Abstract
Vaccination against nicotine is a potential treatment for tobacco smoking. Clinical trials show effect only in high antibody responders; therefore it is necessary to increase the effectiveness of nicotine vaccines. The use of a multivalent vaccine that activates several B cell populations is a possible approach to increase antibody response. The aim of this study was to investigate whether three different nicotine immunogens could be mixed to generate independent responses resulting in additive antibody titers, and whether this would alter nicotine distribution to a greater extent than antibodies generated by a monovalent vaccine. When immunogens were administered s.c. with alum adjuvant, the trivalent vaccine generated significantly higher titers and prevented the distribution of an i.v. nicotine dose to brain to a greater extent than an equivalent dose of a monovalent vaccine. The number of rats with antibody titers >1:10,000 was significantly increased in the trivalent group compared to the monovalent group. There were no correlations between the titers generated by the different nicotine immunogens in the trivalent vaccine, supporting the hypothesis that the immunogens generated independent responses from distinct populations of B cells. In contrast, when administered i.p. in Freund’s adjuvant, the trivalent nicotine vaccine was not more immunogenic than its component monovalent vaccine. Vaccine immunogenicity was suppressed if unconjugated protein was added to the monovalent vaccine formulated in Freund’s adjuvant, compared to monovalent vaccine alone. These data suggest a protein–protein interaction that affects titers negatively and is apparent when the vaccines are formulated with Freund’s adjuvant. In summary, a trivalent nicotine vaccine formulated with alum showed significantly higher efficacy than a dose-matched monovalent vaccine and may offer a strategy for increasing nicotine vaccine immunogenicity. This approach may be generalizable to other nicotine immunogens or vaccines for other addictive drugs.
Keywords: Nicotine, Vaccine, Multivalent, Antibody, Tobacco, Immunogen
1. Introduction
Tobacco smoking is the leading preventable cause of premature mortality in the world [1]. Nearly 6 million people are killed every year from tobacco related illnesses and the number is increasing [2].
Nicotine is the major addictive component of tobacco smoke [3]. Current treatments for tobacco dependence such as nicotine replacement therapy, bupropion and varenicline improve the chances for abstinence, but even with these treatments the great majority of smokers relapse within a year [4–7]. An alternative treatment approach is active immunization against nicotine. Nicotine conjugate vaccines elicit nicotine specific antibodies that sequester nicotine in the blood and reduce nicotine distribution to the brain. There is a large body of evidence showing the preclinical efficacy of nicotine vaccines. Studies in rodents have shown that nicotine vaccines reduce nicotine levels in brain by 13–90% [8–16], prevent nicotine-induced dopamine release in the nucleus accumbens [17], alter acquisition and maintenance of nicotine self-administration [18,19], and prevent reinstatement of nicotine-seeking behavior [20]. However, phase II and III clinical trials of three nicotine vaccines have either showed limited efficacy or failed to meet their primary endpoint of increased smoking cessation rates compared to placebo [21–25]. Despite the overall negative results increased cessation rates were reported in the subgroups of subjects with the highest antibody concentrations [21,22]. These trials therefore provide proof-of-concept that nicotine vaccines can enhance smoking cessation rates but it is clear that higher antibody concentrations need to be generated in order for nicotine vaccines to be effective.
One possible approach for enhancing antibody levels is to use a multivalent vaccine. We have previously shown that two structurally distinct nicotine immunogens, trans-3′-aminomethyl-(±)-nicotine conjugated to recombinant Pseudomonas aeruginosa exoprotein A (3′-AmNic-rEPA) and 6-(carboxymethylureido)-(±)-nicotine conjugated to keyhole limpet hemocyanin (6-CMUNic-KLH), generate antibodies that bind nicotine but which have different specificities [26]. This is evident by the minimal cross-reactivity of antibodies generated by each immunogen with the other, and was true whether the immunogens were administered alone or together as a bivalent vaccine. These observations showed that even a small molecule such as nicotine (M = 162 g/mol) can provide several distinct epitopes that independently activate different B cell populations. A third distinct nicotine immunogen, 1′-N-(2-mercaptoethyl) pentanamide-(−)-nicotine conjugated to KLH (1′-SNic-KLH) has been identified, that generates antibodies that do not appreciably cross-react in ELISA with either of the two other immunogens [16]. This introduces the possibility of using a trivalent vaccine to further increase the total antibody response. However, it is unclear if there is an advantage in using a multivalent vaccine as supposed to just increasing the dose of a monovalent vaccine.
In this study, we co-administered the three previously studied nicotine immunogens and compared their combined effects to that of 3′-AmNic-rEPA alone (Fig. 1). 3′-AmNic-rEPA is of particular interest as a comparator because it has been tested clinically and shown to have efficacy in subjects exhibiting the most robust antibody responses. We investigated whether the trivalent nicotine vaccine would generate higher antibody titers than the dose-matched monovalent vaccine, and whether the trivalent vaccine would prevent nicotine from entering the brain to a greater extent than the monovalent vaccine.
Fig. 1.
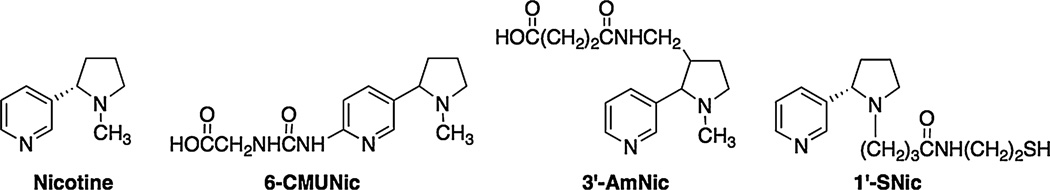
Structures of (S)-nicotine and the nicotine haptens, 3′-AmNic, 6-CMUNic and 1′-SNic, including the linkers to which the carrier protein is conjugated.
2. Materials and methods
2.1. General methods
2.1.1. Animals
Male HsdHot:Holtzman®SD® rats (Harlan Laboratories, USA) were housed in solid bottom cages in a temperature-controlled environment. Experimental protocols were approved by the Minneapolis Medical Research Foundation Institutional Animal Care and Use Committee (IACUC).
2.1.2. Immunogens and immunization protocols
Three previously described nicotine haptens (Fig. 1) were used in this study, i) trans-3′-aminomethyl-(±)-nicotine (3′-AmNic), ii) 6-(carboxymethylureido)-(±)-nicotine (6-CMUNic) and iii) 1′-N-(2-mercaptoethyl) pentanamide-(−)- nicotine (1′-SNic). The nicotine haptens differed both in linker attachment position on the nicotine molecule and in linker structure. The 3′-AmNic hapten was conjugated to recombinant Pseudomonas aeruginosa exoprotein-A (rEPA), the 6-CMUNic was conjugated to keyhole limpet hemocyanin (KLH) via amide linkage and the 1′-SNic hapten was conjugated to malemide activated KLH via thioether linkage [16]. All nicotine immunogens has previously been shown to generate antibodies with high nicotine selectivity [8,12,16].
