Abstract
The cyclic-nucleotide 3’,5’-cyclic AMP (cAMP) is an ancient and wide spread regulatory molecule. Previous studies have shown that fimbria production and secondary metabolite production are inhibited by cAMP in the prokaryote Serratia marcescens. This study used genetic manipulations to test the strain specificity of cAMP-CRP regulation of fimbria production and of the red pigment, prodigiosin. A surprising amount of variation was observed, as multicopy expression of the cAMP-phosphodiesterase gene, cpdS, conferred either an increase or decrease in fimbriae-dependent yeast agglutination and prodigiosin production depending upon the strain background. Mutation of crp, the gene coding for the cAMP-receptor protein similarly conferred strain-dependent phenotypes. This study shows that three distinct biological properties, modulated by a conserved genetic regulatory molecule, can vary significantly among strains. Such variation can complicate the functional analysis of bacterial phenotypic properties which are dependent upon global genetic regulators such as cAMP.
Keywords: genetic analysis, biofilm, secondary metabolite, opportunistic pathogen, pili
Introduction
The cyclic-nucleotide, cyclic 3’, 5’-AMP (cAMP), is a key global regulator in prokaryotes (Botsford and Harman 1992). It is generally made in response to environmental carbon and helps bacteria respond to their environment (Bruckner and Titgemeyer 2002). In the Enterobacteriaceae, levels of cAMP are controlled in part by extracellular glucose concentration, when glucose levels are low, adenylate cyclases are activated to catalyze generation of cAMP (Botsford and Harman 1992; Lory et al. 2004). Conversely, cyclic-AMP phosphodiesterases hydrolyze cAMP to maintain homeostasis (Richter 2002). The cAMP signal is bound by cyclic-AMP receptor protein (CRP), a transcription factor which can act as a positive or negative regulator of gene expression (Botsford and Harman 1992).
Previous studies have indicated that cAMP regulates many processes in the opportunistic pathogen Serratia marcescens. These include regulation of surface structures: positive regulation of flagella production through cAMP-CRP control of flhDC (Stella et al. 2008; Kalivoda et al. 2010), and negative regulation of fimbriae (type I pili) (Kalivoda et al. 2008). In this study “fimbriae” is used only to describe type I fimbriae derived from fimABCD genes (Labbate et al. 2007). Secondary metabolites, prodigiosin and serratamolide, are inhibited by cAMP-CRP (Kalivoda et al. 2010; Shanks et al. 2012b), as is the protease serralysin (Shanks et al. 2013b). This effect on secondary metabolite production is partially regulated through indirect control of the transcription factor PigP by cAMP-CRP (Shanks et al. 2013a). Similarly, the S. marcescens cAMP-phosphodiesterase gene (cAMP-PDE), cpdS, was recently identified and shown to regulate cAMP homeostasis, and fimbria production (Kalivoda et al. 2013). These studies were largely performed using S. marcescens isolate, PIC3611.
It was previously shown that the effect of cAMP on fimbria production by Escherichia coli varied from strain to strain (Eisenstein et al. 1981), and that S. marcescens isolates can exhibit phenotypic variation (Grimont and Grimont 1978). Therefore, we sought to determine whether the cAMP-mediated phenotypes of S. marcescens are well conserved among environmental, laboratory, and clinical isolates through genetic manipulation of genes involved in homeostasis of cAMP and cAMPmediated regulation. This study was designed to have relevance beyond the genus Serratia, as cAMP, the cAMP-receptor protein class of transcription factors, and pili are conserved among many Gram-negative bacteria. Whereas prodigiosin is produced by fewer organisms, this antibiotic pigment has been considered a model secondary metabolite representing secondary metabolites in general (Williams 1973; Coulthurst et al. 2004).
