481 | 0.307 | 0.123 |
| 70% Isopropanol | −0.770 | 0.186 | 4.675 | 0.415 |
| 95% Isopropanol | −0.272 | 0.023 | 4.435 | 0.196 |
| 2.4% GTA | −0.856 | 0.179 | −0.041 | 0.014 |
| 3.4% GTA | −0.306 | 0.232 | −0.145 | 0.232 |
| 0.55% OPA | 0.017 | 0.200 | 0.109 | 0.180 |
| Phenol | −0.319 | 0.380 | 4.218 | 0.144 |
| 0.25% PAA-silver | −0.857 | 0.195 | 1.359 | 0.408 |
| 1.2% PAA-silver | 5.150 | 0.971 | 4.946 | 0.548 |
| 0.525% Hypochlorite | 4.862 | 0.623 | 5.087 | 0.413 |
All tests were performed at least five times and the averages are shown.
Infection and RT-qPCR infectivity testing
HaCaT cells, an immortalized keratinocyte line kindly provided by Norbert Fusenig (German Cancer Research Center), were seeded onto 24-well plates at a density of 50 000 cells/well. Cells were grown and maintained in the HaCaT culture medium mentioned previously and incubated at 37°C in 5% CO2. After 48 h, the disinfectant-treated virus was added to the subconfluent cells. Virus and cells were incubated for 48 h at 37°C. The ability to infect HaCaT cells after 48 h of incubation was determined by the presence of the spliced HPV16 E1^E4 mRNA species. Total mRNA was harvested with the SurePrep TrueTotal RNA Purification Kit (Fisher Scientific) and diluted to a final concentration of 100 ng/μL with 500 ng of total RNA in each PCR. Amplification of both the viral target and endogenous cellular control target was performed as described previously, except all PCRs were performed in a 7900HT SDS thermal cycler and 96-well optical reaction plates (Applied Biosystems).5 Relative expression was calculated using the 2–ΔΔCt method, as efficiency of both the housekeeping gene and the target gene was found to be similar.
Results
The disinfectants tested in this study were 70% and 95% ethanol and isopropanol, 2.4% and 3.4% GTA, 0.55% OPA, a triple phenolic, a 0.25% and 1.2% PAA-silver-based disinfectant and 0.525% hypochlorite (Table 1). The alcohols were chosen due to their widespread use as surface disinfectants and hand sanitizers, both in public and clinical settings. Considering previous studies that show high levels of HPV DNA on fingers, especially in patients with current genital infections, we sought to determine whether alcohols were effective against HPV.6,7 GTA and OPA were tested due to their widespread use as disinfectants, and in the case of GTA also as a sterilant, in medical and dental healthcare facilities. The remaining disinfectants were chosen as representatives of other common disinfectant types.
In HPV16 authentic viruses, only hypochlorite (4.86 log10 reduction) and the 1.2% PAA-silver-based disinfectant (5.15 log10 reduction) were able to produce >99.99% reduction in infectivity. All other disinfectants showed slight or no reduction in infectivity (Table 1).
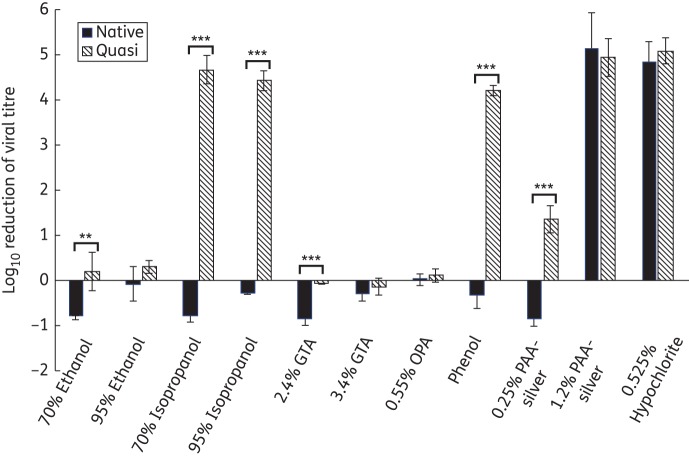
Comparing the two systems we used to produce virus particles, the organotypic culture system is more time-consuming and more expensive. This has made the recombinant particle technology very attractive to researchers. However, in organotypic cultures, the tissue differentiation along with the accompanying physiological conditions that occur in stratified tissue are more similar to what would be found in mucosal tracts normally infected by HPV16. We sought to investigate whether differences exist, with respect to disinfectant susceptibility, between HPV16 quasivirus and HPV16 organotypic-produced virus. HPV16 quasivirions were subjected to the same disinfectants and conditions as the raft virions to compare their susceptibility profiles (Table 1 and Figure 1). The results suggest that quasivirions are generally more susceptible to disinfection than organotypic tissue-derived virus. Specifically, quasivirions showed a susceptibility to both 70% (4.68 log10 reduction) and 95% (4.44 log10 reduction) isopropanol and the triple phenolic (4.22 log10 reduction) disinfectant that was not seen in the raft-produced virions. Also, the lower percentage PAA-silver-based disinfectant (0.25%) produced a moderate reduction in infectivity (1.36 log10 reduction) of quasivirus that was not seen with organotypic tissue-derived virus.
