Abstract
Hypoparathyroidism is associated with abnormal structural and dynamic skeletal properties. We hypothesized that parathyroid hormone(1–84) [PTH(1–84)] treatment would restore skeletal properties toward normal in hypoparathyroidism. Sixty-four subjects with hypoparathyroidism were treated with PTH(1–84) for 2 years. All subjects underwent histomorphometric assessment with percutaneous iliac crest bone biopsies. Biopsies were performed at baseline and at 1 or 2 years. Another group of subjects had a single biopsy at 3 months, having received tetracycline before beginning PTH(1–84) and prior to the biopsy (quadruple-label protocol). Measurement of biochemical bone turnover markers was performed. Structural changes after PTH(1–84) included reduced trabecular width (144 ± 34 µm to 128 ± 34 µm, p = 0.03) and increases in trabecular number (1.74 ± 0.34/mm to 2.07 ± 0.50/mm, p = 0.02) at 2 years. Cortical porosity increased at 2 years (7.4% ± 3.2% to 9.2% ± 2.4%, p = 0.03). Histomorphometrically measured dynamic parameters, including mineralizing surface, increased significantly at 3 months, peaking at 1 year (0.7% ± 0.6% to 7.1% ± 6.0%, p = 0.001) and persisting at 2 years. Biochemical measurements of bone turnover increased significantly, peaking at 5 to 9 months of therapy and persisting for 24 months. It is concluded that PTH(1–84) treatment of hypoparathyroidism is associated with increases in histomorphometric and biochemical indices of skeletal dynamics. Structural changes are consistent with an increased remodeling rate in both trabecular and cortical compartments with tunneling resorption in the former. These changes suggest that PTH(1–84) improves abnormal skeletal properties in hypoparathyroidism and restores bone metabolism toward normal euparathyroid levels.
Keywords: Hypoparathyroidism, PTH(1–84), Bone histomorphometry, Bone turnover markers, Remodeling
Introduction
Hypoparathyroidism is due to deficient or absent parathyroid hormone (PTH). It occurs most commonly after thyroid or parathyroid surgery or as a consequence of autoimmune disorders.(1–3) Independent of etiology, the typical biochemical constellation in untreated hypoparathyroidism includes low or absent circulating PTH levels, hypocalcemia, relatively high urinary calcium excretion, hyperphosphatemia, and reduced levels of 1,25-dihydroxyvitamin D3.(4,5) In addition to the biochemical abnormalities, bone remodeling is markedly reduced in hypoparathyroidism.(6–9) Chronically low bone turnover typically leads to bone mass that is higher than that of age- and sex-matched control individuals.(6,10–13) We have reported that subjects with hypoparathyroidism have greater cancellous bone volume, trabecular width, and cortical width biopsy specimens.(6) Dynamic skeletal indices, including mineralizing surface and bone-formation rate, were profoundly suppressed in the hypoparathyroid subjects.(6) The abnormalities in cancellous microarchitecture were further confirmed by 3D assessment with micro–computed tomography (µCT).(14)
Treatment of hypoparathyroidism with PTH(1–84) helps to normalize calcium metabolism and leads to changes in BMD.(15) With PTH(1–84) treatment, lumbar spine BMD increases, whereas distal 1/3 radius BMD decreases.(15) How these densitometric changes relate to bone quality is not known. We hypothesized that administration of PTH(1–84) in hypoparathyroidism would reverse the atypical dynamic and structural skeletal properties that we had observed previously in untreated subjects. Our findings provide new insight into the fundamental importance of PTH in the maintenance of skeletal structure and function.
Methods
Subjects
We studied 64 subjects with documented hypoparathyroidism. The diagnosis of hypoparathyroidism was established by the simultaneous presence of serum calcium and PTH concentrations below the lower limits of normal on at least two occasions separated by an interval of at least 30 days. Hypoparathyroidism had to have been present for at least 3 years so as to represent a chronic state of PTH deprivation. Subjects were excluded if they had been on a bisphosphonate within 5 years prior to study entry or for greater than 6 months at any time or if they were women within 5 years of the onset of menopause. Subjects also were excluded if they used any of the following medications: estrogens, progestins, raloxifene, calcitonin, systemic corticosteroids, fluoride, lithium, statins, loop diuretics, or methotrexate. Potentially confounding disorders also were exclusionary criteria, if present: Paget’s disease of bone, diabetes mellitus, chronic liver or renal disease, acromegaly, Cushing syndrome, rheumatoid arthritis, or multiple myeloma.
Forty-five sex- and age-matched control subjects were randomly selected from four previous studies, which included 22 postmenopausal women,(16) 12 premenopausal women,(17)and 11 men.(18) There was no history of low-trauma fracture in any of the control individuals, as well as no history of medical illness or drug therapy known to affect bone metabolism.
Subjects were recruited from the Metabolic Bone Diseases Unit of Columbia University Medical Center and from the Hypoparathyroidism Association. The study was approved by the institutional review boards of Columbia University Medical Center and Helen Hayes Hospital. All subjects gave written informed consent.
