Abstract
Purpose
Larger social networks have been associated with lower breast cancer mortality. The authors evaluated how levels of social support and burden influenced this association.
Methods
We included 2,264 women from the Life After Cancer Epidemiology study who were diagnosed with breast cancer between 1997–2000, and provided data on social networks (spouse or intimate partner, religious/social ties, volunteering, time socializing with friends, and number of first-degree female relatives), social support, and caregiving. 401 died during a median follow-up of 10.8 years follow-up with 215 from breast cancer. We used delayed entry Cox proportional hazards regression to evaluate associations.
Results
In multivariate-adjusted analyses, social isolation was unrelated to recurrence or breast cancer-specific mortality. However, socially isolated women had higher all-cause mortality (HR=1.34, 95%CI:1.03–1.73) and mortality from other causes (HR=1.79, 95%CI:1.19–2.68). Levels of social support and burden modified associations. Among those with low, but not high, levels of social support, lack of religious/social participation (HR=1.58, 95%CI: 1.07–2.36, p=0.02, p-interaction=0.01) and lack of volunteering (HR=1.78, 95%CI: 1.15–2.77, p=0.01, p-interaction=0.01) predicted higher all-cause mortality. In cross-classification analyses, only women with both small networks and low levels of support (HR=1.61, 95%CI:1.10–2.38) had a significantly higher risk of mortality than women with large networks and high levels of support; women with small networks and high levels of support had no higher risk of mortality (HR=1.13, 95%CI:0.74–1.72). Social networks were also more important for caregivers vs. noncaregivers.
Conclusions
Larger social networks predicted better prognosis after breast cancer, but associations depended on the quality and burden of family relationships.
Keywords: Social networks, social support, social strain, caregiving, social burden, breast cancer, survival, mortality, women, social ties
Introduction
Social networks are defined as the web of social relationships that surround an individual[1]. The most commonly examined characteristic in the epidemiologic literature on social networks and breast cancer survival is social network size, i.e. the number of network members. Previous studies have found that greater social network size measured close in time or prior to diagnosis is associated with better survival after a breast cancer diagnosis[2–6]. In the Nurses’ Health Study (NHS) of 2,835 postmenopausal women with any stage breast cancer, Kroenke and colleagues found that socially isolated women, i.e., those with small networks, were twice as likely to die of their breast cancer than socially integrated women[4]. However, in the Women’s Health Initiative, Kroenke and colleagues found that associations between social networks and breast cancer outcomes depended on levels of social support and burden in relationships[7]. Counter to expectation, larger social networks, particularly larger networks of first-degree relatives, were related to higher levels of mortality among women with low levels of support, high levels of relationship strain, or caregiving responsibilities[7].
Thus, the impact of social networks on breast cancer mortality may depend on the quality of relationships within naturally occurring networks as social support interventions have not improved survival[8–12]. While results from the WHI are intriguing, the investigators were unable to adjust for breast cancer treatment potentially compromising validity of findings. Their findings need replication in a cohort of survivors with complete treatment data.
We hypothesized that larger social network size would be related to lower mortality in women with breast cancer, consistent with previous work. However, consistent with results reported in the WHI, we hypothesized that associations would differ by levels of support and social burden in relationships. We examined associations in 2,264 women with invasive breast cancer from the Life After Cancer Epidemiology (LACE) Study.
METHODS
Study population
The LACE Study cohort consisted of 2,264 women diagnosed with invasive breast cancer between 1997 and 2000 who were recruited primarily from the Kaiser Permanente Northern California (KPNC) Cancer Registry (83%) and the Utah Cancer Registry (12%) between 2000 and 2002. Further details are provided elsewhere[13]. In brief, eligibility criteria included: 1) ages between 18–70 years at enrollment; 2) diagnosis of early-stage primary breast cancer (stage I ≥ 1 cm, II, or IIIA); 3) enrollment between 11–39 months post-diagnosis; 4) completion of breast cancer treatment (except adjuvant hormonal therapy); 5) freedom from recurrence; and 6) no history of other cancers within 5 years prior to enrollment. Of the 2,264 women, 383 had a recurrence and 401 died of any cause, with 215 (51.8%) from breast cancer. The study was approved by the institutional review boards of KPNC and the University of Utah.
Data collection
Breast cancer ascertainment
Information on clinical factors was obtained through electronic data sources available from KPNC or from medical chart review for the non-KPNC participants. Data included tumor size, number of positive lymph nodes, hormone receptor status, and treatment (i.e., chemotherapy, radiation therapy, and hormonal therapy). Tumor stage was calculated according to criteria of the American Joint Committee on Cancer (AJCC) (4th edition).
Recurrences were ascertained by a mailed semi-annual or annual (after April 2005) health status questionnaire asking participants to report any events occurring in the preceding 6 or 12 months, respectively. Recurrences included a locoregional cancer recurrence, distant recurrence/metastasis, or development of a contralateral breast primary. Nonrespondents were called by telephone to complete questionnaires. Medical records were reviewed to verify reported outcomes.
Mortality
Participant deaths were determined through KPNC electronic data sources, a family member responding to a mailed questionnaire, or a phone call to the family. Copies of death certificates were obtained to verify primary and underlying causes of death (International Classification of Diseases, 9th revision). All-cause mortality included death from any cause. Breast cancer-specific death included death attributable to breast cancer as a primary or underlying cause on the death certificate. Death from causes other than breast cancer included all other deaths. A physician reviewer was consulted when the cause of death was unclear.
Social networks
Social networks included five components: a spouse or intimate partner, number of first-degree female relatives (living mother, number of biological daughters, number of full sisters), friendship ties, religious/social ties, and community ties.
