Abstract.
The aim of the present prospective study was to evaluate the impact of laser phototherapy (LPT) on the healing of oral ulcers. Different power densities were used on oral wounds in Wistar rats () randomly divided into three groups: control (), laser, and laser. Ulcers (3 mm in diameter) were made on the dorsum of the tongue with a punch. Irradiation with an indium-gallium-aluminum-phosphide laser (660 nm; output power: 40 mW; spot size: ) was performed once a day in close contact with the ulcer for 14 consecutive days. A statistically significant acceleration in healing time was found with wounds treated with LPT. Moreover, striking differences were found in the ulcer area, healing percentage, degree of reepithelialization, and collagen deposition. The most significant changes occurred after 5 days of irradiation. Based on the conditions employed in the present study, LPT is capable of accelerating the oral mucosa wound-healing process. Moreover, faster and more organized reepithelialization and tissue healing of the oral mucosa were achieved with an energy density of in comparison to .
Keywords: healing, repair, ulcer, laser, diode laser, phototherapy
1. Introduction
Oral ulcers, whether originating from traumatic, immunological, or other pathological processes, are one of the most common complaints involving the oral mucosa and can cause mild to severe pain. These lesions are characterized by damage to both the epithelium and connective tissue (lamina propria), which are usually repaired within weeks if the etiological factor is removed. The aim of treatment is to relieve the symptoms and accelerate the repair process, as patients with oral ulcers, such as recurrent aphthous stomatitis and mucositis, report a reduction in oral health-related quality of life.1,2
Wound healing is a dynamic and complex process that involves biochemical and physiological phenomena, and is made up of three main overlapping phases: inflammation, proliferation, and remodeling.3,4 Different therapeutic protocols, such as analgesics, corticosteroids, anti-inflammatory agents, and phytotherapy, have been tested to accelerate the wound healing process and reduce pain.5–10
The laser phototherapy (LPT) can be employed to modulate a number of biological processes in a phenomenon known as photobiomodulation.11,12 The LPT increases cell metabolism and is reported to induce analgesia, anti-inflammatory action, and tissue repair.13 Mester14 and collaborators were one of the first groups that describe enhanced healing and pain relief achieved with LPT. Studies involving this method of therapy report its effects on myofibroblasts, lymphoid cell proliferation,11,15,16 and collagen synthesis15,17 as well as its anti-inflammatory properties,18,19 neo-vascularization potential,20,21 and the release of growth factors.22 The effects of LPT on the healing process of skin wounds have been studied, particularly using the dorsum skin model to investigate the effect of different irradiation parameters.11,20,21,23–27A growing body of evidence suggests that LPT has anti-inflammatory action and accelerates tissue repair.18,19,21,22 However, these effects are dependent on irradiation parameters such as wavelength, output power,28,29 and energy density.15,28 Moreover, the same parameters can have different effects on different cell types.16
The impact of LPT on wound healing of the oral mucosa is not completely understood. It is therefore of paramount importance to determine the correct combination of parameters to achieve desirable effects regarding the healing of oral ulcers. The aim of the present study was to evaluate the effects of different LPT energy densities on clinicopathological aspects of oral ulcers. Herein, the present study showed the acceleration in the healing process, as demonstrated by clinical and histological findings, in function of the laser energy density protocol, with achieving better results.
2. Methods
2.1. Animal Model
All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals30 and received approval from the ethics committee of the Porto Alegre University Hospital (Brazil) under the process number 12-0338. Seventy-two male Wistar rats weighing 150 to 200 g were kept under standard conditions of temperature (20 to 24°C) and light/dark cycle with solid chow and water ad libitum. The animals were randomly divided into three cohorts of 24 animals each: control group (), laser group, and laser group.
2.2. Wound Procedure
Under aseptic conditions, the three groups were anesthetized with an intraperitoneal administration of ketamine () and xylazine (). Traumatic ulcers measuring 3 mm in diameter were made on the dorsum of the tongue using a standard punch biopsy technique.
2.3. Laser Irradiation
Laser irradiation was delivered with a continuous-wave indium-gallium-aluminum-phosphide (InGaAlP) diode laser (MM Optics Ltd., São Carlos, SP, Brazil) with a spot size of , operating at a wavelength of 660 nm and an output power of 40 mW in punctual and contact modes. Irradiation was performed perpendicularly to the mucosa in two points with 3-mm distance to each other in the two opposite borders of the ulcer. The energy densities were (energy per point of 0.16 J, total energy of 0.32 J) and (energy per point of 0.8 J, total energy of 1.6 J) with respective exposure times of 4 and 20 s on each point of the ulcer. The LPT was applied after the immediately the wound procedure. The same investigator applied the LPT once a day on the wounds under isoflurane inhalant anesthesia during 14 consecutive days. The control group was treated under identical conditions but with the laser equipment switched off.