When Freund’s adjuvant was used, immunogens were diluted in 0.1 M sterile phosphate buffered saline, pH = 7.2–7.4 (PBS) to appropriate concentration, mixed 1:1 (v/v) with Freund’s adjuvant and emulsified by vigorous shaking for 10 min. Freund’s Complete adjuvant (FCA, Calbiochem, San Diego, CA) was used for the primary immunization and Freund’s Incomplete adjuvant (FIA, Sigma-Aldrich, St. Louis, MO) for the booster immunizations.
For the alum formulations, immunogens were adsorbed to Alhydrogel85, 2% (Brenntag Biosector). Rats received 3 immunizations intraperitoneally (i.p.) in Freund’s adjuvant or subcutaneous (s.c.) in alum in a total volume of 0.4 ml, on days 0, 21 and 42.
2.1.3. ELISA
3′-AmNic conjugated to polyglutamate (pGlu), 6-CMUNic conjugated to bovine serum albumin (BSA) or 1′-SNic conjugated to ovalbumin (OVA) were used as coating antigens to prevent cross-reactivity to the carrier proteins used in the immunizations. Costar 9018 EIA/RIA 96 well polystyrene plates (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were coated with the coating antigens diluted in 0.05 M carbonate buffer pH 9.6 at 4 °C over night. Each well was coated with separate coating antigens. Plates were washed 5 times with 0.05 M phosphate buffered saline tween-20 pH 7.2–7.4 (PBST). This washing procedure was repeated between all steps in the ELISA. Plates were blocked with 1% gelatin in PBST for 1 h in room temperature. From this point on the ELISAs were run using two different protocols depending on convenience. The two methods produced corresponding titers. Protocol 1: Plates were stored at 4 °C over night and various dilutions of sera in 0.05 M PBST were added to the wells the next day. Plates were incubated at room temperature for 2 h. The secondary antibody, an Fc-specific goat anti-rat IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted in 0.05 M PBST, was added and plates incubated at 4 °C over night. Protocol 2: Immediately after blocking, sera diluted in 0.05 M PBST were added and the plates incubated at 37 °C for 1 h. Plates were then incubated with the secondary antibody, diluted as above, at 37 °C for 1 h. For both protocols the enzyme substrate o-phenylenediamine (OPD) was used (SIGMAFAST™ tablet set, Sigma Life Science, S:t Louis, MO). After 30 min of incubation in the wells, 3 M HCl was added to stop the enzymatic reaction. Plates were read at 492 nm on a BioTek PowerWave XS (BioTek Instruments Inc., Winooski, VT) using the KC4 version 3.4 software. Titers were determined as the dilution resulting in 50% of the maximum absorbance. Total titers generated by trivalent vaccines were determined by numerically adding titers separately measured for each nicotine hapten. Antibody concentrations (Experiment 1) were assessed by ELISA using a standard curve of serum with known antibody concentration determined by radioimmunoassay [27].
2.1.4. Nicotine distribution
Nicotine distribution experiments were performed 7–10 days after the 3rd immunization. Rats were anesthetized with droperidol/fentanyl (3.3 mg/kg and 67 µg/kg, respectively) or ketamine/dexmedetomidine (100 mg/kg and 0.125 mg/kg, respectively). The method of anesthesia was kept the same within experiment. A jugular vein catheter was inserted and a blood sample for analysis of antibody titers was collected. 30 µg/kg nicotine ((−)-nicotine hydrogen tartrate salt, Sigma-Aldrich, St. Louis, MO) expressed as weight of the free base was diluted in sterile physiologic saline (pH adjusted to 7.2–7.4) and administered intravenously (i.v.) over 10 sec. Three min after nicotine administration, rats were decapitated and trunk blood and brains collected. Sera and brain samples were stored at −20 °C.
2.1.5. Nicotine assay
Serum samples were extracted according to the method of Jacob et al. [28] with the exception that 0.4 ml toluene/butanol (9:1) was added in the last extraction step. Brain nicotine was extracted according to the method of Hieda et al. [8]. In short: 0.5 g of thawed brain was cut into smaller pieces and 2.5 ml 2 M NaOH was added. Samples were vortexed and left on slow mixing over night at room temperature. To 1 ml of the digested brain, nicotine and cotinine internal standards (5-methyl nicotine bis-oxalate and (R,S)-orthocotinine) were added. After addition of 1 ml 1 M H2SO4, the pH was confirmed to be below four and the brain samples were extracted with 3 ml toluene/butanol (7:3). The aqueous phase was collected, 1 ml 2 M NaOH added and pH confirmed to be over nine. From this point the brain samples were treated according to the same protocol as the serum samples, starting from the first toluene/butanol extraction. The solvent layer was analyzed for nicotine using gas chromatography according to the method of Jacob et al. [28] modified for nitrogen–phosphorus detection. 1 µl of sample was injected in splitless mode at a port temperature of 250 °C. Samples were separated on a fused silica capillary column (Rtx®-5, Restek Chromatography Products, Bellefonte, PA) with initial oven temperature of 90 °C. After a 0.5 min hold the temperature was ramped up (25 °C/min) to the detector temperature of 285 °C. Results were analyzed using ChemStation software (Agilent Technologies, Santa Clara, CA).
2.1.6. Correction for blood brain content
The brain nicotine content was corrected for the contribution of blood nicotine in brain [29,30], using a volume fraction of 1.9% blood in brain [31].
2.2. Experiments
2.2.1. Experiment 1: 3′-AmNic-rEPA dose–response curve (Freund’s adjuvant)
The purpose of this experiment was to determine the relationship of 3′-AmNic-rEPA dose to antibody response. Groups of rats were immunized with 3′-AmNic-rEPA at doses of 25, 50, 100 or 200 µg in Freund’s adjuvant. Blood samples for assessment of antibody concentrations were collected 7 days after the 3rd immunization.
2.2.2. Experiment 2: 25 µg Monovalent vaccine vs. 75 µg trivalent vaccine (Freund’s adjuvant)
The purpose of the experiment was to investigate whether three immunogens could be combined in a trivalent vaccine using Freund’s adjuvant without compromising the immunogenicity of the separate components. Because doses of 25–200 µg 3′-AmNic-rEPA all produced comparable antibody levels in Experiment 1, the trivalent vaccine in a total immunogen dose of 75 µg was compared with 25 µg of monovalent vaccine. Groups of rats were immunized with either a trivalent vaccine consisting of 25 µg of each of the nicotine immunogens 3′-AmNic-rEPA, 6-CMUNic-KLH and 1′-SNic-KLH, resulting in a total nicotine immunogen dose of 75 µg, or a monovalent vaccine consisting of 25 µg 3′-AmNic-rEPA in combination with 50 µg KLH. The KLH was added in order to keep total protein concentration and protein to adjuvant ratio constant between groups. Controls were immunized with 25 µg rEPA and 50 µg KLH to match the proteins in the nicotine vaccine groups. Nicotine distribution was assessed 7–10 days after the 3rd immunization.