Women were asked, “What is your current marital status? (married, divorced, living as married, separated, widowed, or never married).” Women were asked further whether their mother was still alive. Additionally, women were asked whether or not they had biological daughters or full sisters, and if yes, how many. Women reported 0–11 first-degree relatives (median=3, SD=1.9).
Questions regarding community, friendship, and religious/social ties were derived from the Arizona Activity Frequency Questionnaire[14]. Though this questionnaire is intended to measure the extent of physical activity, it does so by querying time spent in a variety of activities including social participation. As a measure of community ties, women were asked, “Did you do weekly volunteer work in the past year? (yes, no)” As a measure of friendship ties, women were asked, “On average, how often in the past year did you socialize, visit with friends, or talk on the phone?” As a measure of religious/social participation, women were asked, “On average, how often in the past year did you attend religious, social or service club meetings, sporting events, concerts, movies or shows?” Response options to the latter two questions included: 1) never or less than 1 time per month, 2) 1–3 times per month, 3) 1–2 times per week, 4) 3–5 times per week, and 5) more than 5 times per week. Women were further asked, “Each time you did this activity, how much time did you usually spend doing it?” Responses included: 1) less than 15 minutes, 2) 15–30 minutes, 3) 31–60 minutes, and 4) 61–90 minutes. Hours per week spent in these activities was derived by multiplying frequency times duration, based on the mean values of categories.
The Berkman-Syme Social Network Index (B-SNI)[15] is frequently used to measure social networks in epidemiologic studies though other measures are also used[16]. The B-SNI is computed based on the number and extent of contact with close friends, children, and other relatives; the presence of a spouse or intimate partner; religious participation; and community participation. It heavily weights the number of close friends and relatives in the computation of the index compared with religious and community components and also includes extent of contact in the computation of network size. However, no measure of social networks has been specifically developed in a cohort of breast cancer survivors. Given a lack of theoretical rationale and lack of information on numbers of close friends and relatives, we assigned approximately equal weighting to components, allowing slightly greater weighting to variables in which variability existed. Thus, women were assigned 0 or 1 points depending on whether or not they were married or engaged in volunteer work. We assigned women 0, 1, or 2 points for no, small, and large numbers of female relatives or little, some, and high levels of participation in religious/social activities or socializing with friends. We thus generated an overall measure of social networks by summing points based on numbers of and/or levels of involvement with network members. We then divided the women into tertiles and generated three categories of women: socially isolated, moderately integrated, and socially integrated. This social networks variable has not been validated against the B-SNI, but results from a similarly developed measure were previously published[7]. Because “closeness” was not written into questions and thus not presumed in relationships, we were able to examine associations modified by levels of social support.
Social support
Social support was assessed using six items from the social and family well-being scale from the Functional Assessment of Cancer Therapy–Breast (FACT-B)[17]. Items included: “I get emotional support from my family;” “I get support from my friends and neighbors;” “My family has accepted my illness;” “Family communication about my illness is poor;” “I feel close to my partner (or the person who is my main support);” and “I feel distant from my friends.” Participants ranked on a 5-point scale how true each statement was for them during the past 7 days (not at all, a little, somewhat, quite a bit, very much). The questions regarding distance from friends and family communication were reverse scored and responses summed. The summary score ranged from 0 to 24, with a higher score indicating higher levels of support. We divided women by high and low levels of support based on the total support, based on the median=22 score.
Social burden
We asked women whether they were providing caregiving to an infant, a child or an elderly adult or disabled person. The 34% of women indicated providing care to any of these persons were classified as caregivers.
Other covariates
Information on other covariates was self-reported at baseline. Data on race, education, smoking, reproductive factors, lifestyle factors, and BMI were obtained from the mailed baseline questionnaire.
Statistical analyses
Using analysis of covariance, we regressed potential confounding variables against categories of social network size, adjusted for continuous age (Table 1).
Table 1.
Selected baseline characteristics* by category of social network size, in the Life After Cancer Epidemiology (LACE) cohort (N=2,264).