2.4. Clinical Analysis
Six rats in each group were euthanized using a chamber on days 1, 5, 10, and 14 after the surgical procedure. The wounds were measured and photographed. Ulcer area was calculated based on the length and width using a digital caliper. These measurements were multiplied for the determination of area in square centimeters. The percentage of wound healing and healing time were recorded, as described elsewhere.31 Briefly, percentage of healing was calculated as [(initial area − area at respective evaluation time)/(initial area)] .
2.5. Histopathological Analysis
After euthanasia, the tongues were removed and fixed in a 10% buffered formalin solution for 48 h. After washing with water, the specimens were dehydrated and embedded in paraffin. Slices measuring 5 μm in thickness were obtained and stained with hematoxylin-eosin.
Descriptive analyses of each group/evaluation time were performed, followed by a semi-quantitative analysis. The degree of reepithelialization was determined by a grading system (0 to 4), as described elsewhere:32 Grade on the margins of the wound; Grade covering less than half of the wound; Grade covering more than half of the wound; Grade covering the entire wound with irregular thickness; and Grade covering the entire wound with normal thickness. Inflammation was also evaluated using a grading system as described elsewhere:33,34 Grade inflammation (pyogenic membrane); Grade of acute diffuse inflammation; Grade of chronic inflammation (fibroblast proliferation); and Grade and healing (reduction in or disappearance of chronic inflammation, despite the persistence of some inflammatory cells).
2.6. Picrosirius Red Staining for Collagen
Picrosirius red staining was used to analyze the collagen fibers. When examined under polarized light, larger collagen fibers appear strongly birefringent and orange or red in color, whereas thin collagen fibers are weakly birefringent and appear greenish in color. This birefringence is highly specific to collagen.23
2.7. Statistical Analysis
The data were expressed as mean and standard deviation values. The SPSS version 18.0 was employed for the statistical analyses. Groups, evaluation times, and the interaction between group and evaluation time were compared using the generalized estimating equation, followed by a post-hoc Bonferroni correction when necessary. The significance level was set at 5% (). The Kappa coefficient was calculated to determine inter-examiner agreement regarding the scores of reepithelialization and inflammation.
3. Results
3.1. Clinical Analysis
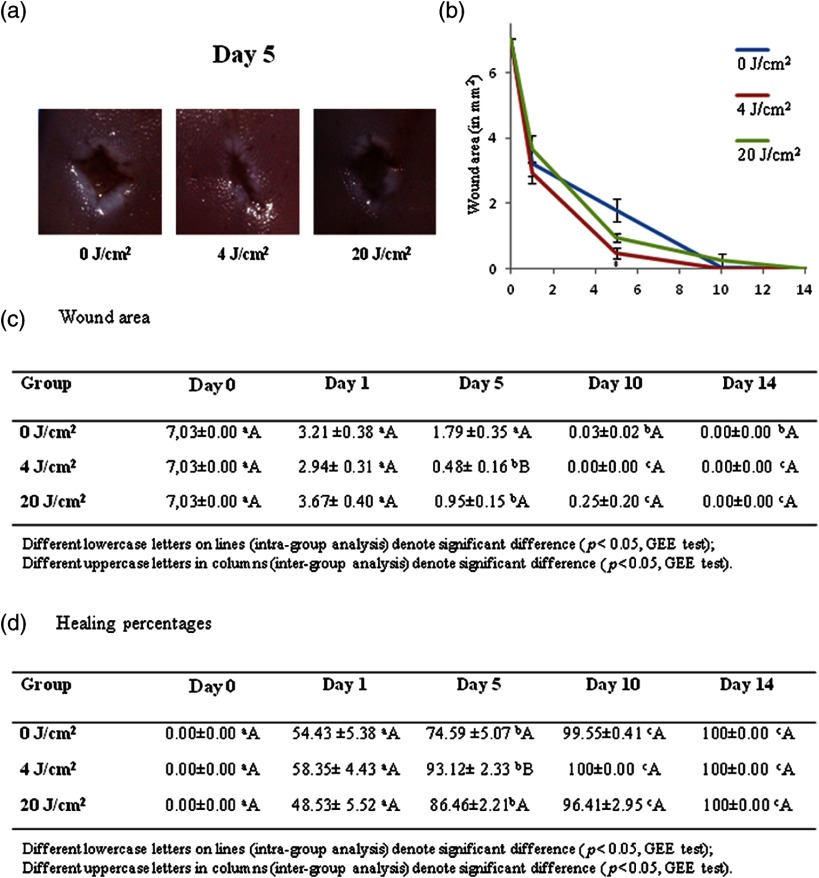
No differences were found in wound area, and no detectable signs of repair were evident on day 1. A decrease in mean wound area was found in all groups on day 5, with the smallest area in the laser group, followed by the 20 and laser groups [Fig. 1(a)]. On day 10, all animals in the laser group exhibited repaired lesions, whereas this clinical situation was only observed at the end of the experiment (day 14) in the other groups. No statistically significant differences were found among groups on days 10 and 14 [Figs. 1(b) and 1(c)].
