Abstract
While stressful experiences are a part of everyone’s life, they can also exact a major toll on health. Stressful life experiences are associated with increased substance abuse, and there exists significant co-morbidity between mental illness and substance use disorders (Volkow & Li, 2004; Koob & Kreek, 2007; Sinha, 2008). The risk for development of mood or anxiety disorders after stress is positively associated with the risk for substance use disorders (Sinha, 2008), suggesting that there are common substrates for vulnerability to addictive and affective disorders. Understanding the molecular and physiological substrates of stress may lead to improved therapeutic interventions for the treatment of substance use disorders and mental illnesses.
Keywords: dopamine, reward, depression, synaptic plasticity, ventral tegmental area
Alterations in reward-related behaviors following stress suggest that the brain circuitry regulating reward and reinforcement may be a critical hub for the effects of stress on behavior. The ventral tegmental area (VTA) is a key player in the brain’s reward system, and dysregulation of this brain region has long been implicated in both depression and addiction (Nestler & Carlezon, 2006; Fields et al., 2007; Kauer & Malenka, 2007; Koob & Volkow, 2010; Wise & Morales, 2010; Luscher & Malenka, 2011). As the sites of information storage, synapses of the VTA are poised to be a crucial site of regulation of reward and aversion by stress. Here we will review recent literature on the role of VTA circuits and synapses in stress-related disorders.
I. VTA function and structure
Dopaminergic neurons in the VTA project to the prefrontal cortex and nucleus accumbens (NAc), as well as to the hypothalamus, amygdala, lateral habenula, pallidum, and bed nucleus of the stria terminalis (BNST) (Kauer & Malenka, 2007; Sesack & Grace, 2010). These neurons have long been implicated in rewarding and reinforcing processes. Release of dopamine from the VTA into the nucleus accumbens is necessary for the rewarding properties of natural stimuli required for survival, such as food and sex (Kelley & Berridge, 2002) as well as of drugs of abuse (Di Chiara & Imperato, 1988). Recent experiments utilizing optogenetic tools to activate VTA dopaminergic neurons selectively have demonstrated that phasic activity of these cells is sufficient to induce robust behavioral conditioning (Tsai et al., 2009) and optical self-stimulation (Witten et al., 2011). While this evidence is consistent with the conventional view of dopaminergic neuron activity signaling rewarding or incentive salient stimuli (Robinson & Berridge, 2000), aversive and stressful events are also clearly capable of enhancing dopaminergic function within the mesolimbic system (Tidey & Miczek, 1996; Anstrom & Woodward, 2005; Berton et al., 2006; Brischoux et al., 2009; Ungless et al., 2010). For example, dopamine release in the NAc and prefrontal cortex increases during social threat (Tidey and Miczek, 1996), and VTA dopaminergic neurons are phasically excited by footshock and during acute restraint stress (Anstrom and Woodward, 2005; Brischoux et al., 2009). The precise meaning of neuronal signals within the mesolimbic dopamine system is still a topic of debate, but it is clear that this circuit is crucial for both rewarding and aversive experiences.
The VTA is made up of a mixture of dopaminergic, GABAergic, and glutamatergic neurons. Roughly 60–65% of these neurons are dopaminergic and 35% GABAergic, with a small population of glutamatergic neurons (Nair-Roberts et al., 2008; Sesack & Grace, 2010). Additional complexity is added by the fact that dopaminergic neurons can co-release glutamate (Stuber et al., 2010; El Mestikawy et al., 2011; Hnasko et al., 2012) or GABA (Tritsch et al., 2012; Stamatakis et al., 2013). Dopaminergic neurons of the VTA are highly heterogeneous anatomically and physiologically; however, recent work defining subclasses based on projection target has identified two useful broad divisions of dopaminergic cells (Ford et al., 2006; Margolis et al., 2006; Lammel et al., 2008; Margolis et al., 2008; Lammel et al., 2011; Lammel et al., 2012; Lammel et al., 2013). In VTA slices, dopaminergic neurons known to project to the lateral shell of the nucleus accumbens exhibit the electrophysiological properties conventionally used to identify dopaminergic neurons, including a large h-current (Ih) that contributes to a slow, pacemaker firing rate. These neurons are found predominantly in the lateral portion of the VTA, and glutamatergic synapses on these cells have a low AMPA receptor/NMDA receptor ratio under basal conditions (AMPA/NMDA ratio) (Lammel et al., 2013). In contrast, dopaminergic neurons projecting to the prefrontal cortex, basolateral amygdala, and core of the nucleus accumbens exhibit a negligible Ih, rapid firing rate, and a high AMPA/NMDA ratio under basal conditions, and are predominantly found in the medial VTA (Lammel et al., 2013).
Both excitatory and inhibitory synapses control the firing rates and patterns of VTA dopaminergic neurons. In vivo recordings show that activation of glutamatergic neurons projecting from the prefrontal cortex to the VTA increases bursting of VTA DA neurons (Gariano & Groves, 1988; Murase et al., 1993; Tong et al., 1996). Bursting is typically not observed in dopaminergic neurons in the slice preparation, likely because excitatory afferents have been severed; however, bath application of NMDA induces bursting (Johnson et al., 1992; Mereu et al., 1997). GABAergic synapses also play a major role in shaping the activity of dopaminergic neurons. Both pharmacological and optogenetic studies demonstrate that the firing rate of dopaminergic neurons is profoundly reduced by activation of GABAA receptors on these neurons (Johnson & North, 1992; Paladini & Tepper, 1999; Tan et al., 2012; van Zessen et al., 2012).