| Social network size | ||||
|---|---|---|---|---|
| Socially† isolated | Moderately integrated | Socially integrated | p-trend* | |
| N | 797 | 706 | 761 | |
| Person-years | 8,117 | 7,123 | 7,897 | |
| Family history of breast cancer (%) | 20.6 | 19.9 | 21.2 | 0.84 |
| Breast cancer-specific mortality (%) | 10.6 | 8.4 | 9.4 | 0.34 |
| Non breast cancer mortality (%) | 10.2 | 9.1 | 5.3 | <0.001 |
| Demographic variables | ||||
| Age (mean years) | 58.9 | 58.0 | 57.8 | 0.10 |
| Ethnicity (%) | ||||
| Caucasian | 79.1 | 78.6 | 82.4 | 0.12** |
| African-American | 4.1 | 5.7 | 5.1 | |
| Asian | 6.4 | 6.7 | 5.7 | |
| Hispanic/Latino | 7.0 | 6.4 | 3.7 | |
| Other | 3.4 | 2.6 | 3.2 | |
| Education ≥ college (%) | 33.4 | 35.2 | 36.9 | 0.36 |
| Severity of disease | ||||
| Stage | ||||
| 1 (%) | 47.4 | 45.8 | 46.3 | 0.97** |
| 2 (%) | 49.3 | 51.1 | 50.8 | |
| 3 (%) | 3.3 | 3.1 | 2.9 | |
| Nodal involvement (%) | 36.2 | 36.1 | 38.0 | 0.69 |
| Tumor size (cm) | 2.1 | 2.1 | 2.1 | 0.78 |
| ER positive tumor (%) | 81.6 | 83.1 | 80.2 | 0.36 |
| HER-2-neu receptor + (%) | 14.7 | 16.0 | 12.4 | 0.14 |
| Treatment | ||||
| Chemotherapy (%) | 54.6 | 55.2 | 61.9 | 0.002 |
| Radiation (%) | 63.7 | 63.7 | 61.5 | 0.57 |
| Tamoxifen (%) | 60.5 | 61.7 | 63.0 | 0.59 |
| Behavioral factors | ||||
| Body mass index (kg/m2) | 27.4 | 27.4 | 27.7 | 0.50 |
| Physical activity (METhr/wk) | 45.4 | 51.3 | 59.5 | <0.001 |
| Never smokers (%) | 47.5 | 50.9 | 60.0 | <0.001 |
| Alcohol (svg/wk) | 3.1 | 2.7 | 2.1 | 0.02 |
| Reproductive factors | ||||
| Age at menarche < 12 y (%) | 12.6 | 12.6 | 12.6 | 0.88 |
| Age at first birth > 30 y (%) | 24.3 | 24.7 | 24.2 | 0.29 |
| Parity (Any pregnancies ≥ 5 months (%)) | 76.6 | 83.9 | 91.8 | <0.001 |
| Postmenopausal (%) | 74.5 | 72.5 | 74.2 | 0.40 |
| Social variables | ||||
| High levels of support (%) | 46.2 | 49.3 | 55.6 | <0.001 |
| Living mother (%) | 32.8 | 38.3 | 43.9 | <0.001 |
| Other first-degree female relatives (mean) | 1.8 | 2.4 | 3.2 | <0.001 |
| Married (%) | 51.3 | 66.2 | 87.9 | <0.001 |
| Hours/wk socializing with friends | 1.8 | 2.7 | 3.5 | <0.001 |
| Religious, social, and cultural participation (%) | 41.9 | 83.9 | 99.4 | <0.001 |
| Volunteering (%) | 5.5 | 16.5 | 44.8 | <0.001 |
Except for age, all variables age-adjusted
p-value, Mantel-Haenszel chi square test
Means were imputed when data on a social network were missing. Ranges of values were 0–4 for socially isolated women, 4.1–<5.5 for moderately integrated women, and 5.5–8.0 for socially integrated women. This distribution reflects approximate tertiles.
Imputation of missing items
Of the total sample, 1,727 women (76.3%) had complete information on social networks at baseline. Most previous analyses of social networks and health-related outcomes have employed complete case analysis, but elimination of this large fraction (23.7%) of the sample substantially reduced study efficiency. Additionally, complete case analysis has been shown to be substantially biased[18]. Therefore, we retained participants with missing data, imputing mean values for each network measure with missing data, in the generation of an overall social networks measure. All but seven women reported marital status suggesting general completion of questionnaires. Of the full sample, 15% were missing more than one social network item; 12% were missing three items measured by the physical activity questionnaire suggesting women didn’t complete the questionnaire. However, when examined, other items unrelated to social participation were completed suggesting that missing data meant the participant omitted completing personally irrelevant questions. Nevertheless, we imputed data in three ways. We conducted analyses assuming that missing data meant the absence of the social network member. We also employed mean imputation, assigning the sample mean for the specific social network item when data were missing for that item, and multiple imputation (SAS PROC MI) for missing social network items. In analyses of separate individual network members, we did not impute data and sample size varied by analysis.
Analyses of social networks and outcomes
We employed delayed entry Cox proportional hazards models (SAS PROC PHREG; SAS Institute, Cary, NC) for failure-time data to assess associations of approximate tertiles of social networks, assessed at study onset, with time to event. We evaluated associations of social networks with time to recurrence, breast cancer death, death from causes other than breast cancer, and all-cause mortality[19, 20]. Person-years of follow-up were counted from the date of study entry until the date of death or end of follow-up, whichever came first. We conducted tests for linear trend or continuous variables, as indicated, computing Wald statistics.
Minimally-adjusted results were compared with those adjusted for multiple covariates. Initial analyses were adjusted for age and time between social assessment and breast cancer diagnosis. Analyses were adjusted additionally for factors considered a priori to be important potential confounding variables of the relationship between social network size and breast cancer outcomes including: disease severity (stage, tumor size, grade, nodal status, estrogen-receptor status, and HER-2 status), treatment (radiation, chemotherapy, tamoxifen), education, ethnicity, reproductive variables (age at menarche, age at first birth, parity, menopausal status), and behavioral and related factors (body mass index (BMI), physical activity, alcohol intake, smoking status) (see Tables 1–3).
Table 3.
Relative risk of all-cause mortality by social network component and by level of social support among 2,264 participants diagnosed with breast cancer from the LACE cohort, 1997–2000.