Materials and Methods
Bacterial strains and growth medium
bacteria were routinely grown in lysogeny broth (LB, per liter: 5 g yeast extract, 10 g tryptone, 5 g NaCl) (Bertani 2004). Where noted M9 minimal medium (Adams 1959) was supplemented with casein amino acids (0.06%) and either glucose or glycerol (0.2% for liquid medium and 0.4% for agar plates). Kanamycin was used at 100 µg / ml for S. marcescens and 50 µg / ml for E. coli; tetracycline and gentamicin were used at 10 µg / ml. Bacteria were grown at 30°C. Strains are described in Table 1. Bacteria were grown in test tubes on a New Brunswick TC-7 tissue culture rotor set to speed setting 8 (~62 rpm), and optical density was measured at 600 nm in a 1 cm cuvette for the cpdS multicopy expression experiments using a Molecular Devices Spectramax Plus spectraphotometer, and in a 96 well plate using 150 µl of culture broth for the crp mutants using a Biotek Synergy 2 plate reader.
Table 1.
Strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| S17-1 pir | thi pro hsdR hsdM+ ΔrecA RP4-2::TcMu-Km::Tn7 pir | (Miller and Mekalanos 1988) |
| S. marcescens | ||
| PIC3611 | Presque Isle Cultures strain number 3611 | (Shanks et al. 2007; Kalivoda et al. 2008; Kalivoda et al. 2013) |
| CMS1687 | crp, PIC3611 with crp-Δ4 null allele from pMQ260 | (Kalivoda et al. 2010) |
| CHASM | Pigmented Environmental Isolate | (Kalivoda et al. 2010) |
| Db11 | Non-pigmented insect pathogen | (Flyg et al. 1980) |
| Nima | Pigmented Laboratory Strain | (Goldschmidt and Williams 1968) |
| K904 | Pigmented contact lens clinical isolate | (Kalivoda et al. 2010) |
| CMS2859 | K904 with crp-Δ4 null allele from pMQ260 | This study |
| K912 | Non-pigmented keratitis isolate | Regis Kowalski |
| K945 | Non-pigmented keratitis isolate | Regis Kowalski |
| K1028 | Pigmented keratitis isolate | Regis Kowalski |
| K1469 | Non-pigmented keratitis isolate | Regis Kowalski |
| K1538 | Pigmented keratitis isolate | Regis Kowalski |
| K1739 | Pigmented keratitis isolate | Regis Kowalski |
| K1937 | Pigmented keratitis isolate | Regis Kowalski |
| K2019 | Pigmented keratitis isolate | Regis Kowalski |
| UC1SER | Non-pigmented isolate from neonate | (Morowitz et al. 2011) |
| Plasmids | ||
| pMQ131 | oripBBR1, aphA-3, oriT, URA3, CEN6/ARSH4 | (Shanks et al. 2009) |
| pMQ132 | oripBBR1, aacC1, oriT, URA3, CEN6/ARSH4 | (Shanks et al. 2009) |
| pMQ166 | pMQ131 + crp | (Kalivoda et al. 2010) |
| pMQ172 | pMQ132 + cpdS | (Kalivoda et al. 2013) |
| pEJK7 | pMQ131 + cpdA-His8 | (Kalivoda et al. 2013) |
| pMQ236 | allelic replacement vector oriR6K, I-SceI site, nptII | (Shanks et al. 2009) |
| pMQ240 | I-SceI expression vector, oripSC101ts, aacC1, I-SceI | (Shanks et al. 2009) |
| pMQ260 | pMQ236 + crp-Δ4 mutant allele | (Kalivoda et al. 2010) |
Plasmids, mutagenesis, and immunoblots
plasmids were transferred into S. marcescens by conjugation as follows. A 0.5 ml aliquot of an overnight culture of donor E. coli, strain S17-1 pir, with a plasmid was pelleted by centrifugation and the supernatant was removed. A 0.5 ml aliquot of acceptor, S. marcescens strain was added to the microfuge tube with the donor strain, pelleted by centrifugation, and the supernatant was removed. Fifty microliters of LB broth was added to the microfuge and the tube was placed at 42°C for 15 minutes. The bacteria were mixed by pipetting and were spotted on an LB agar plate that was incubated overnight at 30°C. The mixture was then streaked out on an LB plate containing tetracycline (10 µg / ml) to prevent growth of the donor strain, and either kanamycin (100 µg / ml) or gentamicin (10 µg / ml) were used to select for transformed S. marcescens.