Not surprisingly, the subclasses of neurons projecting to NAc or prefrontal cortex (mPFC) receive distinct sets of afferent synapses with roles that are being unraveled using optogenetic activation. Dopaminergic neurons projecting to the accumbens shell receive excitatory glutamatergic and cholinergic inputs from the laterodorsal tegmental nucleus (LDT)(Lammel et al., 2012), and inhibitory GABAergic input from the rostromedial tegmental nucleus (RMTg) (Goncalves et al., 2012; Lammel et al., 2012). The mPFC-projecting dopaminergic neurons, in contrast, receive glutamatergic input from the lateral habenula. The lateral habenula also sends excitatory projections to the RMTg, which in turn inhibits the NAc shell-projecting dopaminergic neurons (Lammel et al., 2012; Stamatakis & Stuber, 2012). A minority of dopaminergic neurons found in the medial VTA also receive an inhibitory GABAergic input from the BNST. Although the projection target of these neurons has not been demonstrated, they lack Ih and are likely to be mPFC-projecting neurons (Jennings et al., 2013).
In addition to these defined subcircuits of the VTA, several other regions strongly innervate the VTA, including glutamatergic inputs from the prefrontal cortex and lateral hypothalamus (Kempadoo et al., 2013) and GABAergic inputs from the ventral pallidum (Hjelmstad et al., 2013). A small population of dopaminergic neurons also receives excitatory inputs from the BNST (Jennings et al., 2013). VTA dopaminergic neurons also receive inhibitory input from GABAergic neurons within the VTA (Tan et al., 2012; van Zessen et al., 2012). The nucleus accumbens sends a dense GABAergic projection to the VTA (Nauta et al., 1978; Kalivas et al., 1993), however recent optogenetic studies indicate that these projections make relatively weak GABAA synapses onto VTA dopaminergic neurons with no identified projection target (Bocklisch et al., 2013) and make no GABAA or GABAB synapses onto dopaminergic neurons that project to the nucleus accumbens (Xia et al., 2011). These findings are consistent with earlier literature suggesting that activating GABAA receptors in the VTA increases dopamine release locally and in nucleus accumbens (Kalivas et al., 1990; Klitenick et al., 1992; Xi & Stein, 1998), supporting the idea that activation of GABAergic afferents from the NAc primarily disinhibits VTA dopaminergic cells.
Considerably less is known about the connectivity and diversity of the non-dopaminergic neurons of the VTA, but GABAergic neurons within the VTA play a significant role in modulating dopaminergic cell activity and driving behavior. For example, optogenetic activation of VTA GABAergic neurons is sufficient to support conditioned place aversion and to interrupt reward consumption (Tan et al., 2012; van Zessen et al., 2012). VTA GABAergic neurons innervate local dopaminergic neurons, but also project to the nucleus accumbens, although there is debate over whether they synapse solely on cholinergic interneurons (Brown et al., 2012) or on medium spiny neurons as well (Ishikawa et al., 2013a; Ishikawa et al., 2013b). VTA GABAergic neurons receive inhibitory input from medium spiny neurons of the nucleus accumbens (Xia et al., 2011; Bocklisch et al., 2013) and the BNST (Jennings et al., 2013). In addition, roughly half of VTA GABAergic neurons have excitatory synapses originating in the BNST (Jennings et al., 2013). Furthermore, recent studies have identified a population of “hybrid” VTA neurons projecting to the lateral habenula that are positive for dopaminergic markers, but do not release detectable levels of dopamine. Instead, these neurons release GABA, which inhibits the lateral habenula and promotes reward seeking (Stamatakis et al., 2013).
II. Projection-specific plasticity in reward and aversion
Repeated studies of synaptic plasticity in the VTA have consistently shown that glutamatergic inputs onto dopaminergic neurons are potentiated in brain slices from animals exposed in vivo to psychostimulants (Ungless et al., 2001; Faleiro et al., 2003; Saal et al., 2003; Dong et al., 2004; Bellone & Luscher, 2006; Argilli et al., 2008; Chen et al., 2008). Similar potentiation of glutamatergic synapses onto dopamine neurons is also observed after in vivo or ex vivo exposure to nicotine, morphine or ethanol (Mansvelder & McGehee, 2000; Saal et al., 2003).
Cocaine-induced potentiation is mediated by an NMDAR-dependent increase in AMPA receptors at the synapse, reported as an increased AMPA/NMDA ratio (Ungless et al., 2001). This increase in AMPA/NMDA ratio corresponds with increased rectification of AMPA receptor currents and increased sensitivity to polyamines, suggesting an increase in GluA2-lacking AMPA receptors (Bellone & Luscher 2006; Argilli et al 2008). In addition, NMDA receptor currents induced by photo-uncaging at single synapses appear to be reduced by cocaine, further amplifying the change in AMPA/NMDA ratio (Mameli et al., 2011), although no difference is observed in the response to exogenously applied NMDA (Ungless et al., 2001). NMDA currents in cocaine-treated animals also are more sensitive to ifenprodil, a selective inhibitor of GluN2B, and less sensitive to zinc, a selective inhibitor of GluN2A, suggesting an alteration in the ratio of GluN2A/GluN2B receptors (Yuan et al., 2013). GluN2A/2B receptor switches often accompany significant changes in circuit properties, e.g. during developmental critical periods, powerfully altering synaptic Ca2+ entry and synaptic plasticity thresholds (Quinlan et al., 1999; Kopp et al., 2007). Cocaine treated animals appear to have increased insertion of GluN3a receptors, as indicated by reduced calcium permeability and magnesium sensitivity (Yuan et al., 2013). Studies in GluN3a knockout animals and utilizing shRNA to GluN3a indicated that this subunit is required for the increase in AMPA/NMDA ratio following cocaine (Yuan et al., 2013). These changes in both NMDARs and AMPARs significantly alter calcium permeability and calcium dynamics at the synapse, allowing stimulation protocols that are not sufficient for LTP induction in naïve animals to induce robust LTP following cocaine exposure (Mameli et al., 2011).