| N | All-cause mortality | Low support | 95% CI | All-cause mortality | High support | 95% CI | |
|---|---|---|---|---|---|---|---|
| Social network size* | |||||||
| Socially integrated | 761 | 48 | 1.00 | 61 | 1.00 | ||
| Moderately integrated | 706 | 64 | 1.11 | (0.75–1.63) | 58 | 1.00 | (0.68–1.47) |
| Socially isolated | 797 | 105 | 1.51 | (1.05–2.18) | 65 | 1.08 | (0.73–1.59) |
| p-value** | 0.006 | 0.71 | |||||
| p-interaction | 0.41 | ||||||
| Married | |||||||
| Yes | 1533 | 117 | 1.00 | 112 | 1.00 | ||
| No | 724 | 100 | 1.08 | (0.81–1.45) | 72 | 1.32 | (0.95–1.85) |
| p-value | 0.59 | 0.10 | |||||
| p-interaction | 0.07 | ||||||
| First-degree female relatives | |||||||
| 3 or more | 1051 | 98 | 1.00 | 77 | 1.00 | ||
| 1–2 | 844 | 76 | 1.20 | (0.69–2.11) | 74 | 1.47 | (0.61–3.53) |
| 0 | 158 | 18 | 1.14 | (0.66–1.97) | 12 | 1.06 | (0.74–1.51) |
| p-value | 0.43 | 0.55 | |||||
| p-interaction | 0.41 | ||||||
| Any volunteering | |||||||
| Yes | 420 | 24 | 1.00 | 38 | 1.00 | ||
| No | 1515 | 156 | 1.78 | (1.15–2.77) | 118 | 0.89 | (0.60–1.32) |
| p-value | 0.01 | 0.57 | |||||
| p-interaction | 0.01 | ||||||
| Religious, social, cultural participation | |||||||
| ≥1 hour/wk | 749 | 48 | 1.00 | 61 | 1.00 | ||
| Up to 1 hr/wk | 648 | 60 | 1.33 | (0.90–1.97) | 53 | 0.95 | (0.64–1.40) |
| Not at all | 522 | 70 | 1.58 | (1.07–2.36) | 38 | 0.90 | (0.58–1.39) |
| p-value | 0.08 | 0.68 | |||||
| p-interaction | 0.04 | ||||||
| Socializing with friends | |||||||
| ≥2 hour/wk | 985 | 83 | 1.00 | 85 | 1.00 | ||
| 0.5–<2 hour/wk | 744 | 66 | 1.08 | (0.78–1.51) | 62 | 1.26 | (0.90–1.77) |
| 0–<0.5 h/wk | 197 | 28 | 1.82 | (1.14–2.91) | 9 | 0.81 | (0.40–1.64) |
| p-value | 0.82 | 0.39 | |||||
| p-interaction | 0.26 |
Models adjusted for age at diagnosis, time between diagnosis and assessment of social networks, race (white, nonwhite[ref]), education (<college graduate [ref], college graduate), any family history of breast cancer (no history [ref], yes), cancer stage at diagnosis (1 [ref], 2, 3), tumor size (continuous), HER-2 neu status (positive, negative[ref]), nodal status (no involvement [ref], any involvement), estrogen-receptor status (positive, negative [ref]), chemotherapy (yes, no [ref]), radiation (yes, no [ref]), tamoxifen (never, past, current [ref]), age at menarche (continuous), age at first birth (never had pregnancy lasting at least 5 months, <20 [ref], 20–29,30–39, ≥40 years [ref]), parity (0, 1, 2, 3, 4+ [ref] pregnancies ≥ 5 months), menopausal status (pre[ref], post), smoking status (never [ref], past, current), body mass index (<25 [reference] 25–29, 30+ kg/m2), physical activity (quartiles, quartile 1 [ref]), and alcohol intake (0 [ref], >0 svg/wk).
p-value, continuous variable
Stratified analyses
We employed Kaplan-Meier curves to evaluate survival for categories of the cross-classification of large, medium, and small social networks and high and low levels of social support, using the Log rank test to evaluate significance.
We conducted stratified analyses for analyses of social networks and all-cause mortality by high and low levels of social support as well as by caregiving status. When associations differed across strata, we used Wald tests to evaluate interaction terms of dichotomous stratification variables and the continuous social network variable.
RESULTS
Study participants contributed 23,137 person-years follow-up. Follow-up ranged from 1.3 to 13.9 years with a median of 10.8 years.
Women with larger social networks had higher levels of physical activity, lower alcohol intake, and were more likely to be never smokers. They were more likely to be married, have children, to participate in religious/social activities, to volunteer, and to have a larger number of female relatives. Disease characteristics were unrelated to social network size though women with larger networks were more likely to receive chemotherapy. Other reproductive factors were unrelated to social network size (Table 1).
Social networks and mortality after breast cancer
In minimally-adjusted analyses, larger social networks were unrelated to risk of recurrence or breast cancer mortality, but were associated with lower mortality from other causes and all-cause mortality. This was true whether we used mean imputation (Table 2), multiple imputation (data not shown), or assumed that nonresponse meant the participant didn’t have a particular social network member (data not shown). Adjustment for covariates attenuated associations somewhat but associations remained significant, regardless of method of imputation (Table 2).
Table 2.
Hazard ratio of breast cancer event by category of social network size in the LACE cohort (N=2,264).