2.2.3. Experiment 3: Monovalent vaccine vs. trivalent vaccine–dose-matched (Freund’s adjuvant)
In order to confirm the benefit of using a trivalent vaccine seen in Experiment 2, the trivalent vaccine was compared to an equal dose monovalent vaccine. Rats were immunized with 24 µg or 75 µg of trivalent vaccine or 24 µg or 75 µg of monovalent 3′-AmNic-rEPA immunogen alone. The trivalent vaccine consisted of the three nicotine immunogens at doses of 8 µg or 25 µg each (resulting in total immunogen doses of 24 µg or 75 µg, respectively). Immunogens were administered in Freund’s adjuvant. Seven to ten days after the 3rd immunization, a subset of rats receiving total immunogen doses of 24 µg underwent nicotine distribution experiments; the rats not in that subset were sacrificed and trunk blood collected. Control rats for the nicotine distribution experiment were immunized with 8 µg rEPA and 16 µg KLH in Freund’s adjuvant.
2.2.4. Experiment 4a: Effect of adding KLH to monovalent vaccine (Freund’s adjuvant)
Because Experiments 2 and 3 showed contradictory results indicating interference between KLH and the monovalent vaccine or between immunogens in the trivalent vaccine, Experiment 4a studied whether the addition of unconjugated KLH would interfere with the immune response to a single nicotine immunogen. Rats were immunized with 25 µg of 3′-AmNic-rEPA, with or without 50 µg KLH, in Freund’s adjuvant. Nicotine distribution experiments were performed 7–10 days after the 3rd immunization.
2.2.5. Experiment 4b: Effect of adding KLH or BSA to monovalent vaccine (Freund’s or alum adjuvant)
This experiment was performed in order to explore whether the protein–protein interaction seen in Experiment 4a would extend to other proteins and adjuvants. 25 µg of 3′-AmNic-rEPA was administered alone or mixed with 50 µg BSA or 50 µg KLH, in Freund’s adjuvant or alum. The Freund’s formulations were mixed as above. For the alum formulations, the immunogens were mixed together and diluted in sterile physiological saline pH adjusted to 7.2–7.4 and then alum was added to a concentration of 1 mg Al(OH)3 per dose (corresponding to 0.35 mg Al). The pH was confirmed to be 6.5–7. Nicotine distribution experiments were performed 7–10 days after the 3rd immunization.
2.2.6. Experiment 5: Monovalent vaccine vs. trivalent vaccine–dose-matched (alum adjuvant)
Because Experiments 4a and b suggested that immunogen interference was reduced by using alum rather than Freund’s adjuvant, Experiment 5 was performed to compare the efficacy of a trivalent vaccine with a dose-matched monovalent vaccine in alum. In contrast with Experiment 4b, immunogens were separately adsorbed to alum before being mixed together in the trivalent vaccine in order to minimize the opportunity for interactions. In addition, a higher total dose of Al(OH)3 was used in this experiment to allow adequate alum for adsorption of each individual immunogen. 25 µg of each of the three adsorbed immunogens were mixed to form a trivalent vaccine with a total nicotine immunogen dose of 75 µg. The monovalent vaccine consisted of 75 µg of 3′-AmNic-rEPA in alum. Controls were immunized with 25 µg rEPA and 50 µg KLH in alum. All immunogens were diluted in PBS and a total Al(OH)3 dose of 1.4 mg (corresponding to 0.5 mg Al) was administered per immunization.
3. Results
3.1. Experiment 1: 3′-AmNic-rEPA dose-response curve (Freund’s adjuvant) (Table 1)
Table 1.
Antibody concentrations (mean ± SD) following 3 immunizations of 3′-AmNic-rEPA in different doses.
| 3′-AmNic-rEPA dose (µg) | 12.5 | 25 | 50 | 100 | 200 |
| Antibody concentration (µg/ml) | 220 ± 146 | 372 ± 176 | 306 ± 186 | 176 ± 78 | 216 ± 108 |
There were no significant differences in antibody concentrations between the groups receiving 25–200 µg of 3′-AmNic-rEPA in Freund’s adjuvant (Table 1, one-way ANOVA, p = 0.16). Increasing doses were not associated with increasing antibody concentrations.
3.2. Experiment 2) 25 µg monovalent vaccine vs. 75 µg trivalent vaccine (Freund’s adjuvant) (Fig. 2A, Fig. 3)
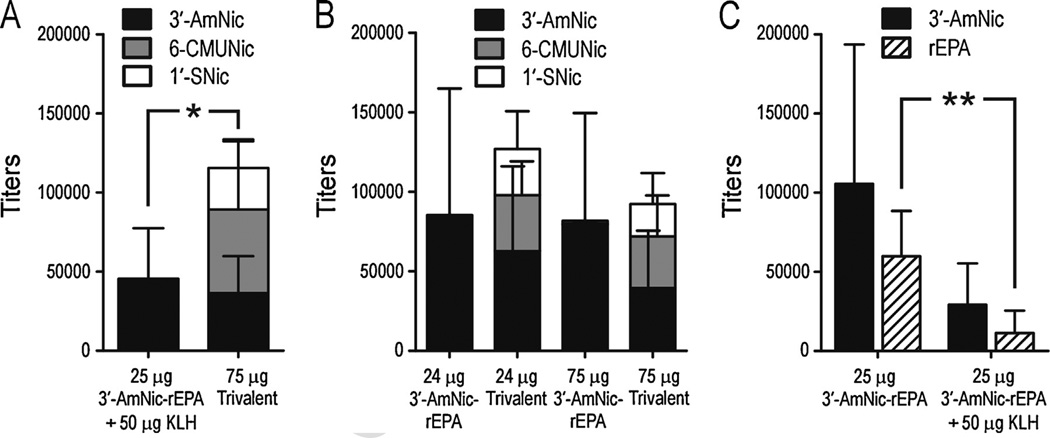
Fig. 2.