The crp gene of K904 was replaced by the crp-Δ4 allele as previously described by allelic replacement (Kalivoda et al. 2010). The pMQ131 and pMQ132 vectors have a pBBR1 medium copy replicon and aphA-3 and aacC1 aminoglycoside resistance genes, respectively (Shanks et al. 2009). The pMQ131 vector was previously modified by the addition of crp to make pMQ166 (Kalivoda et al. 2008). The pMQ132 vector was previously modified by addition of cpdS to make pMQ172 (Kalivoda et al. 2013). Both cloned genes, crp and cpdS, were placed under transcriptional control of the E. coli Plac promoter.
To verify expression of genes from the Plac promoter on a pBBR1-based plasmid among the strains used in this study, PIC3611, CHASM, Nima, K904, K945, K1028, K1538, K1739, K1936, and K2019 were transformed with pEJK7. The pEJK7 plasmid has pMQ131 with a poly-histidine tagged version of cpdA, as a reporter, under control of the E. coli Plac promoter; the cpdA gene is the E. coli homolog of cpdS and is very similar in structure and in hydrolysis of cAMP (Kalivoda et al. 2013). S. marcescens PIC3611 with the vector was used as a negative control. Cultures where grown overnight in LB medium with kanamycin, adjusted to OD600 = 2.0, aliquoted into 100 µl samples, boiled in protein gel loading buffer (Sambrook et al. 1989), centrifuged, 15 µl samples were separated on a PAGE gel, and an immunoblot with anti-his-tag antibodies was performed as previously described (Shanks et al. 2012a). This experiment was performed on three days with consistent results.
Assays for fimbria and prodigiosin production
yeast agglutination to measure type I fimbria production was performed as previously described (Kalivoda et al. 2008). S. marcescens with fimbriae bind to Saccharomyces cerevisiae causing the yeast to agglutinate and fall out of suspension, thereby changing the optical density of the suspension. The change in optical density at 600 nm can be measured to determine the relative abundance of fimbriae for a given bacterial strain (Kalivoda et al. 2008). Bacteria were grown for 18–20 hours in LB broth, washed once with phosphate-buffered saline (PBS) and adjusted to OD600 = 1.0 in PBS. S. cerevisiae (Sigma product no. YSC2) was added to PBS (0.2 g / 10 ml). Bacteria (0.4 ml), yeast (0.5 ml), and PBS (1.5 ml) were combined in a 1 cm cuvette and shaken. The no bacteria control contains only yeast (0.5 ml) and PBS (1.9 ml). Cuvettes were placed in a spectrophotometer and the turbidity (OD600) was measured at 0 and 10 minutes using a Molecular Devices Spectramax Plus spectraphotometer.
Prodigiosin was measured as previously described (Kalivoda et al. 2010; Slater et al. 2003), by ethanol extraction of 18–20 hour cultures followed by centrifugation and measurement of absorbance at 534 nm with a 1 cm path length cuvette using a Molecular Devices Spectramax Plus spectraphotometer.
Statistical analysis
One-way ANOVA followed by Tukey’s pairwise comparison test and Student’s T-tests were performed using Graphpad Prism software.
Results
Mutation of the crp gene confers conserved and strain specific phenotypes
In an effort to expand our understanding of the role of cAMP in biofilm formation and secondary metabolite production by S. marcescens, a crp mutation was made in the ocular clinical isolate strain, K904, by allelic exchange as previously described (Kalivoda et al. 2010). Since crp is necessary for growth on glycerol as a sole carbon source in tested the Enterobacteriaceae including S. marcescens strain PIC3611 (Kalivoda 2008), we tested the K904 crp strain for growth in M9 minimal medium supplemented with glucose or glycerol. Both the previously characterized PIC3611 crp mutant and the K904 crp mutant were defective for growth in M9 glycerol (Fig. 1). Multicopy expression of the wild-type crp gene from PIC3611 from plasmid pMQ166 restored growth in glycerol, whereas the vector control, pMQ131 did not (Fig. 1). Strains behaved similarly with the vector control and without any plasmid (data not shown). These data indicate a conserved role for CRP in regulation of carbon utilization.
Figure 1. Culture density of crp mutants.