LTP of glutamatergic synapses is long-lasting, persisting for a week after acute exposure to cocaine (Ungless et al., 2001; Borgland et al., 2004) and for at least three months after chronic cocaine self-administration (Chen et al., 2008). While operant responding for naturally rewarding substances such as food or sucrose also potentiates glutamatergic synapses in the VTA, their effects are much shorter lived with AMPA/NMDA ratios returning to baseline levels between seven days and three weeks after the final self-administration session (Chen et al., 2008).
Dopamine neurons with differing projection targets exhibit differential alterations in synaptic plasticity following rewarding or aversive stimuli (Lammel et al., 2011). In the medial VTA, dopamine neurons projecting to the prefrontal cortex show a robust increase in AMPA/NMDA ratio in response to an aversive stimulus (hindpaw injection of formalin) but no change in response to a rewarding stimulus (single injection of cocaine). In contrast, VTA neurons projecting to the medial shell of the nucleus accumbens have an increased AMPA/NMDA ratio following cocaine injection, but do not change 24 hours after hindpaw formalin injection. Lateral VTA neurons, which project to the lateral shell of the nucleus accumbens, exhibit a moderate increase in AMPA/NMDA ratio after both rewarding and aversive stimuli (Lammel et al., 2011). These data correspond nicely with the projection-specific functional role of dopamine neurons. Optogenetic stimulation of accumbens-projecting dopamine neurons supports robust place preference (Lammel et al., 2012), suggesting that they contribute to reward. In contrast, activation of PFC-projecting dopamine neurons induces conditioned place aversion suggesting a role in aversion processing (Lammel et al., 2012).
III. Stress and VTA synapses
Both excitatory and inhibitory synapses on VTA dopaminergic neurons express long-term potentiation (LTP) that is altered by exposure to acute stress(Saal et al., 2003; Dong et al., 2004; Niehaus et al., 2010; Graziane et al., 2013), reviewed in (Kauer & Malenka, 2007; Luscher & Malenka, 2011). Parallel changes seen after drug exposure and acute stress may provide a reason why acute stressors precipitate drug-seeking after abstinence. Like exposure to drugs of abuse, acute swim stress increases the AMPA/NMDA ratio of excitatory synapses on VTA dopaminergic neurons (Saal et al., 2003; Dong et al., 2004; Daftary et al., 2009; Graziane et al., 2013). This increase requires GluA1 receptors, and is dependent upon activation of NMDA receptors and glucocorticoid receptors (Saal et al., 2003; Dong et al., 2004) (Figure 1A). AMPA/NMDA ratios are potentiated as soon as 2 hours after stress, and remain potentiated for at least 24 hours (Daftary et al., 2009). Glucocorticoid receptor activation is sufficient to induce potentiation of these glutamatergic synapses, as either in vivo or in vitro dexamethasone increases AMPA/NMDA ratio (Daftary et al., 2009). Local block of both AMPARs and NMDARs in the VTA also prevents stress-induced dopamine efflux in the prefrontal cortex (Butts & Phillips, 2013).
Figure 1.