Antibody titers (mean ± SD) following immunization using Freund’s adjuvant. Nicotine hapten-specific antibody titers are shown in black (3′-AmNic), grey (6-CMUNic) and white (1′-SNic) bars and rEPA-specific titers in diagonal bars. Panel A shows higher total nicotine-specific antibody titers following immunization with 75 µg trivalent vaccine compared to 25 µg 3′-AmNic-rEPA in combination with KLH added to provide a comparable protein dose (p = 0.011, Student’s t-test). Panel B also compares monovalent and trivalent vaccines but differs from panel A in that the total immunogen doses were equal. There were no differences in total antibody titers at total doses of either 24 µg or 75 µg. Panel C shows that the addition of 50 µg KLH to 3-AmNic-rEPA significantly suppresses carrier-specific titers (p = 0.009, Student’s t-test) and trends towards a suppression of hapten-specific titers (p = 0.1, Student’s t-test).
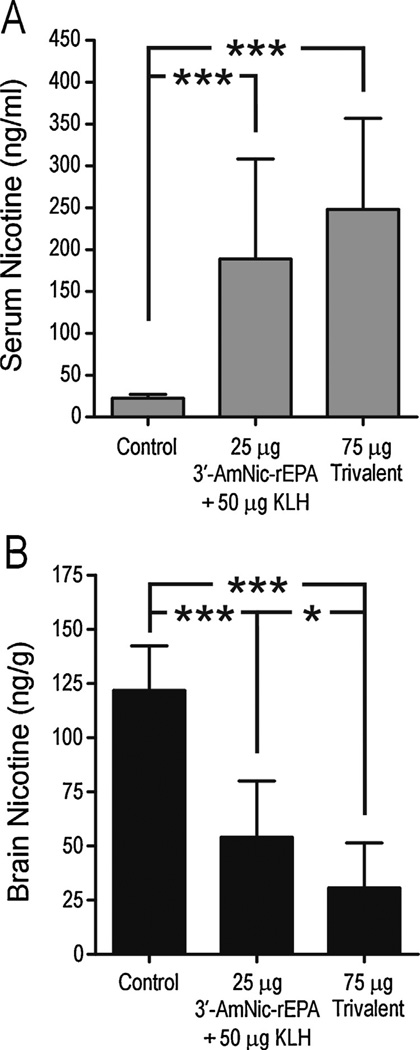
Fig. 3.
Serum and brain nicotine levels (mean ± SD) following a 30 µg/kg i.v. dose of nicotine in rats immunized with 25 µg 3′-AmNic-rEPA and 50 µg KLH or 75 µg trivalent vaccine with Freund’s adjuvant (for corresponding titers see Fig. 2A). The serum nicotine concentration was significantly increased and brain nicotine levels significantly decreased in immunized rats compared to controls. * p < 0.05, *** p < 0.001, one-way ANOVA followed by Bonferroni’s multiple comparison test.
Total antibody titers generated by the trivalent vaccine (25 µg of each immunogen) were significantly higher than those generated by the monovalent vaccine (25 µg 3′-AmNic-rEPA + 50 µg KLH, Student’s t-test, p = 0.011). The 3′-AmNic-specific antibody titers generated by the trivalent vaccine did not differ from the 3′-AmNic-antibodies generated by the monovalent vaccine (Fig. 2, panel A), and 6-CMUNic- and 1′-S-Nic-specific titers were of the same order of magnitude. There were significant correlations between antibody titers generated by the different immunogens in the trivalent vaccine (3′-AmNic titers vs. 6-CMUNic titers r2 = 0.93, p < 0.0001; 3′-AmNic titers vs. 1′-SNic r2 = 0.55, p = 0.006; 1′SNic vs. 6-CMUNic r2 = 0.57, p = 0.004).
Immunization with both the monovalent and trivalent vaccines significantly altered nicotine distribution, increasing serum nicotine concentrations and decreasing brain nicotine levels compared to controls (Fig. 3). There were no differences in serum nicotine concentrations between the immunized groups, but brain nicotine levels in the trivalent vaccine group were significantly lower than in the monovalent group. This experiment suggested an additive antibody response following immunization with 75 µg of trivalent vaccine in Freund’s adjuvant compared to 25 µg of monovalent vaccine with KLH added.
3.3. Experiment 3: Monovalent vaccine vs. trivalent vaccine–dose-matched (Freund’s adjuvant) (Fig. 2B, Fig. 4)
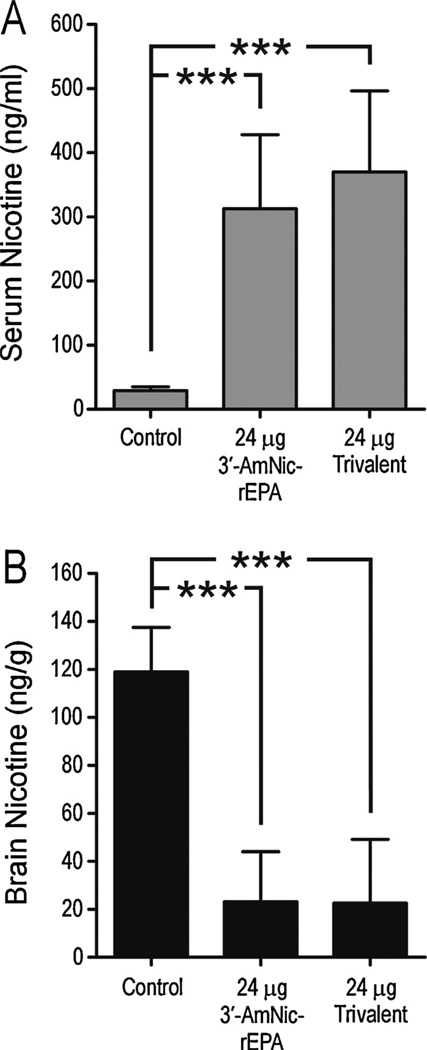
Fig. 4.
Serum and brain nicotine levels (mean ± SD) following a 30 µg/kg i.v. dose of nicotine in rats immunized with 24 µg 3′-AmNic-rEPA or 24 µg trivalent vaccine in Freund’s adjuvant. These animals represent a subset of the 24 µg immunogen dose groups in fig. 2B. The serum nicotine was significantly increased and brain nicotine levels were significantly decreased in immunized rats compared to controls. There was no difference between the monovalent and trivalent vaccine groups. *** p < 0.001, one-way ANOVA followed by Bonferroni’s Multiple Comparison Test.
The total antibody titers generated by the trivalent vaccine were not higher than the titers generated by the dose-matched monovalent vaccine at either total immunogen dose (Fig. 2, panel B). In the 24 µg trivalent group there were significant correlations between antibody titers generated by the different immunogens (3′-AmNic titers vs. 6-CMUNic titers r2 = 0.40, p = 0.001; 3′-AmNic titers vs. 1′-SNic r2 = 0.38, p = 0.002; 1′SNic vs. 6-CMUNic r2 = 0.57, p < 0.0001). In the 75 µg trivalent group, the only significant correlation was between antibody titers against the 6-CMUNic and 1′-SNic haptens (r2 = 0.60, p = 0.003). There were no correlations between 3′-AmNic and 6-CMUNic titers (r2 = 0.08, p = 0.38), or 1v-SNic titers (r2 = 0.27, p = 0.08).