Single colonies were taken from LB agar plates and used to inoculate M9 medium (5 ml) supplemented with casein amino acids (0.06%) and either glucose (0.2%) or glycerol (0.2%). Bacteria were grown for 20 h at 30°C with shaking and the OD600 was measured. The mean and standard deviation of three independent cultures measured on two separate days is shown. p131 = pMQ131, the vector control, and p166 = pMQ166, the vector with the wild-type crp gene.
Type I fimbriae from S. marcescens can be measured indirectly by the yeast agglutination assay (Shanks et al. 2007; Kalivoda et al. 2008; Kalivoda et al. 2013). This convenient assay was used to assess the relative importance of cAMP in surface adhesin production for bacteria grown in LB medium. Mutation of crp in PIC3611 conferred an increase in fimbriae-induced yeast agglutination as previously reported (Kalivoda et al. 2008); a similar trend was observed with the K904 crp mutation (Fig. 2).
Figure 2. Yeast agglutination of crp mutants as a measure of fimbria production.
Bacteria were grown overnight, adjusted to OD600 = 1.0 and assessed for their ability to induce precipitation of S. cerevisiae. Total data from 3 separate experiments are shown representing 5–6 independent biological replicates, except for no bacteria that serves as a negative control (black bar), with 3 independent replicates. p131 = pMQ131, the vector control, and p166 = pMQ166, the vector with the wild-type crp gene. Mean value + SEM is shown. Asterisks indicate significant difference (p < 0.05) as assessed by ANOVA and Tukey’s pairwise comparison test.
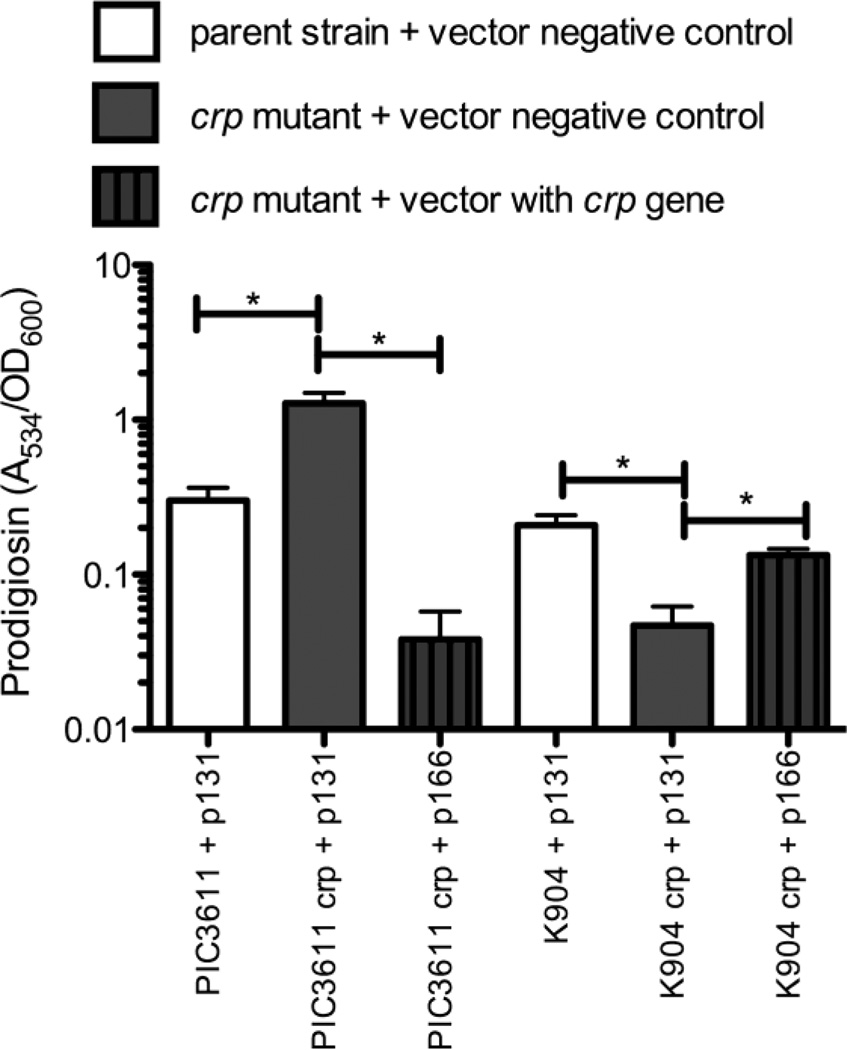
The red pigment associated with S. marcescens, prodigiosin, is a model secondary metabolite. Similar to a previous report, prodigiosin was elevated in the PIC3611 crp mutant (Kalivoda et al. 2010) (Fig. 3). The opposite phenotype was observed with the K904 crp mutant, where mutation of crp reduced pigmentation (Fig. 3).