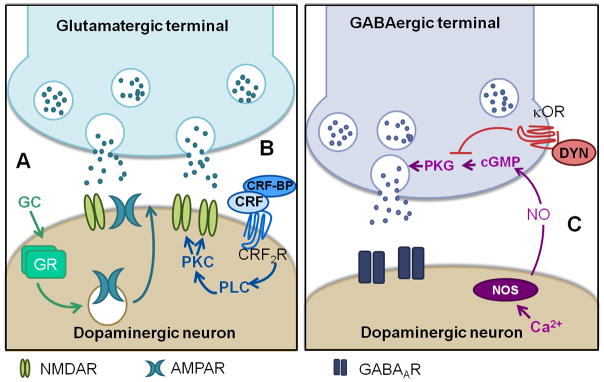
Modulation of VTA synaptic plasticity by stress systems. A) At excitatory synapses, stress-induced activation of glucocorticoid receptors leads to an increase in the AMPA and NMDA ratio. B) CRF potentiates NMDA currents through activation of CRF2 receptors and downstream activation of PLC and PKC. C) Inhibitory synapses are potentiated via the retrograde messenger nitric oxide and activation of cGMP signaling. This plasticity is blocked by stress through activation of kappa opioid receptors. From (Saal et al., 2003; Ungless et al., 2003; Nugent et al., 2007; Daftary et al., 2009; Nugent et al., 2009; Niehaus et al., 2010; Graziane et al., 2013)
GC: glucocorticoid, GR: glucocorticoid receptor, CRF: corticotrophin releasing factor, CRF-BP: CRF-binding protein, CRF2R: CRF receptor type 2, PLC: phospholipase C, PKC: protein kinase C,NOS: nitric oxide synathase, NO: nitric oxide, cGMP: cyclic guanosine monophosphate, κOR: kappa opioid receptor, dyn: dynorphin, PKG: protein kinase G
In addition to glucocorticoids, other stress-modulating signaling molecules such as corticotrophin releasing factor (CRF) can regulate VTA functioning. CRF is a hypothalamic peptide that stimulates the hypothalamic-pituitary-adrenal stress response system and signals the effects of stress throughout the brain (Sarnyai et al., 2001; Bale & Vale, 2004). The paraventricular nucleus of the hypothalamus, the central amygdala, and the BNST all send CRF-positive projections to the VTA (Rodaros et al., 2007). While CRF-containing projections form both glutamatergic and GABAergic synapses, it appears that CRF-containing synapses on dopaminergic neurons are primarily glutamatergic (Tagliaferro & Morales, 2008). Both CRF1R and CRF2R receptors are found in the VTA (Van Pett et al., 2000; Ungless et al., 2003) and CRF promotes firing in both GABAergic and dopaminergic cells (Korotkova et al., 2006). In dopaminergic neurons, the increase in firing rate occurs through alterations in the Ih (Wanat et al., 2008). Bath application of CRF to VTA slices also enhances NMDAR-mediated currents, but not AMPAR-mediated currents (Ungless et al., 2003) (Figure 1B). This increase is mediated by CRF2R receptors, and is dependent on activation of PKC.
Intriguingly, prior exposure to drugs of abuse enhances the effects of CRF on the VTA. Intra-VTA infusion of CRF increases extracellular glutamate levels in cocaine treated but not naïve rats (Wang et al., 2005). Compared to cocaine-naïve rats, cocaine-experienced rats exhibit a greater magnitude and longer lasting potentiation of NMDAR currents induced by CRF (Hahn et al., 2009). Unlike in naïve animals, in which CRF potentiates NMDAR currents solely through CRF2R (Ungless et al., 2003), the potentiation in cocaine-experienced rats is dependent on both CRF receptor subtypes. Furthermore, CRF potentiates AMPAR currents in cocaine-treated rats, but not in naïve animals. In rats that have self-administered cocaine, glutamatergic synapses on dopaminergic neurons are already potentiated, as evidenced by the increased AMPA/NMDA ratio (Chen et al., 2008). That CRF is able to further potentiate these synapses suggests that CRF and cocaine self-administration potentiate distinct populations of glutamatergic synapses, or potentiate excitatory synapses through distinct mechanisms.
CRF also plays an important role in reinstatement of drug seeking after footshock. Footshock increases CRF levels in the VTA and causes a CRF-dependent increase in extracellular glutamate (Wang et al., 2005). Intra-VTA infusion of CRF is also sufficient to reinstate cocaine seeking, an effect that is blocked by a glutamate receptor antagonist (Wang et al., 2005). Footshock-induced reinstatement can be prevented by infusion of antagonists of CRF receptors or of AMPARs and NMDARs into the VTA (Wang et al., 2005). Subsequent work demonstrated that a CRF2R antagonist, but not a CRF1R antagonist (Wang et al., 2007) prevented footshock-induced reinstatement of cocaine seeking. Increased NMDAR currents and footshock-induced reinstatement also both require CRF binding protein (Ungless et al., 2003; Wang et al., 2007).
The dynorphin/kappa opioid receptor (KOR) system is an additional downstream mediator of the stress response that alters VTA synapses (reviewed in (Van’t Veer & Carlezon, 2013). Afferents from several dynorphin-expressing brain regions including the nucleus accumbens, hypothalamus, amygdala and BNST project to the VTA (Fallon et al., 1985; Meredith, 1999; Dong & Swanson, 2003; Chartoff et al., 2009; Poulin et al., 2009). KORs are expressed in the VTA (Speciale et al., 1993; Arvidsson et al., 1995; Mansour et al., 1996) and dynorphin levels and kappa receptor phosphorylation are increased after stress (Nabeshima et al., 1992; Land et al., 2008). KORs have been strongly implicated in stress and aversion related behaviors (Bals-Kubik et al., 1993; Beardsley et al., 2005; Valdez et al., 2007; Land et al., 2008; Land et al., 2009; Beardsley et al., 2010; Bruchas et al., 2010; Van’t Veer & Carlezon, 2013), particularly in stress-induced drug seeking (McLaughlin et al., 2003; Beardsley et al., 2005; McLaughlin et al., 2006; Carey et al., 2007; Redila & Chavkin, 2008; Beardsley et al., 2010; Sperling et al., 2010; Graziane et al., 2013; Van’t Veer et al., 2013)
KORs profoundly affect VTA synaptic transmission. Bath application of a KOR agonist to VTA slices transiently decreases EPSC amplitude on dopaminergic and GABAergic neurons (Margolis et al., 2005), IPSCs on BLA-projecting dopaminergic neurons (Ford et al., 2006), and dopamine-mediated IPSCs (Ford et al., 2007). Our recent studies show that activation of KORs also entirely blocks LTP at GABAergic synapses (LTPGABA) on VTA dopaminergic neurons 24 hours after acute cold water swim stress (Nugent et al., 2007; Nugent et al., 2009; Niehaus et al., 2010; Graziane et al., 2013) (Figure 1C). However, administration of the KOR antagonist nor-BNI prevents the block of LTPGABA by stress without preventing potentiation of excitatory synapses by stress. Linking this finding to drug self-administration, intra-VTA delivery of nor-BNI also prevents reinstatement of cocaine seeking after the same cold water swim stress (Graziane et al., 2013). These data indicate that stress affects excitatory and inhibitory VTA synapses through distinct pathways, and that reinstatement can be prevented without restoring excitatory synapses to their pre-stress state.