Nicotine distribution experiments were performed in a subset of the rats receiving 24 µg trivalent or monovalent vaccines. The total antibody titers did not differ between groups (93 ± 70 × 103 and 86 ± 59 × 103 for the trivalent and monovalent groups, respectively). There was a significant increase in serum nicotine concentrations and a significant decrease in brain nicotine levels in both immunized groups compared to controls (Fig. 4). The monovalent and the trivalent vaccines altered the nicotine distribution to the same degree. This experiment showed no benefit of using a trivalent vaccine compared to a dose-matched monovalent vaccine when administered with Freund’s adjuvant.
3.4. Experiment 4a: Effect of adding KLH to monovalent vaccine (Freund’s adjuvant) (Fig. 2C, Fig. 5)
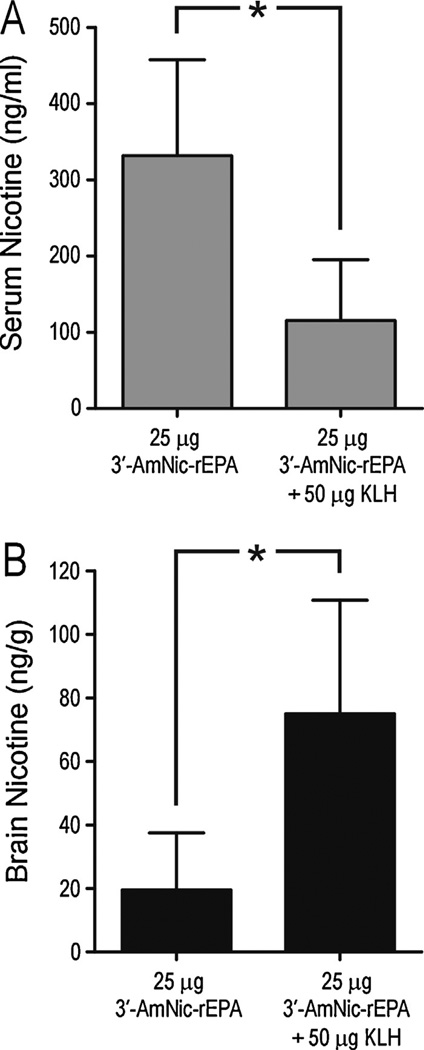
Fig. 5.
Serum and brain nicotine levels (mean ± SD) following a 30 µg/kg i.v. dose of nicotine in rats immunized with 25 µg 3′-AmNic-rEPA alone or in combination with 50 µg KLH in Freund’s adjuvant (for corresponding titers see fig. 2C). Addition of KLH to the immunogen suppressed the ability of the vaccination to result in retention of nicotine in serum (p = 0.012, Student’s t-test) or reduction of its distribution to brain (p = 0.015, Student’s t-test).
When KLH was added to 25 µg of 3′-AmNic-rEPA, there was a significant decrease in carrier protein specific antibodies compared with the 3′-AmNic-rEPA administered alone in Freund’s adjuvant (Fig. 2, panel C), and a non-significant trend toward a decrease in hapten specific antibodies (Student’s t-test, p = 0.1). In addition, there were large and significant differences in both serum and brain nicotine levels between the groups (Fig. 5) with lesser efficacy in the group receiving vaccine with added KLH. This confirmed that unconjugated KLH hampers the immune response directed towards the nicotine immunogen when administered with Freund’s adjuvant.
3.5. Experiment 4b: Effect of adding KLH or BSA to monovalent vaccine (Freund’s and alum adjuvant) (Table 2)
Table 2.
Antibody titers, serum and brain nicotine levels (mean ± SD) following immunization with 3′-AmNic-rEPA with or without added protein.
| Freund’s adjuvant, i.p. immunization | ||||
| Nicotine immunogen | Additional protein | 3′-AmNic titers × 103 | Serum nicotine (ng/ml) | Brain nicotine (ng/g) |
| 25 µg 3′AmNic-rEPA | None | 32 ± 24 | 233 ± 83 | 27 ± 17 |
| 50 µg BSA | 17 ± 6 | 177 ± 59 | 47 ± 21 | |
| 50 µg KLH | 24 ± 17 | 165 ± 97 | 48 ± 29 | |
| Alum, s.c. immunization | ||||
| Nicotine immunogen | Additional protein | 3'-AmNic titers × 103 | Serum nicotine (ng/ml) | Brain nicotine (ng/g) |
| 25 µg 3′AmNic-rEPA | None | 8.1 ± 5.2 | 153 ± 49 | 105 ± 28 |
| 50 µg BSA | 7.1 ± 5.0 | 144 ± 100 | 100 ± 42 | |
| 50 µg KLH | 4.3 ± 2.4 | 91 ± 29* | 144 ± 12* | |
p < 0.05 compared to 3′AmNic-rEPA alone.
This experiment was an extension of experiment 4a in order to explore whether the interference of KLH with 3′-AmNic-rEPA immunogenicity seen in that experiment would extend to other proteins and adjuvants. The effect of adding 50 µg BSA or KLH to 25 µg 3′-AmNic-rEPA in Freund’s or alum adjuvants was investigated. Following immunization with Freund’s adjuvant, there were great variations in titers, and no significant differences between the groups (Table 2). The magnitude of alteration of nicotine distribution corresponded with the titers of nicotine antibodies, and there was a trend toward increased brain nicotine levels (less effect on nicotine distribution to brain) in rats immunized with the nicotine immunogen combined with protein compared to the ones immunized with 3′-AmNic-rEPA alone (Student’s t-test, p = 0.09).
When using alum as adjuvant, titers overall were lower than with Freund’s, as expected. There were no differences in nicotine antibody titers or serum and brain nicotine levels between groups receiving 3′-AmNic-rEPA alone or in combination with BSA (Table 2). Co-administration of immunogen with KLH resulted in significantly lower serum and higher brain nicotine levels than when 3′-AmNic-rEPA was administered alone (Student’s t-test, p = 0.04 and p = 0.02, respectively). This experiment suggested a greater interaction of KLH than of BSA with 3′-AmNic-rEPA pharmacokinetic efficacy, and that interference may occur with either Freund’s or alum adjuvants.
3.6. Experiment 5: Monovalent vaccine vs. trivalent vaccine–dose-matched (alum adjuvant) (Figs. 6–8)
Fig. 6.