Figure 1.
Susceptibility of HPV virions to clinical disinfectants. Both authentic virus and quasivirus were incubated with the indicated disinfectants for a contact time of 45 min. Disinfectants were neutralized and the virus was added to HaCaT cells for infection. Data shown are the averages of at least five independent experiments, with errors shown as the standard deviations of all experiments. **P < 0.01. ***P < 0.001.
It is interesting to note that both GTA and OPA were ineffective in producing any significant reduction in infectivity for both particle types. GTA was further tested at longer contact times (up to 48 h) and still failed to inactivate HPV to any measurable degree (data not shown). One proposed disinfection mechanism for aldehydes is the cross-linking of important lysine residues in viral capsid proteins. Downstream effects of these capsid modifications may include inhibition of viral entry, uncoating and transport of viral genomes to the correct cellular compartments necessary to initiate viral programmes.8 Aldehydes are also effective at denaturing nucleic acids, suggesting that if viral capsid integrity was compromised, the viral DNA or RNA might also be inactivated. Knappe et al.9 showed that there are lysine residues in the L1 capsid protein that are important for efficient infection. Structural data on bovine papillomavirus have also shown lysine residues located on the invading arm of the L1 protein that may be important for pentamer integrity.10 Although quasivirions and organotypic-derived virions differed with respect to their susceptibility to certain disinfectants, their common resistance to the aldehydes leads to the assumption that these residues are either not accessible or do not exhibit the proper spatial separation necessary for cross-linking. This commonality suggests that these two types of particles have some structural similarities. The two disinfectants that were effective against both particle types were the 1.2% PAA-silver-based formulation and the hypochlorite. Both agents are strong oxidizers, suggesting that oxidization-based disinfectants may be effective at inactivating HPV.
GTA- and OPA-based disinfectants are often used in protocols with longer contact times. Therefore, we tested both GTA (3.4%) and OPA (0.55%) at a contact time of 24 h on native HPV16 virions. Testing was completed as described above. The 24 h GTA (−0.039 ± 0.129) and OPA (−0.270 ± 0.620) treatments did not result in a decrease in viral infectivity (Table 2).
Table 2.
Efficacy of disinfectants on HVP16 virions at 24 h contact time
| Disinfectant | Native virion (log10 reduction) | ±SD | Quasivirion (log10 reduction) | ±SD |
|---|---|---|---|---|
| 3.4% GTA | −0.039 | 0.129 | −0.128 | 0.114 |
| 0.55% OPA | −0.270 | 0.620 | 0.058 | 0.052 |
Data shown are the averages of six independent experiments.
The addition of neutralizers alone, coupled with centrifugation through Amicon Ultra centrifugal filters, had no effect on infectivity. All studies shown in Figure 1 and Table 1 were repeated at least five times. All studies in Table 2 were repeated six times. Due to the importance of the results concerning nosocomially and iatrogenically acquired infections, all studies were performed in two locations, by different researchers, with multiple batches of virus and cells.
Discussion
To date, this is the first report of a virus shown to be resistant to inactivation by GTA. Because of its broad range of effectiveness against other microbial pathogens, GTA has been the disinfectant of choice in many clinical situations. It has been proven effective against a large number of non-enveloped viruses, including adenoviruses, parvoviruses, caliciviruses and many enteroviruses.8,11,12 Other than the resistance to GTA and OPA, HPV16 shows many similarities to other highly resistant non-enveloped viruses such as caliciviruses and parvoviruses. These viruses are all susceptible to high concentrations of hypochlorite and a parvovirus in one study was also susceptible to a PAA-silver-based disinfectant as well as PAA alone.11 Non-enveloped viruses are generally mildly or completely resistant to alcohol-based disinfection and that resistance was also seen with organotypic-derived HPV16 in our system.