Fig. 1.
(a) Clinical aspect of oral ulcers in different groups on day 5, smaller ulcer area in group; (b and c) Clinical evaluation of mean and standard error of area (in ); and (d) Clinical evaluation of percentage of wound healing (%).
Figure 1(d) displays the percentages of wound healing. No significant differences among groups were found on day 1. The laser group exhibited a significant increase in the percentage of healing on day 5. On days 10 and 14, most of the wounds were healed and no significant differences were found among the groups at these evaluation times.
3.2. Histopathological Analysis
3.2.1. Descriptive analysis
Exposure of connective tissue was observed on day 1. Discrete or no migration of epithelial cells in the center of the wound was found in all groups. Intense diffuse acute inflammation (polymorphonuclear infiltrate) and red blood cells were noticed in the wound area.
On day 5, reepithelialization covering more than half of the wound was found in the group. Some focal points of acute inflammation were still present in most of the animals, but the number of mononuclear cells was higher than that on day 1. Notably, all animals in the laser group exhibited full reepithelialization at this time. Granulation tissue was evident, and no focal points of acute inflammation were found. Mononuclear infiltrate, neovascularization, and fibroblast proliferation were evident. Collagen fibers were predominantly organized and paralleled to the basal layer of the epithelium. The laser group exhibited reepithelialization covering more than half of the wound in some animals and covering the entire wound with irregular thickness in other animals. Inflammatory infiltrate revealed the formation of granulation tissue in most of the wound beds, and focal points of acute inflammation still occurred in some cases.
On day 10, all groups exhibited reepithelialization covering the entire wound. The control group () exhibited epithelium with irregular thickness, whereas the 4 and laser groups exhibited new epithelium with normal thickness. The formation of granulation tissue was evident in all groups, and rare polymorphonuclear cells were observed.
On day 14, resolution and healing of the wound were observed. Reepithelialization covering the entire wound with normal thickness was observed in all groups as well as a reduction in or the disappearance of chronic inflammation.
3.2.2. Degree of reepithelialization
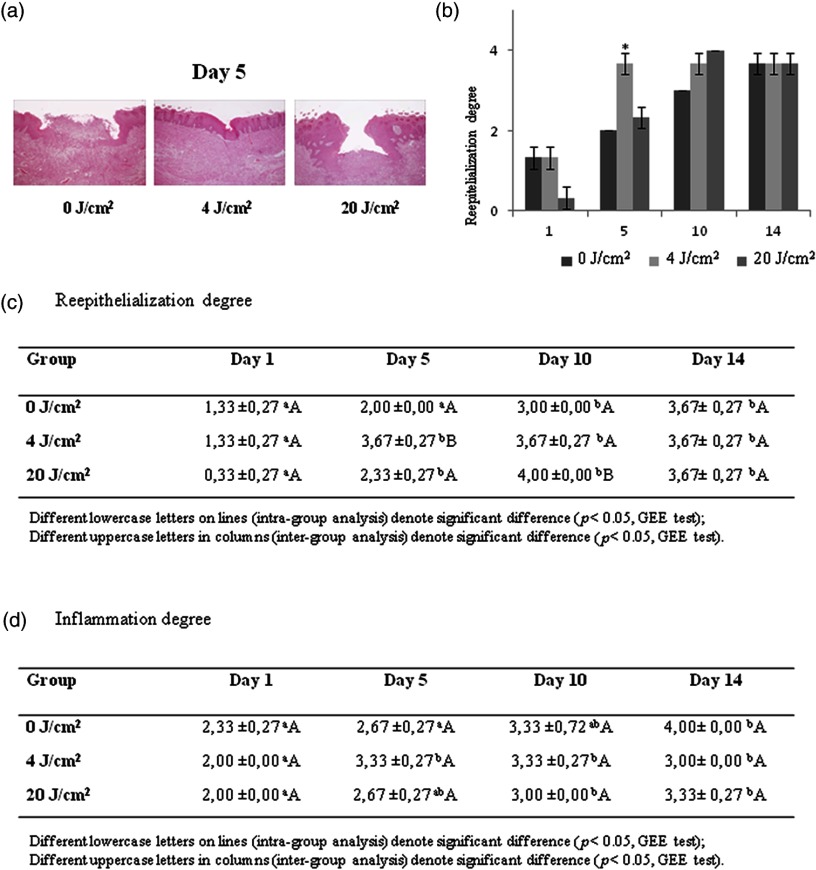
The Kappa coefficient for inter-examiner agreement regarding the degree of reepithelialization was 0.91, indicating a high level of agreement. On day 5, the group exhibited a significantly greater degree of reepithelialization, whereas the 0 and laser groups exhibited similar degrees of connective tissue exposure. On day 10, the laser group exhibited a significantly greater degree of reepithelialization [Figs. 2(a)–2(c)].