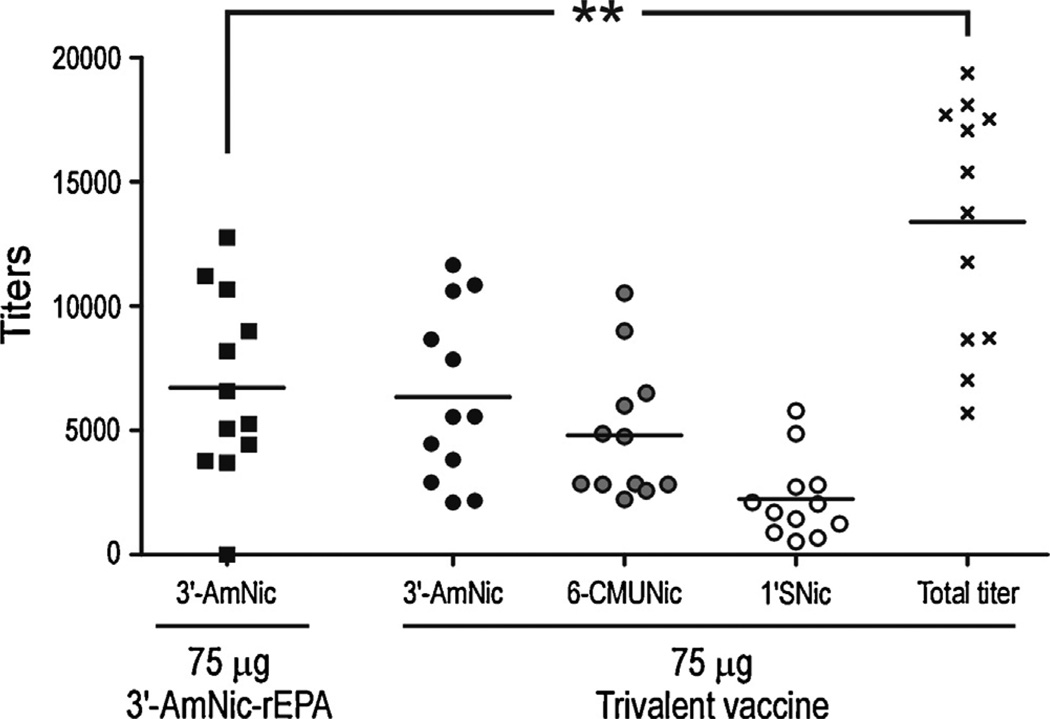
Antibody titers following immunization with 75 µg 3′-AmNic-rEPA or 75 µg trivalent vaccine in alum. Titers from the trivalent group are shown for each separate nicotine hapten (black circles, 3′-AmNic; grey circles, 6-CMUNic and white circles, 1′-SNic) and as total titers (stars, the sum of hapten specific titers measured for each serum). The total nicotine-specific antibody titer was higher following immunization with 75 µg trivalent vaccine compared to 75 µg 3′-AmNic-rEPA (p = 0.001, Student’s t-test).
Fig. 8.
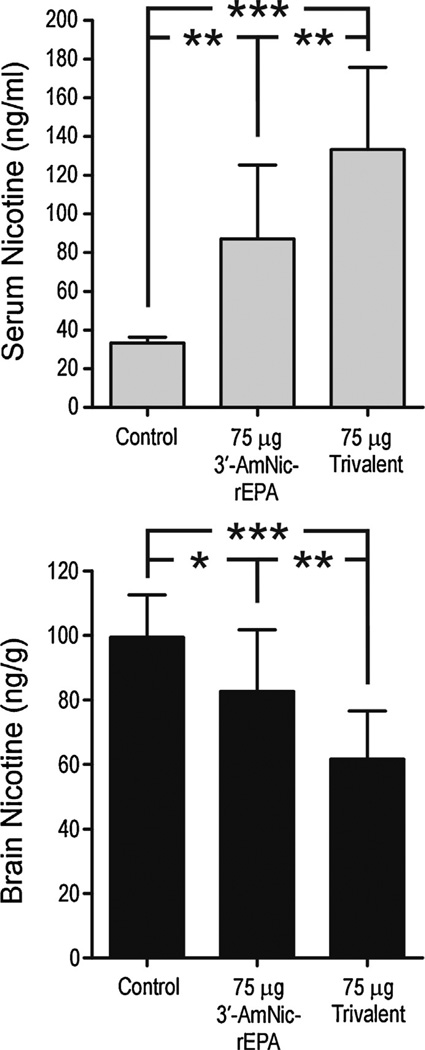
Serum and brain nicotine levels (mean ± SD) following a 30 µg/kg i.v. dose of nicotine in rats immunized with 75 µg 3′-AmNic-rEPA or 75 µg trivalent vaccine in alum. Nicotine distribution was significantly altered in immunized rats compared with controls, and the effects were greatest with the trivalent vaccine compared to 3′-AmNic-rEPA alone. * p < 0.05, ** p < 0.01, *** p < 0.001, one-way ANOVA followed by Bonferroni’s Multiple Comparison Test.
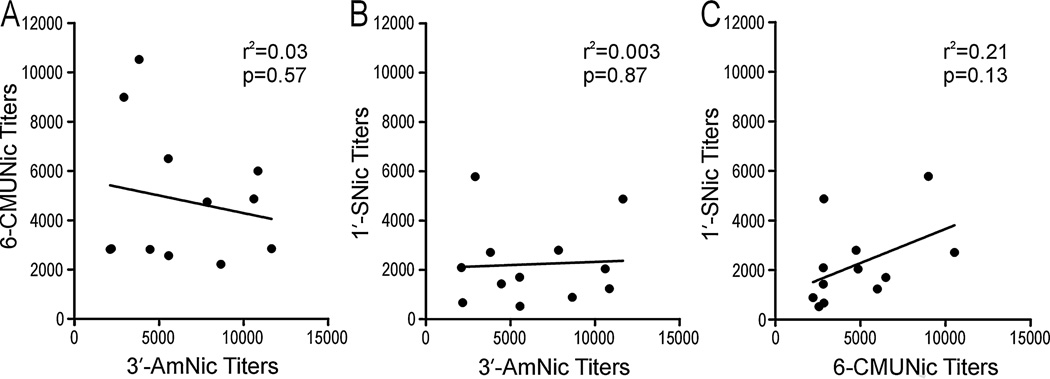
With evidence of immunogen inference when administered in Freund’s adjuvant i.p. and perhaps also alum adjuvant, vaccines were compared when administered in alum s.c. but with the three immunogens of the trivalent vaccine adsorbed separately in alum and then mixed together. The total titers of nicotine-specific antibodies generated by 75 µg of the trivalent vaccine (25 µg of each immunogen) were significantly higher than the titers generated by 75 µg of the monovalent vaccine (Student’s t-test, p = 0.0011, Fig. 6). The numbers of rats that responded with titers above 1:10 000, which in our lab is generally sufficient to alter nicotine distribution, was significantly higher in the trivalent group than in the monovalent group (8/12 and 3/12, respectively, Chi-squared test, p = 0.04). There were no correlations between the titers generated by the different immunogens in the trivalent vaccine (Fig. 7), indicating independent antibody responses. Both vaccinated groups had significantly altered serum nicotine concentrations (Fig. 8A) and brain nicotine levels (Fig. 8B) compared to the control group but effects were greatest in the trivalent group (1-way ANOVA followed by Bonferroni’s Multiple Comparison Test, p < 0.01 compared to the monovalent vaccine). This experiment showed that there was an advantage of using a trivalent vaccine instead of a monovalent vaccine when immunogens were separately adsorbed to alum.