Figure 3. Secondary metabolite production by crp mutants.
Bacteria were grown overnight in LB medium. Culture density was read at OD600, and prodigiosin was extracted with acidified ethanol and measured (A534). Data from three separate experiments are shown with 5–6 independent biological replicates. p131 = pMQ131, the vector control, and p166 = pMQ166, the vector with the wild-type crp gene. Mean value + SEM is shown. Asterisks indicate significant difference (p < 0.05) as assessed by ANOVA and Tukey’s pairwise comparison test.
Multicopy expression of the cAMP-phosphodiesterase gene, cpdS, confers strain specific phenotypes
Because of the opposing phenotypic prodigiosin phenotypes conferred by crp mutation between PIC3611 and K904, we decided to increase the breadth of the study by adding more strains. A rapid screening process for the effect of cAMP on various S. marcescens strains was developed. This approach involved expression of a cAMP-phosphodiesterase from a multicopy plasmid to decrease intracellular cAMP concentrations. This approach was previously used with strain PIC3611, where, multicopy expression of the cpdS using plasmid pMQ172 conferred a significant decrease in intracellular cAMP and a significant increase in biofilm formation and fimbria production that mimic crp mutant phenotypes (Kalivoda et al. 2013).
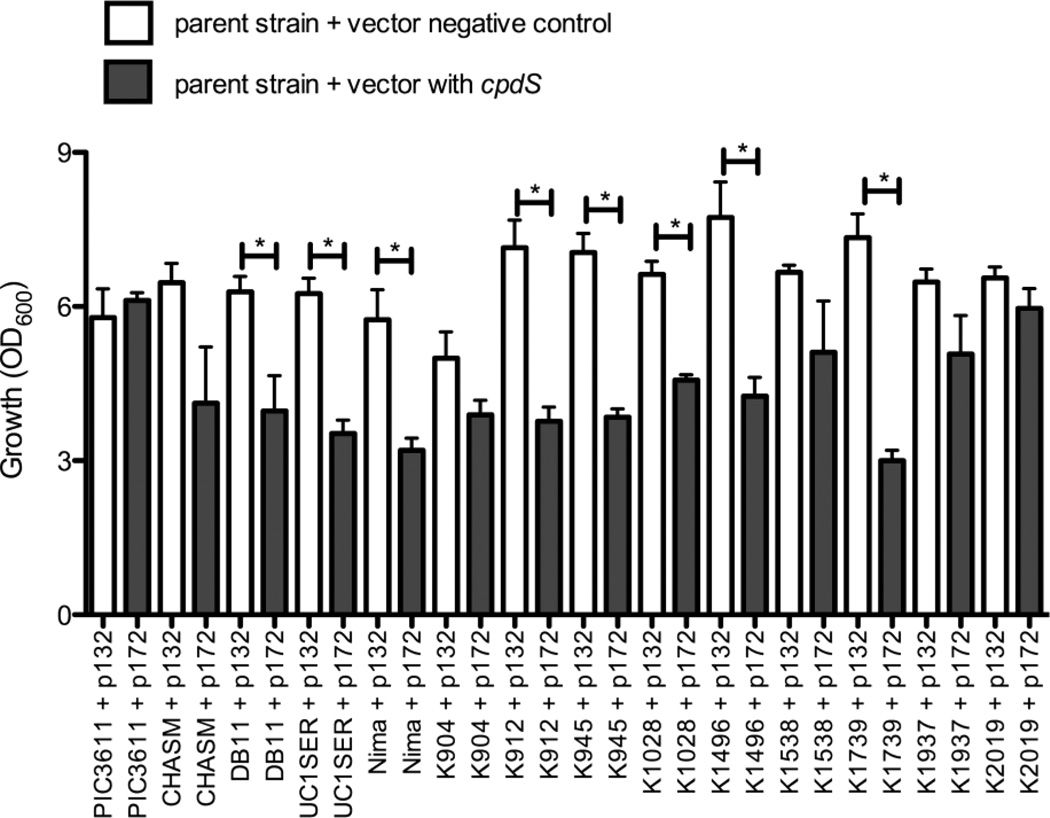
A number of laboratory, environmental, and clinical strains (Table 1), were transformed with pMQ172 and the vector control, pMQ132. Culture turbidity of these strains grown in LB was determined at 20 h. Whereas the multicopy cpdS plasmid did not affect growth of PIC3611, it inhibited growth of many of the other isolates compared to the same strain with the vector control (Fig. 4). As a control to verify expression of cAMP-PDE protein resulting from the plasmid-borne cAMP-PDE expression among these strains, PIC3611 and 9 arbitrarily chosen strains were transformed using pEJK7, which has a poly-histidine tagged version of the cpdS homolog from E. coli, cpdA. Immunoblot analysis supported that the tested strains had similar levels of cAMP-PDE protein (data not shown).