Stress also alters delta opioid peptide effects on VTA synapses. In VTA slices from naïve animals, bath application of a delta opioid receptor (δOR) agonist depresses GABAA receptor-mediated IPSCs (Margolis et al., 2011). In slices from animals exposed to footshock, however; a subset of cells exhibits an enhancement of GABAA synaptic currents following application of a δOR agonist. This enhancement primarily occurs in TH+/Ih+ dopamine neurons. Enhancement of IPSCs by the δOR agonist occurs via postsynaptic modifications, and is dependent on AKT-mediated trafficking of GABAA receptors to the cell surface.
Many intriguing questions remain regarding the regulation of VTA synapses by stress. In contrast to regulation of VTA synapses by cocaine, surprisingly little is known about the mechanism by which stress increases AMPA/NMDA ratios and if stress alters glutamate receptor subunit composition. Additionally, while early work found that either chronic restraint stress or chronic unpredictable stress increases expression of GluA1 and NMDAR1 subunits in VTA (Fitzgerald et al., 1996), few studies since have investigated the effects of chronic stress on VTA synapses. Future studies in this area will be particularly valuable, given that human experience consists of a complex variety of stressors of varying intensities and duration. There is also very little known about effects of stress on projection-target specific dopamine neurons. As mentioned above, hindpaw formalin injection increases AMPA/NMDA ratio selectively on dopamine neurons projecting to the PFC, a circuit alteration that can be interpreted as a stress response. It will be of interest to ascertain whether similar neuroadaptations follow more complex stressors, such as those linked to reinstatement of drug seeking (cold water swim or footshock) or to chronic stress conditions, and whether stress-induced alteration in other forms of plasticity, such as plasticity of GABAergic synapses or potentiation of NMDA receptors by CRF is also restricted to defined subtypes of dopamine neurons.
IV. Stress and Addiction
Animal models have long suggested an interaction between stress and drug-seeking behavior. Acute and chronic stress protocols increase self-administration of psychostimulants, opiates, and, in some studies, alcohol (Hadaway et al., 1979; Piazza et al., 1990; Ramsey & Van Ree, 1993; Goeders & Guerin, 1994; Shaham & Stewart, 1994; Haney et al., 1995; Miczek & Mutschler, 1996; Piazza & Le Moal, 1996; Kosten et al., 2000; Sinha, 2001; Boyce-Rustay et al., 2007; Moffett et al., 2007; Sinha, 2008; Ambroggi et al., 2009; Becker et al., 2011). Additionally, stress reinstates drug-seeking in animals that have extinguished self-administration (Shaham et al., 1994; Shaham & Stewart, 1995; Erb et al., 1996; Le et al., 1998; Shaham et al., 2003; Conrad et al., 2010). Taken together, these studies show that stress significantly alters an animal’s behavior towards addictive drugs, both by increasing initial drug intake and by restoring previously-extinguished drug-seeking behavior.
Social defeat stress, a psychosocial stressor in which rodents are defeated by a conspecific aggressor, has been used as a model of escalated drug-seeking after stress (Miczek et al., 2008). After exposure to an aggressor, defeated animals exhibit increased self-administration of cocaine during a 24 hour binge session and decreased latency to self-administration of cocaine during a binge session (Tidey & Miczek, 1997; Covington & Miczek, 2001; 2005). Defeated animals also have increased conditioned place preference for cocaine (McLaughlin et al., 2006), and increased alcohol preference (Croft et al., 2005; Dong et al., 2011). Social defeat increases activity of dopaminergic neurons in the VTA, reflected in increased dopamine release in the nucleus accumbens (Tidey & Miczek, 1996). Furthermore, changes in behavioral responses to cocaine after social defeat can be reversed by intra-VTA infusion of an NMDAR antagonist or the CRF-1 antagonist, antalarmin (Croft et al., 2005; Covington et al., 2008).
The VTA is required for stress-induced reinstatement of drug seeking. Inactivation of the VTA with baclofen and muscimol prevents footshock induced reinstatement (McFarland et al., 2004), and as discussed above, intra-VTA injections of a KOR antagonist also prevents swim stress-induced reinstatement (Graziane et al., 2013). Similarly, the BNST-VTA pathway is important for reinstatement of place preference by swim stress (Briand et al., 2010). A number of other areas essential for reinstatement including the BNST, PFC, and NAc, converge on the VTA, suggesting that the VTA may be a crucial intersection point between stress and drug seeking (McFarland et al., 2004). Caution should be taken, however, in generalizing circuitry of reinstatement between distinct stressors, underscored by the apparent differences in reinstatement patterns after different stressors. For example, footshock causes a rapid, robust reinstatement (McFarland et al., 2004; Wang et al., 2005) that occurs in the same context where drugs are self-administered, while cold water swim stress, which is contextually and temporally separated from drug self-administration, induces a more mild reinstatement that lasts for several days (Conrad et al., 2010).