Fig. 2.
(a) Photomicrographs of experimental groups on day 5, reepithelialization covering entire wound and more chronic inflammatory infiltrate in the group [hematoxylin-eosin; magnification: (a, c, and e) and (b, d, and f)]; (b and c) Histopathological evaluation of degree of reepitelialization (mean and standard error); and (d) Histopathological evaluation of degree of inflammation (mean and standard error).
3.2.3. Degree of inflammation
The Kappa coefficient for inter-examiner agreement regarding the degree of inflammation was 0.96, indicating a high level of agreement. Although variations were found in the pattern of inflammatory infiltrate, no statistically significant differences were found among the groups [Fig. 2(d)].
3.2.4. Organization and distribution of collagen fibers
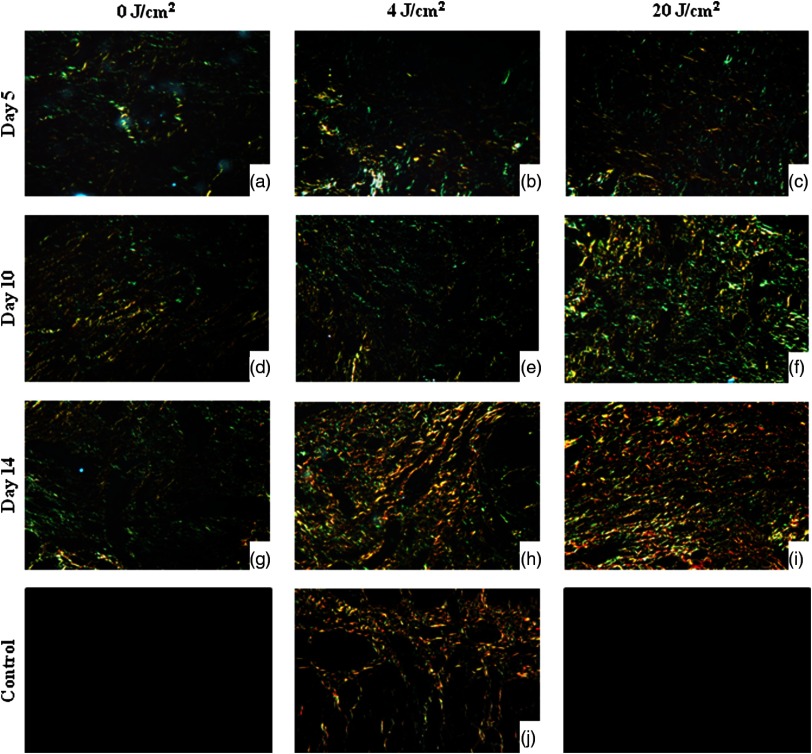
On day 1, Picrosirius red staining revealed no red or green birefringence, indicating the absence of collagen. The wounds were filled with blood clots. On day 5, all groups displayed thin collagen fibers (characterized by green birefringence), which were poorly organized. In the groups treated with laser, some denser collagen fibers, which appeared reddish in color, were noted in the wound. These fibers were parallel to the epithelium and demonstrated a more organized pattern in comparison to the control group. On day 10, collagen fibers had begun to rearrange into bundles among the new muscle fibers in all three groups. On day 14, all groups exhibited organized collagen bundles mainly with red birefringence around the perimysium. The groups treated with laser exhibited an organization pattern similar to normal tongue mucosa, with a predominance of reddish fibers organized parallel to the epithelium in the adjacent lamina propria and bundled in the submucosa area around the muscle fibers (Fig. 3)
Fig. 3.