Numerous studies have suggested, albeit based on limited data, that non-sexual transmission routes exist for HPV. Smith et al.13 evaluated concordance of HPV detection in mothers and infants. They found that among HPV DNA-positive mothers, a small percentage of their infants also had detectable HPV DNA. Interestingly, this study also found that two infants were HPV DNA-positive, whereas their mothers had no detectable HPV DNA. Samples were taken at birth and the PCR assays were performed in a separate facility to lower the risk of maternal inoculation and contamination. These findings suggest that in clinical settings there exists a low risk of nosocomial infection with HPV. Taken together with our disinfectant data, it is conceivable that many of the disinfectants currently used may not be effective in eliminating hospital sources of infectious HPV. Studies looking at prevalence among women with no reported sexual history have yielded mixed results.14,15 Kjaer et al.14 followed a group of virgins for 2 years and at the start of the study all were HPV16 DNA-negative and seronegative to VLP HPV16. Only those who initiated sexual intercourse during the study were found to have detectable HPV16 DNA and/or seroconvert. Tay et al.15 determined the occurrence of HPV infection by colposcopy and histology and found that >50% of the virgins in their study had colposcopic evidence of HPV infection with >80% of those confirmed by histology. The variations seen in these studies may be due to differences in detection methods, sample size, geography or even processes of patient referral. These variations in methods and design are common and often lead to the inability to draw clear conclusions about some aspects of the natural history of HPV infection. Our data support the possibility of HPV fomite-related transmission, autoinoculation and nosocomial transmission, even via instruments that were considered ‘sterile’. Because native HPV16 has been shown to be very resistant to chemical disinfectants, especially those routinely used in hand sanitizers and instrument processing, new infection control procedures are warranted. As routine disinfection and hygiene may not be sufficient to remove possible sources of HPV, the risk of reinfection may be compounded in populations with active infections. Additionally, our data show that quasivirions and similar particles may not accurately measure the effectiveness of disinfectants on HPV.
Funding
This study was funded in part by the National Institute of Allergy and Infectious Diseases (R01AI57988) and the BYU Mentoring Environment Grant (MEG) Program.
Transparency declarations
C. M. has received speaker honoraria from Merck, Quest Diagnostics, GSK, Wyeth and Bristol-Myers Squibb, and has performed research funded by Merck, The Phillip Morris External Research Program, NexMed, GSK, OriGenix and Interferon Sciences Inc. R. R. has performed research funded by sBioMed, Inc. All other authors: none to declare.
Acknowledgements
Excellent technical help was provided by Ms Janice Melici and Terri Bills.
References
- 1.Buck CB, Pastrana DV, Lowy DR, et al. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 2.Meyers C, Frattini MG, Hudson JB, et al. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992;257:971–3. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 3.Visalli RJ, Courtney RJ, Meyers C. Infection and replication of herpes simplex virus type 1 in an organotypic epithelial culture system. Virology. 1997;230:236–43. doi: 10.1006/viro.1997.8484. [DOI] [PubMed] [Google Scholar]
- 4.Meyers C, Mayer TJ, Ozbun MA. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J Virol. 1997;71:7381–6. doi: 10.1128/jvi.71.10.7381-7386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway MJ, Alam S, Christensen ND, et al. Overlapping and independent structural roles for human papillomavirus type 16 L2 conserved cysteines. Virology. 2009;393:295–303. doi: 10.1016/j.virol.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnex C, Strauss S, Gray JJ. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Infect. 1999;75:317–9. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winer RL, Hughes JP, Feng Q, et al. Detection of genital HPV types in fingertip samples from newly sexually active female university students. Cancer Epidemiol Biomarkers Prev. 2010;19:1682–5. doi: 10.1158/1055-9965.EPI-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambon M, Archimbaud C, Bailly JL, et al. Virucidal efficacy of glutaraldehyde against enteroviruses is related to the location of lysine residues in exposed structures of the VP1 capsid protein. Appl Environ Microbiol. 2004;70:1717–22. doi: 10.1128/AEM.70.3.1717-1722.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knappe M, Bodevin S, Selinka HC, et al. Surface-exposed amino acid residues of HPV16 L1 protein mediating interaction with cell surface heparan sulfate. J Biol Chem. 2007;282:27913–22. doi: 10.1074/jbc.M705127200. [DOI] [PubMed] [Google Scholar]
- 10.Wolf M, Garcea RL, Grigorieff N, et al. Subunit interactions in bovine papillomavirus. Proc Natl Acad Sci USA. 2010;107:6298–303. doi: 10.1073/pnas.0914604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eterpi M, McDonnell G, Thomas V. Disinfection efficacy against parvoviruses compared with reference viruses. J Hosp Infect. 2009;73:64–70. doi: 10.1016/j.jhin.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza DH, Su X. Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage. Foodborne Pathog Dis. 2010;7:319–26. doi: 10.1089/fpd.2009.0426. [DOI] [PubMed] [Google Scholar]
- 13.Smith EM, Parker MA, Rubenstein L, et al. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol. 2010;2010:326369. doi: 10.1155/2010/326369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjaer SK, Chackerian B, van den Brule AJC, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidem Biomar. 2001;10:101–6. [PubMed] [Google Scholar]
- 15.Tay SK, Ho TH, Sookim LT. Is genital human papillomavirus infection always sexually-transmitted? Aust NZ J Obstet Gyn. 1990;30:240–2. doi: 10.1111/j.1479-828x.1990.tb03223.x. [DOI] [PubMed] [Google Scholar]