Further investigation of mechanisms by which stress modifies synapses in the VTA may prove a rich vein for identifying novel treatment targets for addiction. Important molecular players in stress-induced drug seeking such as CRF receptors and the dynorphin-KOR system significantly alter excitatory and inhibitory synapses. Further defining these pathways, as well as finding new ways to manipulate VTA synapses, in animals and eventually in humans, may prove highly beneficial in treating addiction.
V. Depression
Anhedonia and appetite disturbance are core symptoms of depression that involve alterations in reward signaling, suggesting that stress-induced disruptions of the brain’s reward circuitry may underlie some symptoms of depression (Nestler & Carlezon, 2006). Several studies of VTA dopaminergic neurons in depression have utilized the chronic social defeat stress model. In this model, repeated exposure to aggressive animals over a ten day period results in decreased social interaction, decreased sucrose preference, and a number of other behavioral abnormalities that may be related to major depressive disorder (Krishnan et al., 2007). This behavioral model has several intriguing features. First, only a subset of animals exhibit these behavioral changes (termed susceptible animals), while the others are behaviorally unaffected (resilient), despite a fairly homogeneous genetic background. Second, the abnormal behaviors observed in susceptible animals can be reversed after a two-week but not a single-dose antidepressant treatment (Cao et al., 2010). Many behavioral consequences of chronic social defeat stress seem to be encoded by alterations in VTA function.
Studies distinguishing between animals that are resilient and susceptible to social defeat have found that dopaminergic neurons in susceptible mice, but not resilient mice, exhibit increased firing rates (Berton et al., 2006; Krishnan et al., 2007; Cao et al., 2010; Razzoli et al., 2011). The alteration in firing rate is pathway specific, with accumbens-projecting neurons exhibiting an increased firing rate in susceptible animals and PFC-projecting neurons exhibiting a decreased firing rate (Chaudhury et al., 2013) (recall that NAc shell-projecting dopaminergic neurons contribute to conditioned place reinforcement, while mPFC-projecting neurons contribute to conditioned place aversion (Lammel et al., 2012)). Most significantly, normalizing the firing rate of dopaminergic neurons reverses the social interaction deficits and sucrose preference deficits of susceptible mice (Krishnan et al., 2007; Cao et al., 2010; Chaudhury et al., 2013). These studies indicate a causal, projection-specific role for dopaminergic neurons in expression of anhedonic behavior after social defeat stress, and suggest the possibility that alterations in dopaminergic neurons and synapses on these cells also play a causal role in depression in human patients.
However, the role of VTA dopaminergic neurons in stress responding appears to be more complex. A recent study utilizing the chronic mild stress model in mice and rats over a longer time period found a markedly different result, instead reporting a decrease in dopaminergic neuron bursting after stress. Animals were exposed to randomly chosen unpredictable stressors such as crowding, isolation, and food deprivation, twice a day for eight to twelve weeks. Optogenetically stimulating VTA dopaminergic neurons reversed the stress-induced behavioral deficits in sucrose preference, the tail suspension test, and the forced swim test (Tye et al., 2013). Although dopaminergic neurons in this study were not distinguished by projection target, the antidepressant effect of stimulating dopaminergic neurons was blocked by dopamine receptor antagonists in the NAc, suggesting that accumbens-projecting neurons played a role. Thus, in one study, susceptible mice that had undergone social defeat stress exhibited reduced dopamine cell firing rate and decreased sucrose preference, both alleviated by driving dopamine cell firing (Chaudhury et al., 2013), while in the second study, stressed mice instead had reduced dopamine neuron firing and decreased sucrose preference, both alleviated by driving dopamine neuron firing (Tye et al., 2013). If driving dopaminergic neurons in opposite directions in fact restores sucrose preference, either the dopaminergic cells were in different “states” following the two distinct stress protocols, or the somewhat different temporal windows over which sucrose preference was tested contributed to the differences. Clearly, future work will help to identify the relevant differences between stress protocols, species, and methods of optogenetic stimulation. What is obvious from this body of work is the essential role of the VTA-nucleus accumbens circuit in stress-related and depression-related behaviors such as anhedonia.
It remains unknown whether VTA synapses are altered in depression models. The discovery that the NMDAR antagonist ketamine has a rapid antidepressant effect has increased interest in synaptic mechanisms of antidepressant action (Zarate et al., 2006; Li et al., 2010; Autry et al., 2011; Mathews & Zarate, 2013). It will be intriguing to see whether VTA synapses are required for effectiveness of the rapid-acting antidepressants, and whether alterations in these synapses are necessary to promote the development of depressive-like behaviors in animal models. It will be of particular interest to investigate specific sources of synapses onto VTA dopaminergic neurons that are known to be implicated in depression. As one example, the lateral habenula, which sends projections to the VTA (Lammel et al., 2012), has been implicated in depression, and deep brain stimulation of this region was shown to induce remission in a patient with severe treatment refractory depression (Sartorius et al., 2010). This role for the habenula in depression is also seen in animal models, where potentiated excitatory synapses on VTA-projecting neurons were seen in the rat acute and congenital learned helplessness models of depression (Li et al., 2011). Deep brain stimulation in these animals, using the same protocol that showed efficacy in the human patient, suppressed excitatory synapses on habenula neurons and reversed the helpless phenotype of the rats (Li et al., 2011).