Photomicrographs of Picrosirius red staining in experimental groups on days 5, 10, and 14 and in normal mucosa (original magnification ). On day 5, all groups displayed poorly organized thin collagen fibers (characterized by green birefringence) (a, b, and c). On day 10, no difference was observed between all groups. Collagen fibers were begun to rearrange into bundles among the new muscle fibers (d, e, and f). On day 14, the groups treated with laser (h and i) exhibited an organization pattern similar to the normal oral mucosa (j) with a predominance of reddish fibers organized parallel to the epithelium. Control group revealed a more immature collagen (g).
4. Discussion
The effects of LPT on the healing process of skin wounds have been studied with different irradiation parameters.11,20,21,24–29 A growing body of evidence suggests that LPT has anti-inflammatory action and accelerates tissue repair.18,19,21,22 However, these effects are dependent on wavelength, output power,28,35 and energy density.15,28 Moreover, the same parameters can have different effects on different cell types, and few studies have been conducted to analyze the influence of LPT on the wound-healing process involving the oral mucosa.36 It is therefore of paramount importance to determine the correct combination of parameters to achieve desirable effects on oral ulcers.
In the present investigation, two different energy densities were tested (4 and ) with the same wavelength, spot size, power output, irradiation frequency, and interval between irradiations. Moreover, both methods were performed in contact with the ulcer and using the punctual irradiation mode. The choice of testing two very different energy densities was based on the previous studies, where better results regarding wound healing with smaller energy densities were observed in comparison to larger energy densities.15,20,24,27,37,38 Indeed, such findings followed the Arndt–Schultz law, which states that mild stimulus excites physiological activity, whereas strong stimulus can inhibit such activity.39 Therefore, was used as the low-energy density, and was used as the high-energy density in the present study. The dose has been widely used in clinical practice and studied in clinical trials.40,41 However, few clinical studies have been conducted analyzing higher doses of LPT.42,43 Other important aspects regarding the laser parameter are the energy per point that seems to be relevant because it considers the output power and the time of tissue exposure to laser, improving the study reproducibility. In our study, the laser group with 0.16 J of energy per point showed better results in oral wound healing than the laser group with 0.8 J of the total energy.
The in vivo results demonstrated that LPT at the same power density induced the acceleration of the healing process as a function of energy density. Irradiation with 660 nm, 40 mW, and accelerated the healing of oral ulcers in comparison to 0 and . On day 5, the laser group exhibited positive clinical behavior with a greater decrease in the mean area of the oral ulcers and higher percentages of healing in comparison to the control and higher energy density laser groups. Moreover, the histopathological analysis revealed a more advanced stage of repair in the laser group on day 5. This finding is supported by the degree of reepithelialization, with the epithelium covering the entire wound in all animals, and by the histopathological aspect of the connective tissue. Chronicity of the inflammatory infiltrate was also noticed in this group on day 5, whereas the 0 and laser groups still exhibited focal areas of acute inflammation. These histopathological aspects may be associated with the clinical finding of a reduction in ulcer area and greater percentage of healing on day 5, demonstrating that the irradiation with led to the faster and more organized healing pattern. Similar results have been obtained for excisional wound healing in mice, in which a biphasic relationship has been found with positive effects at and an inhibitory effect at .44 However, studies have predominately attributed the inhibitory effect to higher power levels rather than energy density per se.45
It is difficult to compare the present findings with observations of wound healing in the literature, as most studies were performed using different tissue models, such as skin, which has a different repair process than the oral mucosa healing. Furthermore, many studies only report the power and wavelength of the laser irradiation, while energy density is not mentioned and cannot be calculated due to the absence of other important parameters such as spot size and irradiation time. Nonetheless, an increase in energy density has been shown to impair wound healing in vivo.20,44
The LPT with for skin wound repair has demonstrated a decrease in polymorphonuclear infiltrate and neovascularization21,24 as well as greater deposition of collagen24,26,27 and elastic fibers.27 The irradiation with can promote a greater number of newly formed epithelial layers,27 and the irradiation with 3 and is capable of increasing the growth rate of cultured epithelial cells.46 Moreover, mononuclear infiltrate, neovascularization, and fibroblast proliferation were evident in 5 days when the tissue was irradiated with the . This was expected since other authors have shown increased production of growth factors, such as bFGF, as a result of red laser irradiation with small energy densities.47,48 Additionally, the irradiation with for wound healing induces the formation of more fusiform cells expressing desmin and alpha-smooth muscle actin,24 decreases the levels of mRNA,25 increases populations of intact and degranulated mast cells,21 type I collagen, and fibronectin deposition,26 and enhances synthesis activity.29
Low-intensity laser irradiation is absorbed by cellular photosensitizers, such as cytochromes and flavins, which promote a cascade event that results in flux, affecting the levels of cyclic nucleotides, interfering with DNA and RNA syntheses, and modulating cell proliferation.49 A further increase in dose inducts cellular antioxidant activity and can cause the destruction of photoreceptors, which is accompanied by growth inhibition and cell lethality, as expected from the Arndt–Schultz law.39,49
Overall, LPT has been found to accelerate wound healing as well as to reduce pain and the inflammatory response. In the present study, LPT with the energy density of and the other parameters applied caused important oral mucosa wound-healing effects, accelerating two of the most substantial healing phases: inflammation and proliferation. This protocol decreases acute inflammation and induces reepithealization, fibroplasia, and granulation tissue formation. However, the correlation between the results obtained with this animal model and clinical outcomes remains to be established. Therefore, care should be taken before extrapolating these results to clinical practice without additional testing.