Fig. 7.
There were no correlations between the hapten-specific antibody titers generated by the individual nicotine immunogens in the trivalent vaccine when alum adjuvant was used.
4. Discussion
The principal finding of this study was that a trivalent vaccine administered with alum generated significantly higher titers and prevented nicotine distribution to the brain to a greater extent than a dose-matched monovalent vaccine. In addition, the number of high titer responders was significantly greater in the trivalent group than in the monovalent group. This shows that the three nicotine immunogens, adsorbed separately to alum, can be mixed and administrated as a trivalent vaccine without compromising the immunogenicity of the separate components.
Raising the dose of monovalent vaccine beyond what was used in this study is unlikely to be helpful because the 3′-AmNic-specific antibody titers generated by 25 µg (part of the trivalent vaccine) and 75 µg of 3′-AmNic-rEPA (monovalent vaccine) were not different when vaccines were administered with alum adjuvant. This indicates that there is an advantage of using a trivalent vaccine instead of increasing the dose of monovalent vaccine, when using alum as an adjuvant. The functional significance of the increased antibody titers after immunization with the trivalent vaccine was confirmed by the significantly greater effect of the trivalent vaccine on nicotine distribution. There were no correlations between any of the hapten-specific titers generated by the trivalent vaccine, which is consistent with each of the immunogens generating independent responses from distinct B cell populations. This suggests that subjects that are low responders to one immunogen may still respond well to one or both of the others. In support of this, the number of high responders in the trivalent group was significantly greater than in the monovalent group. The large number of low responders in clinical trials has posed a considerable challenge [21–24]. If the nearly 3-fold higher number of high responders in the current study translates to the clinical situation, a trivalent nicotine vaccine could have a significant impact on smoking cessation.
Freund’s adjuvant is not acceptable for human use; however, it has been widely used for preclinical studies of nicotine vaccines. Initial experiments combining nicotine immunogens were performed using Freund’s adjuvant administered i.p. to correspond with our previous preliminary studies of this approach [26]. When using Freund’s adjuvant, results were seemingly contradictory in that the trivalent vaccine (75 µg in total, 25 µg of each immunogen) was superior to 25 µg 3′-AmNic-rEPA (Experiment 2) but not superior to 75 µg of the same monovalent immunogen in Experiment 3, even though the 25 and 75 µg doses of 3′-AmNic-rEPA had been shown to be equally immunogenic. We postulated that the KLH added to 3′-AmNic-rEPA in Experiment 2, to provide a total protein dose comparable to that of the trivalent vaccine, may have suppressed the response to monovalent 3′-AmNic-rEPA and given the mistaken impression that the trivalent vaccine enhanced overall immunogenicity. This was supported by findings that KLH added to 3′-AmNic-rEPA markedly reduced its immunogenicity (Experiment 4a). Although not statistically significant, trends in Experiment 4b suggested that this interference was not specific to KLH because antibody titers were also lower in animals receiving added BSA. In addition, the titers generated by 25 µg 3′-AmNic-rEPA as a component of the trivalent vaccine in Freund’s adjuvant (75 µg total dose) were lower than the titers generated by this immunogen alone. This difference suggests that, apart from the interference found when unconjugated protein was added, the conjugated immunogens in the trivalent vaccine may have also interfered with each other’s immunogenicity.
The reasons for the observed interferences when Freund’s adjuvant was used are not clear but may have been due to a protein–protein interaction, possibly related to increased concentration of immunogens in the vaccine formulation. Increased protein/immunogen concentration may either result in some kind of aggregation of proteins, masking antigen epitopes on the nicotine immunogen, or adversely alter the immunogen to adjuvant ratio. That might also provide an explanation to the correlations generally seen between the hapten-specific titers generated by the different nicotine immunogens in the trivalent vaccine when administered with Freund’s adjuvant, in contrast to when alum was used. An immunogen–immunogen or immunogen–protein interaction is likely to hamper the antibody response to all components of a multivalent vaccine and might result in a correlation between the titers against the different haptens.
Some interference was initially also observed with alum adjuvant, when the nicotine immunogen and KLH were mixed prior to being adsorbed to alum (Experiment 4b). However when immunogens were adsorbed separately to alum before mixing there was no interference and the trivalent vaccine was clearly more effective than the dose-matched monovalent vaccine. Freund’s adjuvant is a water-in-oil emulsion whereas alum is aggregated Al(OH)3 to which immunogens are adsorbed. It is likely that adsorption of immunogens to alum prevented their interaction and preserved their immunogenicity.
In summary, these data support the hypothesis that it is possible to combine structurally distinct nicotine haptens to get an additive immune response and greater efficacy than an equivalent dose of a single immunogen. This approach may be useful for other nicotine immunogens or vaccines for other addictive drugs as well. For example, a number of structurally distinct cocaine haptens [32–36], as well as morphine haptens [37–41] has been described. The cross-reactivities between these different haptens have not been fully characterized but it is possible that, like the nicotine haptens described, they activate distinct populations of B cells and could be combined in a multivalent vaccine. It is also possible that other nicotine immunogens which use the three linker positions described here but different carrier proteins or nanoparticle scaffolds [42–45] might also benefit from combined use. The use of a multivalent nicotine vaccine formulated with alum may potentially be a beneficial way to increase antibody levels and the number of responders in the clinical setting.
Acknowledgments
Funding
Supported by NIDA grant DA10714 and fellowship support (SdV) from the University of Minnesota/Karolinska Institutet/Mayo Clinic Frontiers in Biomedical Research Partnership. The funding sources had no involvement in study design, collection, analysis or interpretation of data, or writing of this report.
Footnotes
Conflict of interest
Authors have no conflict of interest.