Figure 4. Culture density of strains expressing cAMP-PDE from a multicopy plasmid.
Bacteria were grown overnight in LB for 20 h at 30°C, and the optical density (OD600) was measured. Data from 4 separate experiments, with 3–4 independent biological replicates per strain. p132 = pMQ132, the vector control, and p172 = pMQ172, the vector with the wild-type cpdS gene. Mean value + SEM is shown. Asterisks indicate significant difference (p < 0.05) as assessed by ANOVA and followed by an unpaired two-tailed Student’s T-test.
Fimbriae-dependent yeast agglutination was increased in the PIC3611 strain with pMQ172 compared to the vector control, as previously shown (Kalivoda et al. 2013). Similarly, the K904 strain produced more yeast agglutination, consistent with the behavior of the crp mutant. Most strains did not show stimulation by pMQ172, and one strain was reproducibly reduced in yeast agglutination potential, K1937 (Fig. 5).
Figure 5. Yeast agglutination of strains expressing cAMP-PDE from a multicopy plasmid.
Bacteria were grown overnight, adjusted to OD600 = 1.0 and assessed for their ability to induce precipitation of S. cerevisiae. Total data from 3 separate experiments are shown representing 5–6 independent biological replicates per strain. No bacteria is the negative control (black bar). p132 = pMQ132, the vector control, and p172 = pMQ172, the vector with the wild-type cpdS gene. Mean value + SEM is shown. Asterisks indicate significant difference (p < 0.05) as assessed by ANOVA and followed by an unpaired two-tailed Student’s T-test.
Prodigiosin was measured from the pigmented strains, and it was elevated in strain PIC3611 with pMQ172 compared to pMQ132 (Fig. 6). Some strains, PIC3611, K904, Nima, and CHASM produced pigment in both liquid culture medium and on plates, and prodigiosin was measured from the liquid cultures from these strains (Figure 6). Other strains produced pigment only on agar plates (K1028, K1538, K1735, K1937, and K2019); these strains were compared qualitatively on LB agar plates (data not shown). Surprisingly, prodigiosin was severely reduced by pMQ172 in 6 of the 9 pigmented strains, with no red pigment being observed from any of the strains except Nima which exhibited reduced pigment with pMQ172, K904 which did not appreciably change, and PIC3611 which produced more prodigiosin.
Figure 6. Secondary metabolite production by strains expressing cAMP-PDE from a multicopy plasmid.
Bacteria were grown overnight in LB medium. Culture density was read at OD600, and prodigiosin was extracted with acidified ethanol and measured spectrophotometrically (A534). Data from 7 independent biological replicates from two experiments are shown. p132 = pMQ132, the vector control, and p172 = pMQ172, the vector with the wild-type cpdS gene. Mean value + SD is shown. Asterisks indicate significant difference (p < 0.05) as assessed by ANOVA and followed by an unpaired two-tailed Student’s T-test.