| Social network size | ||||
|---|---|---|---|---|
| Socially isolated | Moderately integrated | Socially integrated | p-value** | |
| N† | 797 | 706 | 761 | |
| Person-years | 8,117 | 7,123 | 7,897 | |
| Recurrence | 148 | 111 | 124 | |
| Age-adjusted* 95% CI | 1.16 (0.91–1.47) | 1.00 (0.77–1.29) | 1.00 | 0.21 |
| MV-adjusted model 1‡ 95% CI | 1.09 (0.85–1.41) | 0.94 (0.72–1.22) | 1.00 | 0.55 |
| MV-adjusted model 2 95% CI | 1.08 (0.84–1.40) | 0.95 (0.71–1.27) | 1.00 | 0.66 |
| MV-adjusted model 3 95% CI | 1.03 (0.73–1.42) | 1.08 (0.83–1.41) | 1.00 | 0.36 |
| Breast cancer mortality | 85 | 59 | 71 | |
| Age-adjusted 95% CI | 1.15 (0.84–1.57) | 0.91 (0.64–1.29) | 1.00 | 0.24 |
| MV-adjusted model 1 95% CI | 1.08 (0.77–1.52) | 0.83 (0.53–1.18) | 1.00 | 0.61 |
| MV-adjusted model 2 95% CI | 1.12 (0.79–1.60) | 0.91 (0.61–1.36) | 1.00 | 0.66 |
| MV-adjusted model 3 95% CI | 1.16 (0.76–1.77) | 1.04 (0.72–1.49) | 1.00 | 0.50 |
| All-cause mortality | 170 | 122 | 109 | |
| Age-adjusted 95% CI | 1.48 (1.16–1.88) | 1.23 (0.95–1.59) | 1.00 | <0.001 |
| MV-adjusted model 1 95% CI | 1.34 (1.03–1.73) | 1.08 (0.83–1.41) | 1.00 | 0.008 |
| MV-adjusted model 2 95% CI | 1.34 (1.03–1.75) | 1.11 (0.62–1.51) | 1.00 | 0.02 |
| MV-adjusted model 3 95% CI | 1.50 (1.11–2.03) | 1.13 (0.86–1.49) | 1.00 | 0.004 |
| Mortality from other causes | 85 | 63 | 38 | |
| Age-adjusted 95% CI | 2.07 (1.41–3.03) | 1.77 (1.18–2.65) | 1.00 | <0.001 |
| MV-adjusted model 1 95% CI | 1.79 (1.19–2.68) | 1.45 (0.95–2.21) | 1.00 | <0.001 |
| MV-adjusted model 2 95% CI | 1.68 (1.09–22.58) | 1.36 (0.87–2.14) | 1.00 | 0.005 |
| MV-adjusted model 3 95% CI | 1.97 (1.25–3.10) | 1.23 (0.80–1.89) | 1.00 | <0.001 |
Age-adjusted model adjusted for age (continuous) and time between diagnosis and assessment of social networks. Age-adjusted model employed mean imputation. Multivariate-adjusted (MV-) adjusted model 1 employed mean imputation. MV-adjusted model 2 employed multiple imputation. MV-adjusted model 3 employed the assumption of meaningful nonresponse, that nonresponse signified the participant did not have a social network member. For mean and multiple imputation, ranges of values were 0–4 for socially isolated women, 4.1–<5.5 for moderately integrated women, and 5.5–8.0 for socially integrated women. For the assumption of meaningful nonresponse, the ranges were 0–<4, 4–<6, and 6–8. These distributions reflect approximate tertiles.
p-value, continuous variable
N based on values derived with mean imputation.
Multivariate-adjusted models adjusted for age at diagnosis, time between diagnosis and assessment of social networks, race (white, nonwhite[ref]), education (<college graduate [ref], college graduate), any family history of breast cancer (no history [ref], yes), cancer stage at diagnosis (1 [ref], 2, 3), tumor size (continuous), HER-2 neu status (positive, negative[ref]), nodal status (no involvement [ref], any involvement), estrogen-receptor status (positive, negative [ref]), chemotherapy (yes, no [ref]), radiation (yes, no [ref]), tamoxifen (never, past, current [ref]), age at menarche (continuous), age at first birth (never had pregnancy lasting at least 5 months, <20 [ref], 20–29,30–39, ≥40 years [ref]), parity (0, 1, 2, 3, 4+ [ref] pregnancies ≥ 5 months), menopausal status (pre[ref], post), smoking status (never [ref], past, current), body mass index (<25 [reference] 25–29, 30+ kg/m2), physical activity (quartiles, quartile 1 [ref]), and alcohol intake (0 [ref], >0 svg/wk).
Stratified analyses
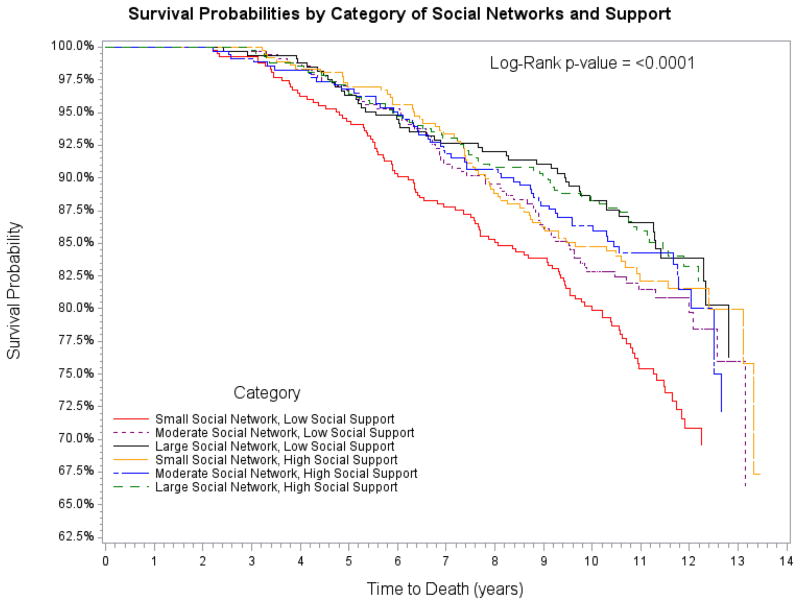
Using Kaplan-Meier curves (using mean imputation), women with small social networks and low levels of social support had the highest risk of all-cause mortality over follow-up (Figure). In cross-classified analyses of support and network size, only women with both small social networks and low levels of support (HR=1.61, 95%CI: 1.10–2.38), and not other women characterized by level of social network size and support (medium networks/low support, HR=1.11, 95%CI:0.73–1.69; large networks/low support, HR=0.97, 95%CI:0.63–1.49; small networks/high support, HR=1.13, 95%CI:0.74–1.72; medium networks/high support, HR=1.11, 95%CI: 0.73–1.70), had an elevated risk of all-cause mortality, compared to women with both large networks and high social support. Other methods of imputation provided similar results (data not shown).