5. Conclusion
Based on the conditions employed in the present study, LPT using red laser (660 nm) and an output power of 40 mW is capable of accelerating the oral mucosa wound-healing process. Moreover, faster and more organized reepithelialization and tissue healing of the oral mucosa were achieved with an energy density of in comparison to .
Acknowledgments
The authors are grateful to the Postgraduate Research Group of the Porto Alegre University Hospital (GPPG/FIPE: 12-0338) and the National Institutes of Health (NIH/NCI) P50-CA97248 partially for funding this study.
References
- 1.Hapa A., et al. , “Does recurrent aphthous stomatitis affect quality of life? A prospective study with 128 patients evaluating different treatment modalities,” J. Dermatol. Treat. 22(4), 215–220 (2011). 10.3109/09546631003675450 [DOI] [PubMed] [Google Scholar]
- 2.Cheng K. K., et al. , “Severe oral mucositis associated with cancer therapy: impact on oral functional status and quality of life,” Support Care Cancer. 18(11), 1477–1485 (2010). 10.1007/s00520-009-0771-7 [DOI] [PubMed] [Google Scholar]
- 3.Mandelbaum S. H., Di Santis E. P., Mandelbaum M. H. S., “Cicatrization: current concepts and auxiliary resources—part I,” An. Bras. Dermatol. 78(4), 393–408 (2003) (in Portuguese). [Google Scholar]
- 4.Mendonça R. J. D., Coutinho-Netto J., “Cellular aspects of healing,” An. Bras. Dermatol. 84(3), 257–262 (2009) (in Portuguese). 10.1590/S0365-05962009000300007 [DOI] [PubMed] [Google Scholar]
- 5.Field E. A., Allan R. B., “Review article: oral ulceration—etiopathogenesis, clinical diagnosis and management in the gastrointestinal clinic,” Aliment. Pharmacol. Ther. 18(10), 949–962 (2003). 10.1046/j.1365-2036.2003.01782.x [DOI] [PubMed] [Google Scholar]
- 6.Leão J. C., Gomes V. B., Porter S., “Ulcerative lesions of the mouth: an update for the general medical practitioner,” Clinics (Sao Paulo) 62(6), 769–780 (2007). 10.1590/S1807-59322007000600018 [DOI] [PubMed] [Google Scholar]
- 7.Miziara I. D., “The treatment of recurrent aphthous stomatitis still intrigues,” Rev. Assoc. Med. Bras. 55(2), 96 (2009) (in Portuguese). 10.1590/S0104-42302009000200001 [DOI] [PubMed] [Google Scholar]
- 8.Martins M. D., et al. , “Healing properties of papain-based gel on oral ulcers,” Braz. J. Oral. Sci. 10(2), 120–123 (2011). [Google Scholar]
- 9.Fernandes K. P. S., et al. , “Healing and cytotoxic effects of Psidium guajava (Myrtaceae) leaf extracts,” Braz. J. Oral Sci. 9(4), 449–454 (2010). [Google Scholar]
- 10.Martins M. D., Marques M. M., Bussadori S. K., “Comparative analysis between Chamomill arecutita and corticosteroids on wound healing. An in vitro and in vivo study,” Phytother. Res. 23(2), 274–278 (2009). 10.1002/ptr.v23:2 [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro M. A. G., et al. , “Immunohistochemical assessment of myofibroblasts and lymphoid cells during wound healing in rats subjected to laser photobiomodulation at 600 nm,” Photomed. Laser Surg. 27(1), 49–55 (2009). 10.1089/pho.2007.2215 [DOI] [PubMed] [Google Scholar]
- 12.Reddy G. K., “Review photobiological basis and clinical role of low-intensity lasers in biology and medicine,” J. Clin. Laser Med. Surg. 22(2), 141–150 (2004). 10.1089/PLT.2004.22.issue-2 [DOI] [PubMed] [Google Scholar]
- 13.Bourguignon-Filho A. M., et al. , “Use of low level laser therapy on wound healing. Literature review,” Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 46(1), 37–43 (2005) (in Portugese). [Google Scholar]
- 14.Mester E., et al. , “Effect of laser rays on wound healing,” Am. J. Surg. 122(4), 532–535 (1971). 10.1016/0002-9610(71)90482-X [DOI] [PubMed] [Google Scholar]