VI. The VTA: a stress hub? Conclusions and future directions
Early on, it was clear that brief acute stress produces changes in VTA synapses that parallel those caused by addictive drugs (Saal et al., 2003). Since this time, the parallels have continued to be observed at the synaptic level, although it is clear that synapses on discrete subpopulations of dopamine neurons are differentially altered by drug exposure or by a brief stressor. Newer data addressing the links between behaviors and optogenetic activation of VTA dopamine neurons have clarified a key role of these neurons: to detect stress and modify behaviors accordingly. The management of stress by the reward/reinforcement circuitry may provide a unifying concept linking addiction-related behaviors with depression-related behaviors. In light of the high rate of co-morbidity between depression and addiction, targeting stress responses within the VTA may represent a highly useful therapeutic target for both disorders.
Chronic exposure to drugs of abuse persistently alters brain circuitry and synapses, perhaps accounting for the difficulty in returning to the pre-addicted state. Similarly, animal models of affective disorders have shown lasting differences in circuitry, and mood disorders are often chronic, recurrent illnesses. While reversing the cellular and molecular changes that occur after drug exposure or exposure to stressors may not be possible, there is growing evidence that these changes throughout the brain can be circumvented or counterbalanced by therapeutic neuroadaptations or treatments (Mameli et al., 2007; Wang et al., 2007; Conrad et al., 2008; Wee et al., 2009; Russo et al., 2012; Chaudhury et al., 2013; Graziane et al., 2013; Kallupi et al., 2013; Loweth et al., 2013; Tye et al., 2013).
Stressors have multiple effects on cells and circuits. Some of these may be persistent and have negative consequences on behavior, e.g anhedonia or metabolic effects. However, some downstream sequelae of stress may be positive or protective (Russo et al., 2012). The exact balance of these positive and negative consequences can vary considerably within a population. While all individuals experience the negative consequences of stress, the severity will depend on the individual. Conversely, the positive effects of stress occur independently of the negative effects, and do not necessarily reverse them. Some positive effects of stress may occur only a subset of individuals, granting them resilience to stressors. Resilience to stress may therefore represent a set of active mechanisms by which the negative consequences of stress can be compensated for and overcome, suggesting the importance of differences in individual behavioral responses to a given stress protocol in animal models of addiction and mood disorders. While studies continue to unravel mechanisms by which stress impacts behavior, stress is only one of several interacting factors contributing to the development of addiction and mental illness. Our growing understanding of genetic and environmental factors that contribute to predispositions to illness will allow us to integrate our understanding of how stressors act to trigger maladaptive responses.
Recent years have seen rapid and exciting advances in the neuroscience of stress, reward and aversion. This new knowledge has the potential to significantly impact our understanding of addiction and depression, but many questions remain. We are gaining a solid understanding of the regulation of synapses on VTA dopaminergic neurons, but much less is known about the synapses on other neurons of this region that have key functional roles in driving behavior. Optogenetic tools provide a tremendous opportunity for precise correlations of specific cell populations with complex behaviors, and will continue to identify neural pathways and cell types involved in specific behaviors. The complexity of the data already arising from optogenetic studies of the mesolimbic dopamine circuitry simply emphasizes the complexity of the behaviors relevant to human addiction and depression. As tools and approaches are refined, we will develop a more sophisticated understanding of how to modify circuits that have been altered in the addicted or depressed brain, and how to limit the negative effects of further stressful experience. Pinpointing signaling molecules and receptors that control synapses within these specific circuits will provide pharmacological tools as well as potential sites for deep brain and transcranial magnetic stimulation through which these circuits can be targeted for treatment of disease.
Acknowledgments
This work was supported by NIH Grants DA011289 and AA007459.
Footnotes
The authors declare that they do not have any conflict of interest.