Figure 1.
Kaplan-Meier curves of the cross-classification of large, medium, and small social networks and low and high levels of social support from a spouse/partner, family, friends and neighbors. Women with small social networks and low levels of social support had a significantly elevated risk of all-cause mortality over follow-up (HR=1.61, 95%CI: 1.10–2.38), compared with women with large networks and high levels of support (reference) but no other groups exhibited an elevated risk compared with the reference group.
Similarly, in stratified analyses (mean imputation), among those with low social support, those who were socially isolated had a significantly higher risk of all-cause mortality. The association was not observed among those with high social support though the interaction by level of support was nonsignificant (Table 3). Among women with low, but not high, levels of social support from friends, family, or a spouse, those who did not engage in volunteering or participate in religious activities had higher mortality than those who had community or religious ties. We did not observe differences in other network members (Table 3).
Caregiver status appeared to modify the association between social network size and all-cause mortality. Among caregivers, those who were socially isolated (HR=2.30, 95%CI: 1.23–4.29) or moderately integrated (HR=1.47, 95%CI: 0.90–2.41) had a higher risk of mortality, compared to those who were socially integrated (p-continuous=0.01); this relationship was weaker in noncaregivers. More specifically, caregivers who were not married or in an intimate relationship had a higher risk of mortality (HR=1.82, 95%CI: 1.16–2.84, p=0.009) compared with those who were in such relationships, though this was not true in noncaregivers (HR=1.08, 95%CI: 0.81–1.43, p=0.62). Additionally, caregivers indicating no volunteering work at baseline had a higher risk of mortality (HR=1.90, 95%CI: 1.09–3.32, p=0.02) whereas this relationship was not seen in noncaregivers (HR=1.03, 95%CI: 0.73–1.45, p=0.88). Among noncaregivers (HR=1.58, 95%CI: 1.02–2.47) but not caregivers (HR=0.64, 95%CI: 0.24–1.72, p=0.38), the lowest tertile of time spent socializing vs. the highest tertile predicted higher mortality. This interaction was borderline significant (p-interaction=0.06) though all other interaction terms were nonsignificant (data not shown).
DISCUSSION
Consistent with expectation, larger social networks were related to longer survival after breast cancer diagnosis. However, associations depended entirely on levels of social support and burden within relationships. Our observation that women with small social networks and low levels of social support had higher mortality, but that women with small networks and high levels of social support did not have a significantly higher risk of mortality compared with those with large networks and high support suggests that relationship quality, and not just network size, matters to survival.
Researchers have generally found that larger network size is predictive of lower post-diagnosis mortality[2–6]. Similar to Beasley[2], we found that smaller social networks were related to higher all-cause mortality but not to breast cancer-specific mortality. In both studies, social network measures were assessed approximately two years after diagnosis, when the most advanced cases of breast cancer may have already died. In long-term breast cancer survivors, social networks may still be important for non-breast cancer specific causes, most notably cardiovascular disease. Since breast cancer treatments can be cardiotoxic, it is possible that social relationships may help protect against adverse cardiovascular effects, through oxytocin-mediated reductions in cardiovascular reactivity[21, 22], reductions in inflammation[23–26] effects on endothelial function[27], the cardiovascular benefits of buffering of stress[28], and increased physical activity related to social participation.
Though naturally occurring social networks may serve to prolong life after a breast cancer diagnosis, research has not yet illuminated what aspects of social networks are most important. These and other recent findings[7] suggest that the importance of specific network members depends on family dynamics and the context of women’s responsibilities. Networks in aging populations may center around family and may be less likely to include social ties outside the family[29, 30] even as ties with families decline[31]. However, women in LACE with low levels of family support appeared to gain health advantages from developing community and religious ties. Though interventions designed to improve family relationship quality could impact breast cancer outcomes, women whose family relationships are of poor quality may also benefit from extrafamilial relationships. Poor quality family relationships may be related to poorer health outcomes[32, 33], and religious and community participation has generally been related to better health outcomes[34] and lower mortality[35–37].
Though large social networks may increase the odds that women have friends and family to rely on for instrumental (e.g., rides to the hospital, trips to the pharmacy, assistance with exercise, or provision of healthy meals[38, 39]) and social-emotional support, they can also increase the likelihood of caregiving obligations[40, 41] to network members and associated adverse health outcomes[42–46]. Though larger relative networks were related to higher mortality among caregivers in the WHI[7], a greater number of network members in the LACE cohort appeared to buffer the stress of caregiving responsibilities, either by providing emotional support or taking on responsibilities that relieved the caregivers of some of their burden[47–50]. Unfortunately, we did not have information on subjective feelings regarding caregiving so it is unclear whether caregiving responsibilities were or were not stressful.
A study strength was the ability to adjust for variables related to breast cancer severity including stage, tumor size, nodal status, hormone receptor status, and HER2 status as well as breast cancer treatment particularly since social networks were measured after treatment was complete. Though social network size does not change substantially over time and network members may influence treatment decisions, the ability to adjust for treatment helped overcome concerns that some aspect of treatment might be related to both social withdrawal and higher mortality risk. A second strength was the ability to adjust carefully for reproductive history and lifestyle, demographic, and socioeconomic variables.