Discussion
Two genetic approaches, deletion of the crp gene and multicopy expression of a cAMPPDE gene were used to determine whether cAMP-CRP regulated phenotypes of S. marcescens are conserved among strains. Creation of a crp null mutation in a clinical strain confirmed the fundamental role of CRP in catabolite control, as the tested crp mutant strains were able to grow in minimal medium with glucose, but not glycerol, as a sole carbon source. The crp mutation in K904, similar to PIC3611 stimulated fimbria production as assessed by yeast agglutination. Surprisingly, mutation of crp increased prodigiosin production by PIC3611, but reduced it in strain K904.
It was observed that all strains expressing cpdS from pMQ172 achieved lower culture turbidity, with the exception of PIC3611. Multicopy expression of cpdS could be expected to reduce growth through reduction of cAMP and thereby reducing the metabolic repertoire of the strain. For the most part, type I fimbria production was not affected by pMQ172, with the exception of PIC3611 and K904 that produced elevated yeast agglutination, and K1937, which had the opposite phenotype. PIC3611 with multicopy cpdS produced more of the secondary metabolite, prodigiosin, than with the vector alone. However, unlike yeast agglutination where most strains were indifferent to multicopy expression of cpdS, all but PIC3611 produced less prodigiosin with pMQ172 than with the vector control.
The results here present an example of a surprising phenotypic variation across strains of a single species following manipulations of cAMP signaling. While the role of a specific protein or signaling system can be highly conserved across strains of Serratia and other genera (Labbate et al. 2007; Shanks et al. 2007; Shanks et al. 2013a), similar, seemingly opposite phenotypes elicited from the same stimulus or mutation have been observed. Notably, the example of cAMP-induced fimbriae differences among E. coli strains has been documented (Eisenstein et al. 1981). CRP can act as both a positive and negative regulator of different genes, so it is possible that it could act in the opposite manner in a strain-dependent fashion. However, our unpublished and published data indicate that CRP does not directly regulate the prodigiosin or fimbriae operons, but rather control these through intermediate transcription factors (Kalivoda et al. 2010; Shanks et al. 2013a). Therefore, it is reasonable to expect that a complex interplay of several signals are involved in control of these processes, of which cAMP-dependent signaling is only one. Additionally, factors such as phase variation and epigenetic factors may be different among strains.
Whereas there are clear instances of strain-dependent phenotypes following genetic manipulations between strains of a given species (Taylor et al. 1987; Guvener and McCarter 2003; Coulthurst et al. 2004; Muller et al. 2009; Zielinska et al. 2011), this phenomenon is likely to be more widely spread than is appreciated. There are examples of researchers utilizing multiple strains to test the importance of a particular factor in their process of interest (Fuchs et al. 2010; Murdoch et al. 2011; Zegans et al. 2012), but more often than not, a single laboratory strain is used thereby limiting the power of these studies. Nevertheless, the major implication of this study is that experiments performed on a single strain, no matter how elegant and thoroughly controlled, should not be used to make blanket statements about the behavior of a given species.
Acknowledgements
The authors thank Regis Kowalski for clinical isolates and Kimberly Brothers, Kristin Hunt, and Eric Kalivoda for critical reading of the manuscript. This work was funded by NIH grant AI5085570. Additional support was provided by NIH Core grant EY08098, the Eye and Ear Foundation of Pittsburgh, and unrestricted funds from Research to Prevent Blindness Foundation.