References
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nature neuroscience. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law PY, Wessendorf MW, et al. The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. The Journal of pharmacology and experimental therapeutics. 1993;264:489–495. [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Pollard GT, Howard JL, Carroll FI. Effectiveness of analogs of the kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl ]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic) to reduce U50,488-induced diuresis and stress-induced cocaine reinstatement in rats. Psychopharmacology. 2010;210:189–198. doi: 10.1007/s00213-010-1846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature neuroscience. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, de Roo M, Tan KR, Luscher C. Cocaine Disinhibits Dopamine Neurons by Potentiation of GABA Transmission in the Ventral Tegmental Area. Science. 2013;341:1521–1525. doi: 10.1126/science.1237059. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain research. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol. 2013;16:1799–1807. doi: 10.1017/S1461145713000187. [DOI] [PubMed] [Google Scholar]
- Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Molecular pharmacology. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but Not Natural Reward Self-Administration nor Passive Cocaine Infusion Produces Persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biological psychiatry. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology. 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology. 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Panksepp J, Dong Y, Saal DB. Stress-induced, glucocorticoid-dependent strengthening of glutamatergic synaptic transmission in midbrain dopamine neurons. Neuroscience letters. 2009;452:273–276. doi: 10.1016/j.neulet.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. The Journal of comparative neurology. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke TK, Lourdusamy A, Smolka MN, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essmann F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, Schumann G. Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. The American journal of psychiatry. 2011;168:1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(-/-) mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nature reviews. Neuroscience. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid AMPAR/NMDAR response to amphetamine: a detectable increase in AMPAR/NMDAR ratios in the ventral tegmental area is detectable after amphetamine injection. Annals of the New York Academy of Sciences. 2003;1003:391–394. doi: 10.1196/annals.1300.032. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM, Cone RI. Dynorphin-containing pathways in the substantia nigra and ventral tegmentum: a double labeling study using combined immunofluorescence and retrograde tracing. Neuropeptides. 1985;5:457–460. doi: 10.1016/0143-4179(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. Journal of neurophysiology. 2007;97:883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain research. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goncalves L, Sego C, Metzger M. Differential projections from the lateral habenula to the rostromedial tegmental nucleus and ventral tegmental area in the rat. The Journal of comparative neurology. 2012;520:1278–1300. doi: 10.1002/cne.22787. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaway PF, Alexander BK, Coambs RB, Beyerstein B. The effect of housing and gender on preference for morphine-sucrose solutions in rats. Psychopharmacology. 1979;66:87–91. doi: 10.1007/BF00431995. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain research. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Xia Y, Margolis EB, Fields HL. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:6454–6459. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Otaka M, Huang YH, Neumann PA, Winters BD, Grace AA, Schluter OM, Dong Y. Dopamine triggers heterosynaptic plasticity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013a;33:6759–6765. doi: 10.1523/JNEUROSCI.4694-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Otaka M, Neumann P, Wang Z, Cook J, Schlueter O, Dong Y, Huang Y. Exposure to cocaine regulates inhibitory synaptic transmission from the ventral tegmental area to nucleus accumbens. The Journal of physiology. 2013b doi: 10.1113/jphysiol.2013.262915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. The Journal of pharmacology and experimental therapeutics. 1990;253:858–866. [PubMed] [Google Scholar]
- Kallupi M, Wee S, Edwards S, Whitfield TW, Jr, Oleata CS, Luu G, Schmeichel BE, Koob GF, Roberto M. Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biological psychiatry. 2013;74:520–528. doi: 10.1016/j.biopsych.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature reviews. Neuroscience. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Luthi A. Experience-dependent changes in NMDA receptor composition at mature central synapses. Neuropharmacology. 2007;53:1–9. doi: 10.1016/j.neuropharm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain research. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, Lacrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nature neuroscience. 2013 doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MT, Luscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nature neuroscience. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. Journal of neurophysiology. 2005;93:3086–3093. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Hjelmstad GO, Fields HL. A novel opioid receptor-mediated enhancement of GABAA receptor function induced by stress in ventral tegmental area neurons. The Journal of physiology. 2011;589:4229–4242. doi: 10.1113/jphysiol.2011.209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DC, Zarate CA., Jr Current status of ketamine and related compounds for depression. The Journal of clinical psychiatry. 2013;74:516–517. doi: 10.4088/JCP.13ac08382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Mereu G, Lilliu V, Casula A, Vargiu PF, Diana M, Musa A, Gessa GL. Spontaneous bursting activity of dopaminergic neurons in midbrain slices from immature rats: role of N-methyl-D-aspartate receptors. Neuroscience. 1997;77:1029–1036. doi: 10.1016/s0306-4522(96)00474-5. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neuroscience letters. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Katoh A, Wada M, Kameyama T. Stress-induced changes in brain Met-enkephalin, Leu-enkephalin and dynorphin concentrations. Life Sci. 1992;51:211–217. doi: 10.1016/0024-3205(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Niehaus J, Murali M, Kauer J. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent F, Penick E, Kauer J. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Niehaus JL, Kauer JA. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology. 2009;34:1829–1842. doi: 10.1038/npp.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–176. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain research. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1356–1365. doi: 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Van Ree JM. Emotional but not physical stress enhances intravenous cocaine self-administration in drug-naive rats. Brain research. 1993;608:216–222. doi: 10.1016/0006-8993(93)91461-z. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal DM. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav Brain Res. 2011;218:253–257. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological reviews. 2001;53:209–243. [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biological psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Rodaros D, Stewart J. Reinstatement of heroin-reinforced behavior following long-term extinction: implications for the treatment of relapse to drug taking. Behav Pharmacol. 1994;5:360–364. doi: 10.1097/00008877-199406000-00015. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speciale SG, Manaye KF, Sadeq M, German DC. Opioid receptors in midbrain dopaminergic regions of the rat. II. Kappa and delta receptor autoradiography. J Neural Transm Gen Sect. 1993;91:53–66. doi: 10.1007/BF01244918. [DOI] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology. 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature neuroscience. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. The Journal of comparative neurology. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain research. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci Biobehav Rev. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. The Journal of pharmacology and experimental therapeutics. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Bechtholt AJ, Onvani S, Potter D, Wang Y, Liu-Chen LY, Schutz G, Chartoff EH, Rudolph U, Cohen BM, Carlezon WA., Jr Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacology. 2013;38:1585–1597. doi: 10.1038/npp.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. The Journal of comparative neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nature reviews. Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. The Journal of physiology. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain research. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain research. 1998;798:156–165. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Mameli M, ECOC, Dey PN, Verpelli C, Sala C, Perez-Otano I, Luscher C, Bellone C. Expression of Cocaine-Evoked Synaptic Plasticity by GluN3A-Containing NMDA Receptors. Neuron. 2013;80:1025–1038. doi: 10.1016/j.neuron.2013.07.050. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]