Missing data complicated interpretation of findings. Poor health, complexity of social network measures, or decisions to omit personally nonrelevant questions, all which may be related to levels of social isolation, may lead to omissions in completing social network survey items[51] and thus to substantial bias[52, 53] if women are omitted from analyses. This is consistent with the nonsignificant result we obtained when we conducted complete case analysis (data not shown) since data did not appear to be missing completely at random. The use of imputed data can also be problematic. However, associations were fairly consistent across analytic approach providing some assurance about the validity of findings. Moreover, these results were consistent with the unimputed associations for specific network members and outcomes. Additional methodological work is needed to evaluate the impact of missing data in analyses of psychosocial factors; future studies of social networks should avoid conducting complete case analysis only.
One limitation potentially compromising the ability to interpret studies of social network size and breast cancer outcomes generally is that social networks may proxy other variables such as personality factors that lead to both smaller social networks and influence breast cancer outcomes. We considered that depressive symptoms could influence both network size and outcomes but adjustment for this variable had little effect on associations (data not shown). Future research should also consider the modifying influence of lifecourse timing. Other limitations include lack of information on numbers of friends and inability to ascertain mechanisms. Additionally, findings may not generalize to women of lower socioeconomic status who were not well represented in this population.
To summarize, larger social networks were related to a lower mortality risk in this cohort of early stage breast cancer survivors. Moreover, levels of social-emotional support within the family strongly influenced the relative importance of specific network members in prolonging survival. Given the rising costs of health care and the aging of the population, there is a growing need to understand how social relationships influence disease progression.
Footnotes
The authors declare that they have no financial conflicts of interest.
References
- 1.Berkman L, Glass TA. Social integration, social networks, social support, and health. In: Berkman L, Kawachi I, editors. Social epidemiology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 2.Beasley JM, Newcomb PA, Trentham-Dietz A, Hampton JM, Ceballos RM, Titus-Ernstoff L, Egan KM, Holmes MD. Social networks and survival after breast cancer diagnosis. J Cancer Surviv. 2010;4(4):372–380. doi: 10.1007/s11764-010-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou AF, Stewart SL, Wild RC, Bloom JR. Social support and survival in young women with breast carcinoma. Psychooncology. 2010 doi: 10.1002/pon.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24(7):1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds P, Boyd PT, Blacklow RS, Jackson JS, Greenberg RS, Austin DF, Chen VW, Edwards BK. The relationship between social ties and survival among black and white breast cancer patients. National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1994;3(3):253–259. [PubMed] [Google Scholar]
- 6.Waxler-Morrison N, Hislop TG, Mears B, Kan L. Effects of social relationships on survival for women with breast cancer: a prospective study. Soc Sci Med. 1991;33 (2):177–183. doi: 10.1016/0277-9536(91)90178-f. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke CH, Michael Y, Tindle H, Gage E, Chlebowski R, Garcia L, Messina C, Manson JE, Caan BJ. Social networks, social support and burden in relationships, and mortality after breast cancer diagnosis. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-1962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel D, Butler LD, Giese-Davis J, Koopman C, Miller E, DiMiceli S, Classen CC, Fobair P, Carlson RW, Kraemer HC. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer. 2007;110(5):1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, Drysdale E, Hundleby M, Chochinov HM, Navarro M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345(24):1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 10.Gellert GA, Maxwell RM, Siegel BS. Survival of breast cancer patients receiving adjunctive psychosocial support therapy: a 10-year follow-up study. J Clin Oncol. 1993;11(1):66–69. doi: 10.1200/JCO.1993.11.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Cousson-Gelie F, Bruchon-Schweitzer M, Atzeni T, Houede N. Evaluation of a psychosocial intervention on social support, perceived control, coping strategies, emotional distress, and quality of life of breast cancer patients. Psychol Rep. 2011;108(3):923–942. doi: 10.2466/02.07.15.20.PR0.108.3.923-942. [DOI] [PubMed] [Google Scholar]
- 12.Helgeson VS, Cohen S, Schulz R, Yasko J. Group support interventions for women with breast cancer: who benefits from what? Health Psychol. 2000;19(2):107–114. doi: 10.1037//0278-6133.19.2.107. [DOI] [PubMed] [Google Scholar]
- 13.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States) Cancer Causes Control. 2005;16(5):545–556. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 14.Staten LK, Taren DL, Howell WH, Tobar M, Poehlman ET, Hill A, Reid PM, Ritenbaugh C. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33(11):1959–1967. doi: 10.1097/00005768-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- 17.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 18.Demissie S, LaValley MP, Horton NJ, Glynn RJ, Cupples LA. Bias due to missing exposure data using complete-case analysis in the proportional hazards regression model. Stat Med. 2003;22(4):545–557. doi: 10.1002/sim.1340. [DOI] [PubMed] [Google Scholar]