- 15.Pereira A. N., et al. , “Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts,” Lasers Surg. Med. 31(4), 263–267 (2002). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 16.Peplow P.V., Chung T., Baxter D., “Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies,” Photomed. Laser Surg. 28(Suppl 1), s3–s40 (2010). 10.1089/pho.2008.2446 [DOI] [PubMed] [Google Scholar]
- 17.Souza T. O. F., et al. , “Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair,” Lasers Med. Sci. 26(6), 803–814 (2011). 10.1007/s10103-011-0951-9 [DOI] [PubMed] [Google Scholar]
- 18.Lim W., et al. , “The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors,” Lasers Surg. Med. 39(7), 614–621 (2007). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 19.Aimbire F., et al. , “Low-level laser therapy induces dose-dependent reduction of TNF-afla levels in acute inflammation,” Photomed. Laser Surg. 24(1), 33–37 (2006). 10.1089/pho.2006.24.33 [DOI] [PubMed] [Google Scholar]
- 20.Corazza A. V., et al. , “Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources,” Photomed. Laser Surg. 25(2), 102–106 (2007). 10.1089/pho.2006.2011 [DOI] [PubMed] [Google Scholar]
- 21.Pereira M. C., et al. , “Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries,” J. Photochem. Photobiol. B. 98(3), 188–192 (2010). 10.1016/j.jphotobiol.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Damante C. A., et al. , “Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts,” Lasers Med. Sci. 24(6), 885–891 (2009). 10.1007/s10103-008-0582-y [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. M., et al. , “Comparative study of 1,064-nm laser-induced skin burn and thermal skin burn,” Cell Biochem. Biophys., Epub ahead of print (2013). 10.1007/s12013-013-9596-6 [DOI] [PubMed] [Google Scholar]
- 24.Medrado A. P., et al. , “Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts,” Lasers Surg. Med. 32(3), 239–244 (2003). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 25.Viegas V. N., et al. , “Effect of low-level laser therapy on inflammatory reactions during wound healing: comparison with meloxicam,” Photomed. Laser Surg. 25(6), 467–473 (2007). 10.1089/pho.2007.1098 [DOI] [PubMed] [Google Scholar]
- 26.Medrado A. P., et al. , “Influence of laser photobiomodulation upon connective tissue remodeling during wound healing,” J. Photochem. Photobiol. B. 92(3), 144–152 (2008). 10.1016/j.jphotobiol.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 27.Pugliese L. S., et al. , “The influence of low-level laser therapy on biomodulation of collagen and elastic fibers,” Pesqui. Odontol. Bras. 17(4), 307–313 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Peplow P.V., Chung T., Baxter G. D., “Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models,” Photomed. Laser Surg. 28(3), 291–325 (2010). 10.1089/pho.2008.2446 [DOI] [PubMed] [Google Scholar]
- 29.Reis S. R. A., et al. , “Effect of 670-nm laser therapy and dexamethasone on tissue repair: a histological and ultrastructural study,” Photomed. Laser Surg. 26(4), 307–313 (2008). 10.1089/pho.2007.2151 [DOI] [PubMed] [Google Scholar]
- 30.Institute for Laboratory Animal Research, Guide for the Care, and Use of Laboratory Animals, National Academies Press, Washington, DC: (2011). [Google Scholar]
- 31.Khorasani G., et al. , “Aloe versus silver sulfadiazine creams for second-degree burns: a randomized controlled study,” Surg. Today. 39(7), 587–591 (2009). 10.1007/s00595-008-3944-y [DOI] [PubMed] [Google Scholar]
- 32.Sinha U. K., Gallagher L. A., “Effects of steel scalpel, ultrasonic scalpel, laser, and monopolar and bipolar electrosurgery on wound healing in guinea pig oral mucosa,” Laryngoscope 113(2), 228–236 (2003). 10.1097/00005537-200302000-00007 [DOI] [PubMed] [Google Scholar]