References
- Adams MH. Bacteriophages. New York, NY: Interscience Publishers Inc; 1959. [Google Scholar]
- Bertani G. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol. 2004;186:595–600. doi: 10.1128/JB.186.3.595-600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner R, Titgemeyer F. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett. 2002;209:141–148. doi: 10.1111/j.1574-6968.2002.tb11123.x. [DOI] [PubMed] [Google Scholar]
- Coulthurst SJ, Kurz CL, Salmond GP. luxS mutants of Serratia defective in autoinducer-2-dependent 'quorum sensing' show strain-dependent impacts on virulence and production of carbapenem and prodigiosin. Microbiology. 2004;150:1901–1910. doi: 10.1099/mic.0.26946-0. [DOI] [PubMed] [Google Scholar]
- Eisenstein BI, Beachey EH, Solomon SS. Divergent effects of cyclic adenosine 3',5'-monophosphate on formation of type 1 fimbriae in different K-12 strains of Escherichia coli. J Bacteriol. 1981;145:620–623. doi: 10.1128/jb.145.1.620-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EL, Brutinel ED, Klem ER, Fehr AR, Yahr TL, Wolfgang MC. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. J Bacteriol. 2010;192:2779–2790. doi: 10.1128/JB.00168-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont PA, Grimont F. Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol. 1978;8:73–83. doi: 10.1128/jcm.8.1.73-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvener ZT, McCarter LL. Multiple regulators control capsular polysaccharide production in Vibrio parahaemolyticus. J Bacteriol. 2003;185:5431–5441. doi: 10.1128/JB.185.18.5431-5441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivoda EJ, Brothers KM, Stella NA, Schmitt MJ, Shanks RM. Bacterial Cyclic AMP-Phosphodiesterase Activity Coordinates Biofilm Formation. PLoS One. 2013;8:e71267. doi: 10.1371/journal.pone.0071267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivoda EJ, et al. Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens. Res Microbiol. 2010;161:158–167. doi: 10.1016/j.resmic.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivoda EJ, Stella NA, O'Dee DM, Nau GJ, Shanks RM. The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl Environ Microbiol. 2008;74:3461–3470. doi: 10.1128/AEM.02733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbate M, et al. Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J Bacteriol. 2007;189:2702–2711. doi: 10.1128/JB.01582-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S, Wolfgang M, Lee V, Smith R. The multi-talented bacterial adenylate cyclases. Int J Med Microbiol. 2004;293:479–482. doi: 10.1078/1438-4221-00297. [DOI] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CM, Aberg A, Straseviciene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5:e1000303. doi: 10.1371/journal.ppat.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, Coulthurst SJ. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol. 2011;193:6057–6069. doi: 10.1128/JB.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W. 3',5' Cyclic nucleotide phosphodiesterases class III: members, structure, and catalytic mechanism. Proteins. 2002;46:278–286. doi: 10.1002/prot.10049. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shanks RM, Dashiff A, Alster JS, Kadouri DE. Isolation and identification of a bacteriocin with antibacterial and antibiofilm activity from Citrobacter freundii. Arch Microbiol. 2012a;194:575–587. doi: 10.1007/s00203-012-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RM, Kadouri DE, MacEachran DP, O'Toole GA. New yeast recombineering tools for bacteria. Plasmid. 2009;62:88–97. doi: 10.1016/j.plasmid.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RM, et al. A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS. PLoS One. 2013a;8:e57634. doi: 10.1371/journal.pone.0057634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RM, Stella NA, Arena KE, Fender JE. Mutation of crp mediates Serratia marcescens serralysin and global secreted protein production. Res Microbiol. 2013b;164:38–45. doi: 10.1016/j.resmic.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RM, et al. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol. 2007;189:7262–7272. doi: 10.1128/JB.00859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks RM, et al. Serratamolide is a Hemolytic Factor Produced by Serratia marcescens. PLoS One. 2012b;7:e36398. doi: 10.1371/journal.pone.0036398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater H, Crow M, Everson L, Salmond GP. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol Microbiol. 2003;47:303–320. doi: 10.1046/j.1365-2958.2003.03295.x. [DOI] [PubMed] [Google Scholar]
- Stella NA, Kalivoda EJ, O'Dee DM, Nau GJ, Shanks RM. Catabolite repression control of flagellum production by Serratia marcescens. Res Microbiol. 2008;159:562–568. doi: 10.1016/j.resmic.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegans ME, et al. Pseudomonas aeruginosa exopolysaccharide Psl promotes resistance to the biofilm inhibitor polysorbate 80. Antimicrob Agents Chemother. 2012;56:4112–4122. doi: 10.1128/AAC.00373-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska AK, et al. Defining the strain-dependent impact of the Staphylococcal accessory regulator ( sarA) on the alpha-toxin phenotype of Staphylococcus aureus. J Bacteriol. 2011;193:2948–2958. doi: 10.1128/JB.01517-10. [DOI] [PMC free article] [PubMed] [Google Scholar]