Contributions
SdV was involved in designing Experiments 3–5, acquiring data for Experiments 3–5, analyzing and interpreting data from all experiments and writing the article. KEC was involved in designing Experiment 4, acquiring, analyzing and interpreting data for Experiment 4. AJT was involved in designing Experiment 1, acquiring, analyzing and interpreting data for Experiment 1. MP was involved in conjugating immunogens, designing Experiment 2, acquiring, analyzing and interpreting data Experiment 2. PRP was involved in designing all experiments and interpreting data from all experiments. All authors critically revised the article and approved its final form.
References
- 1.WHO. Geneva: World Health Organization; 2003. The World Health Report: 2003 Shaping the Future. [Google Scholar]
- 2.WHO. Geneva: World Health Organization; 2012. WHO fact sheet no. 339, may 2012. [Google Scholar]
- 3.US Department of Health and Human Services. Rockville, MD: Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health; 1988. The health consequences of smoking: nicotine addiction, a report of the surgeon general (publication no. CDC 88-8406) [Google Scholar]
- 4.Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. NEJM. 1999;340(9):685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob Res. 2008;10(4):717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- 6.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;3:CD006103. doi: 10.1002/14651858.CD006103.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Fagerstrom K, Hughes J. Varenicline in the treatment of tobacco dependence. Neuropsychiatr Dis Treat. 2008;4(2):353–363. doi: 10.2147/ndt.s927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hieda Y, Keyler DE, Vandevoort JT, Kane JK, Ross CA, Raphael DE, et al. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997;283(3):1076–1081. [PubMed] [Google Scholar]
- 9.Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, et al. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berlin) 1999;143(2):150–157. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- 10.Keyler DE, Hieda Y, st Peter J, Pentel PR. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res. 1999;1(3):241–249. doi: 10.1080/14622299050011361. [DOI] [PubMed] [Google Scholar]
- 11.Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22(10):809–819. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 12.Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65(1):191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 13.Cerny EH, Lévy R, Mauel J, Mpandi M, Mutter M, Henzelin-Nkubana C, et al. Preclinical development of a vaccine ‘against smoking’. Onkologie. 2002;25(5):406–411. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- 14.de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine alters the distribution of nicotine but not the metabolism to cotinine in the rat. Naunyn-Schmiedeberg’s Arch Pharmacol. 2004;370(4):299–304. doi: 10.1007/s00210-004-0960-3. [DOI] [PubMed] [Google Scholar]
- 15.Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, et al. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem Pharmacol. 2011;81(9):1164–1170. doi: 10.1016/j.bcp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, et al. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol. 2012;83(4):543–550. doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Malmerfelt A, Johansson AM, et al. Active immunization against nicotine suppresses nicotine-induced dopamine release in the rat nucleus accumbens shell. Respiration. 2002;69(3):247–253. doi: 10.1159/000063628. [DOI] [PubMed] [Google Scholar]
- 18.LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology. 2006;184(3–4):409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 19.Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol Pharma. 2010;7(2):431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- 20.Lindblom N, de Villiers SH, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration. 2002;69(3):254–260. doi: 10.1159/000063629. [DOI] [PubMed] [Google Scholar]
- 21.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonstad S, Heggen E, Giljam H, Lagerbäck PÅ, Tønnesen P, Wikingsson LD, et al. Niccine(R), a nicotine vaccine, for relapse prevention: a phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt003. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database of Syst Rev. 2012;8:CD007072. doi: 10.1002/14651858.CD007072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahim REF, Kessler PD, Kalnik MW. Therapeutic vaccines against tobacco addiction. Expert Rev Vac. 2013;12(3):333–342. doi: 10.1586/erv.13.13. [DOI] [PubMed] [Google Scholar]
- 26.Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol. 2008;8(11):1589–1594. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller R. Determination of affinity and specificity of anti-hapten antibodies by competitive radioimmunoassay. Methods Enzymol. 1983;92:589–601. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- 28.Jacob P, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3’,3’-d2 in humans. Biol Mass Spectrom. 1991;20(5):247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 29.Khor SP, Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling. Residual tissue blood. I. Theoretical considerations. Drug Metab Dispos Biol Fate Chem. 1991;19(2):478–485. [PubMed] [Google Scholar]
- 30.Khor SP, Bozigian H, Mayersohn M. Potential error in the measurement of tissue to blood distribution coefficients in physiological pharmacokinetic modeling. Residual tissue blood. II. Distribution of phencyclidine in the rat. Drug Metab Dispos Biol Fate Chem. 1991;19(2):486–490. [PubMed] [Google Scholar]
- 31.Triplett JW, Hayden TL, McWhorter LK, Gautam SR, Kim EE, Bourne DW. Determination of gallium concentration in blood-free tissues using a radiolabeled blood marker. J Pharm Sci. 1985;74(9):1007–1009. doi: 10.1002/jps.2600740922. [DOI] [PubMed] [Google Scholar]
- 32.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378(6558):727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 33.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Nat Acad Sci USA. 2001;98(4):1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berlin) 2000;148(3):251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 35.Hrafnkelsdottir K, Valgeirsson J, Gizurarson S. Induction of protective and specific antibodies against cocaine by intranasal immunisation using a glyceride adjuvant. Biol Pharm Bull. 2005;28(6):1038–1042. doi: 10.1248/bpb.28.1038. [DOI] [PubMed] [Google Scholar]
- 36.Danger Y, Devys A, Gadjou C, Galons H, Blanchard D, Follea G. Development of monoclonal antibodies directed against cocaine and cocaethylene: potential new tools for immunotherapy. Hybrid Hybridomics. 2004;23(4):212–218. doi: 10.1089/1536859041651286. [DOI] [PubMed] [Google Scholar]
- 37.Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;178(67):1290–1292. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- 38.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252(5485):708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 39.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24(16):3232–3240. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 40.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, et al. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54(14):5195–5204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, et al. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine. 2012;30(31):4617–4624. doi: 10.1016/j.vaccine.2012.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Villiers S. Thesis: active immunization against nicotine dependence. Stockholm: Karolinska Institutet; 2010. ISBN 978-91-7409-829-7 http://www.hdl.handle.net/10616/39817. [Google Scholar]
- 43.Kishimoto K, Altreuter D, Johnston L, Keller P, Pittet L. SEL-068 a fully synthetic nanoparticle vaccine for smoking cessation and relapse prevention; SRNT’s 18th Annual Meeting; 2012. [Google Scholar]
- 44.McCluskie MJ, Pryde DC, Gervais DP, Stead DR, Zhang N, Benoit M, et al. Enhancing immunogenicity of a 3′aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. Int Immunopharmacol. 2013;16(1):50–6. doi: 10.1016/j.intimp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg J, De B, Hicks M, Janda K, Kaminsky S, Worgall S, et al. Suppression of nicotine-induced pathophysiology by an adenovirus hexon-based anti-nicotine vaccine. Hum Gene Ther. 2013;24:595–603. doi: 10.1089/hum.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]