- 19.Cox D. Regression models and life-tables. J Royal Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 20.Cupples LA, D’Agostino RB, Anderson K, Kannel WB. Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med. 1988;7(1–2):205–222. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- 21.Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biol Psychol. 2012;90 (1):1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 23.Glei DA, Goldman N, Ryff CD, Lin YH, Weinstein M. Social relationships and inflammatory markers: an analysis of Taiwan and the U. S Soc Sci Med. 2012;74 (12):1891–1899. doi: 10.1016/j.socscimed.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford ES, Loucks EB, Berkman LF. Social integration and concentrations of C-reactive protein among US adults. Ann Epidemiol. 2006;16(2):78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Loucks EB, Berkman LF, Gruenewald TL, Seeman TE. Relation of social integration to inflammatory marker concentrations in men and women 70 to 79 years. Am J Cardiol. 2006;97(7):1010–1016. doi: 10.1016/j.amjcard.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 26.Loucks EB, Sullivan LM, D’Agostino RB, Sr, Larson MG, Berkman LF, Benjamin EJ. Social networks and inflammatory markers in the Framingham Heart Study. J Biosoc Sci. 2006;38(6):835–842. doi: 10.1017/S0021932005001203. [DOI] [PubMed] [Google Scholar]
- 27.Peuler JD, Scotti MA, Phelps LE, McNeal N, Grippo AJ. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol Behav. 2012;106(4):476–484. doi: 10.1016/j.physbeh.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 29.Shaw BA, Krause N, Liang J, Bennett J. Tracking changes in social relations throughout late life. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):S90–99. doi: 10.1093/geronb/62.2.s90. [DOI] [PubMed] [Google Scholar]
- 30.Fiori KL, Antonucci TC, Cortina KS. Social network typologies and mental health among older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61(1):P25–32. doi: 10.1093/geronb/61.1.p25. [DOI] [PubMed] [Google Scholar]
- 31.Lyyra TM, Lyyra AL, Lumme-Sandt K, Tiikkainen P, Heikkinen RL. Social relations in older adults: Secular trends and longitudinal changes over a 16-year follow-up. Arch Gerontol Geriatr. 51(3):e133–138. doi: 10.1016/j.archger.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Holt-Lunstad J, Birmingham W, Jones BQ. Is there something unique about marriage? The relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Ann Behav Med. 2008;35 (2):239–244. doi: 10.1007/s12160-008-9018-y. [DOI] [PubMed] [Google Scholar]
- 33.Yates BC, Bensley LS, Lalonde B, Lewis FM, Woods NF. The impact of marital status and quality on family functioning in maternal chronic illness. Health Care Women Int. 1995;16(5):437–449. doi: 10.1080/07399339509516197. [DOI] [PubMed] [Google Scholar]
- 34.Fothergill KE, Ensminger ME, Robertson J, Green KM, Thorpe RJ, Juon HS. Effects of social integration on health: A prospective study of community engagement among African American women. Soc Sci Med. 2011;72(2):291–298. doi: 10.1016/j.socscimed.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oman D, Kurata JH, Strawbridge WJ, Cohen RD. Religious attendance and cause of death over 31 years. Int J Psychiatry Med. 2002;32(1):69–89. doi: 10.2190/RJY7-CRR1-HCW5-XVEG. [DOI] [PubMed] [Google Scholar]
- 36.Strawbridge WJ, Shema SJ, Cohen RD, Kaplan GA. Religious attendance increases survival by improving and maintaining good health behaviors, mental health, and social relationships. Ann Behav Med. 2001;23(1):68–74. doi: 10.1207/s15324796abm2301_10. [DOI] [PubMed] [Google Scholar]
- 37.Strawbridge WJ, Cohen RD, Shema SJ, Kaplan GA. Frequent attendance at religious services and mortality over 28 years. Am J Public Health. 1997;87(6):957–961. doi: 10.2105/ajph.87.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirschman KB, Bourjolly JN. How do tangible supports impact the breast cancer experience? Soc Work Health Care. 2005;41(1):17–32. doi: 10.1300/J010v41n01_02. [DOI] [PubMed] [Google Scholar]
- 39.Woloshin S, Schwartz LM, Tosteson AN, Chang CH, Wright B, Plohman J, Fisher ES. Perceived adequacy of tangible social support and health outcomes in patients with coronary artery disease. J Gen Intern Med. 1997;12(10):613–618. doi: 10.1046/j.1525-1497.1997.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arno PS. The economic value of informal caregiving, US, 2000. American Association for Geriatric Psychiatry; Florida: 2002. [Google Scholar]
- 41.Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Aff (Millwood) 1999;18(2):182–188. doi: 10.1377/hlthaff.18.2.182. [DOI] [PubMed] [Google Scholar]
- 42.Cannuscio CC, Jones C, Kawachi I, Colditz GA, Berkman L, Rimm E. Reverberations of family illness: a longitudinal assessment of informal caregiving and mental health status in the Nurses’ Health Study. Am J Public Health. 2002;92(8):1305–1311. doi: 10.2105/ajph.92.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S, Kawachi I, Grodstein F. Does caregiving stress affect cognitive function in older women? J Nerv Ment Dis. 2004;192(1):51–57. doi: 10.1097/01.nmd.0000106000.02232.30. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Colditz G, Berkman L, Kawachi I. Caregiving to children and grandchildren and risk of coronary heart disease in women. Am J Public Health. 2003;93(11):1939–1944. doi: 10.2105/ajph.93.11.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am J Prev Med. 2003;24(2):113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 46.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 47.Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil. 2011;33(2):682–687. doi: 10.1016/j.ridd.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Wilks SE, Croom B. Perceived stress and resilience in Alzheimer’s disease caregivers: testing moderation and mediation models of social support. Aging Ment Health. 2008;12 (3):357–365. doi: 10.1080/13607860801933323. [DOI] [PubMed] [Google Scholar]
- 49.Turner HA, Catania JA. Informal caregiving to persons with AIDS in the United States: caregiver burden among central cities residents eighteen to forty-nine years old. Am J Community Psychol. 1997;25(1):35–59. doi: 10.1023/a:1024693707990. [DOI] [PubMed] [Google Scholar]
- 50.Lechner VM. Support systems and stress reduction among workers caring for dependent parents. Soc Work. 1993;38(4):461–469. [PubMed] [Google Scholar]
- 51.Huisman M. Imputation of missing network data: some simple procedures. Journal of Social Structure. 2009;10(1) [Google Scholar]
- 52.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 53.Little RAJ, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]