- 33.Kumar V., Cotran R. S., Robbins S. L., Robbins Basic Pathology, 7th ed., Saunders, Philadelphia, Pennsylvania: (2003) [Google Scholar]
- 34.Camacho-Alonso F., Lopez-Jornet P., “Clinical-pathological study of the healing of wounds provoked on the dorso-lingual mucosa in 186 albino rats,” Otolaryngol. Head Neck Surg. 136(1), 119–124 (2007). 10.1016/j.otohns.2006.06.1243 [DOI] [PubMed] [Google Scholar]
- 35.Almeida-Lopes L., et al. , “Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence,” Lasers Surg. Med. 29(2), 179–184 (2001). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 36.Fahimipour F., et al. , “Effect of low-level laser therapy on experimental wounds of hard palate mucosa in mice,” Indian J. Exp. Biol. 49(5), 357–361 (2011). [PubMed] [Google Scholar]
- 37.van Breugel H. H. F. I., DopBärr P. R., “Power density and exposure time of He–Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro,” Lasers Surg. Med. 12(5), 528–537 (1992). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 38.Walsh L. J., “The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications” Aust. Dent. J. 42(4), 247–254 (1997). 10.1111/j.1834-7819.1997.tb00129.x [DOI] [PubMed] [Google Scholar]
- 39.Lubart R., et al. , “Photochemistry and photobiology of light absorption by living cells,” Photomed. Laser Surg. 24(2), 179–185 (2006). 10.1089/pho.2006.24.179 [DOI] [PubMed] [Google Scholar]
- 40.Antunes H. S., et al. , “Low-power laser in the prevention of induced oral mucositis in bone marrow transplantation patients: a randomized trial,” Blood. 109(5), 2250–2255 (2007). 10.1182/blood-2006-07-035022 [DOI] [PubMed] [Google Scholar]
- 41.Kuhn A., et al. , “Low-level infrared laser therapy in chemotherapy-induced oral mucositis: a randomized placebo-controlled trial in children,” J. Pediatr. Hematol. Oncol. 31(1), 33–37 (2009). 10.1097/MPH.0b013e318192cb8e [DOI] [PubMed] [Google Scholar]
- 42.Lima A. G., et al. , “Efficacy of low-level laser therapy and aluminum hydroxide in patients with chemotherapy and radiotherapy-induced oral mucositis,” Braz. Dent. J. 21(3), 186–192 (2010). 10.1590/S0103-64402010000300002 [DOI] [PubMed] [Google Scholar]
- 43.Nes A. G., Posso M. B., “Patients with moderate chemotherapy-induced mucositis: pain therapy using low intensity lasers,” Int. Nurs. Rev. 52(1), 68–72 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Demidova-Rice T. N., et al. , “Low-level light stimulates excisional wound healing in mice,” Lasers Surg. Med. 39(9), 706–715 (2007). 10.1002/(ISSN)1096-9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.do Nascimento P. M., et al. , “A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats,” Photomed. Laser Surg. 22(6), 513–518 (2004). 10.1089/pho.2004.22.513 [DOI] [PubMed] [Google Scholar]
- 46.Eduardo F. P., et al. , “Cultured epithelial cells response to phototherapy with low intensity laser,” Lasers Surg. Med. 39(4), 365–372 (2007). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 47.Yu W., Naim J. O., Lanzafame R. J., “The effect of laser irradiation on the release of bFGF from 3T3 fibroblasts,” Photochem. Photobiol. 59(2), 167–170 (1994). 10.1111/php.1994.59.issue-2 [DOI] [PubMed] [Google Scholar]
- 48.Saygun I., et al. , “Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts,” Lasers Med. Sci. 23(2), 211–215 (2008). 10.1007/s10103-007-0477-3 [DOI] [PubMed] [Google Scholar]
- 49.Karu T. I., “Molecular mechanisms of therapeutic effect of low-intensity laser radiation,” Laser Life Sci. 2(1), 53–74 (1988). [Google Scholar]