Protocol
Hypoparathyroid subjects self-administered PTH(1–84), provided by NPS Pharmaceuticals (Bedminster, NJ, USA) for 24 months at a subcutaneous dose of 100 µg every other day. This dose was selected because we showed previously that this regimen restores suppressed bone turnover markers in hypoparathyroidism to levels that are in the normal range.(15) Calciotropic parameters and BMD were monitored as described previously.(15) Biochemical markers of bone turnover were measured thrice at baseline [at 1 month, 2 weeks, and 1 day prior to PTH(1–84) treatment] and at months 1, 2, 3, 4, 5, 6, 9, 12, 15, 18, 21, and 24. Subjects were randomly assigned to one of three bone biopsy schedules: (1) paired biopsies with tetracycline double labeling obtained at baseline and after 12 months of PTH(1–84) treatment, (2) paired biopsies with tetracycline double labeling obtained at baseline and after 24 months of PTH(1–84) treatment, and (3) one biopsy with a quadruple-label protocol after 3 months of PTH(1–84) treatment. In the quadruple-label protocol, two sets of tetracycline labels were administered sequentially, before PTH(1–84) initiation and 2 weeks prior to the biopsy, which was obtained after 3 months of PTH(1–84) administration. This method permits a comparison of dynamic formation indices at baseline and after PTH(1–84) treatment by measuring characteristics of each set of double labels separately and then comparing them with each other.(19)
Biochemical markers of bone turnover
Biochemistries were measured by automated techniques. Procollagen type 1 amino-terminal propeptide (P1NP) was measured by RIA (normal range 16 to 96 ng/mL for premenopausal women; IDS, Ltd., Fountain Hills, AZ, USA). Bone-specific alkaline phosphatase (BAP) was measured by enzyme immunoassay (normal range 11.6 to 29.6 U/L in premenopausal women; Metra BAP, Quidel Corp., San Diego, CA, USA). Intra- and interassay variabilities are 4% and 8%, respectively. Osteocalcin (OCN) was measured by ELISA(20) (N-mid Osteocalcin; normal mean ± SD 28.4 ± 9.5 ng/mL for postmenopausal women mean: IDS, Ltd., Scottsdale, AZ, USA). The intra- and inter assay variabilities both are 2.7%. Carboxy-terminal cross-linking telopeptide of bone collagen (CTX) was measured using ELISA (normal range for postmenopausal women 0.142 and 1.351 µg/L; IDS, Ltd., Scottsdale, AZ, USA). Within- and between-run precisions are less than 6% and less than 8%, respectively. TRACP-5b was measured by ELISA (normal range for postmenopausal women 3.2 ± 0.9 U/L; IDS, Ltd., Scottsdale, AZ, USA) with an interassay precision of less than 9%.
Histomorphometry
Two tetracycline labels were administered (Sumycin 250 mg four times daily) using a standard 3-days-on, 12-days-off, 3-days-on regimen immediately prior to initiation of PTH treatment.(19) For the two paired biopsy groups, baseline percutaneous iliac crest biopsies were performed 1 week after labeling. In these groups, the labeling schedule was repeated at either 12 or 24 months of PTH treatment, with percutaneous iliac crest biopsies performed 1 week after the second double-label protocol at either 12 or 24 months. For the quadruple-label protocol, the initial tetracycline labeling prior to PTH treatment was the same, but no baseline biopsy was performed. After 3 months of PTH administration, the tetracycline labeling protocol was repeated using the same schedule but with a different tetracycline (Declomycin 150 mg four times daily). Percutaneous iliac crest biopsies were performed 1 week after the second double-label protocol. This method yields two sets of fluorescent labels representing bone formation before (the first set) and after PTH (the second set) administration.(19) Each set of double labels was easily distinguishable by the color generated by the particular tetracycline under fluorescent light.
Biopsy specimens were processed and subjected to histomorphometric analysis as described in detail previously in our laboratory.(21) Histomorphometry was performed using a digitizing image-analysis system (OsteoMeasure, OsteoMetrics, Inc., Atlanta, GA, USA). Cancellous and cortical bone structures were assessed by measuring cancellous bone volume (BV/TV), trabecular width (Tb.W), trabecular number (Tb.N), trabecular separation (Tb.Sp), cortical width (Ct.W), and cortical porosity area (Ct.Po.Ar). Bone-remodeling activity was evaluated on cancellous, endocortical, or intracortical bone surfaces and expressed by the variables of osteoid perimeter (O.Pm), osteoid width (O.W), mineralizing perimeter (Md.Pm), mineral apposition rate (MAR), adjusted apposition rate (Aj.AR), bone-formation rate (BFR), and eroded surface (ES). Bone-resorption rate (BRs.R) was calculated as (BFR/BS)/(E.Pm/BS). All indices are expressed according to the recommendations of the ASBMR nomenclature committee.(22)
Statistical analysis
All continuous data are presented as mean value ± SD. Student’s t tests were used to assess differences between groups for normally distributed data, and the Mann-Whitney U test–Wilcoxon rank-sum test was used to determine significance of differences for non–normally distributed data. Significance of changes in indices from baseline was assessed with paired t tests. Linear regression analyses were performed to assess the relationship between absolute changes in biochemical markers of bone turnover and histomorphometric parameters. A p value of less than 0.05 was considered significant.
Results
Subjects
The hypoparathyroid subjects were predominantly women, consistent with the demographics of the disease (Table 1). The mean number of years from menopause in the postmenopausal women was 10 ± 7 years (minimum 3 years, maximum 30 years). The most common etiologies of the hypoparathyroidism were postoperative and autoimmune. The patients with autoimmune hypoparathyroidism did not show involvement of other endocrine glands. Eighteen of the subjects underwent only the baseline biopsy, whereas 14 subjects had paired biopsies obtained at baseline and after 12 months of PTH(1–84) treatment, 16 subjects had paired biopsies obtained at baseline and after 24 months of PTH(1–84) treatment, and 16 subjects had the quadruple-labeled biopsy after 3 months of PTH(1–84) treatment. There were no differences among the cohorts who had sampling at the different time points. Over half (54%, n = 35) were on thyroid hormone replacement. Thyroid-stimulating hormone (TSH) levels generally were within normal limits, but in 16 subjects, the TSH level was below normal (range 0.02 to 0.27 µU/mL, mean 0.09 ± 0.1 µU/mL), and in 4 subjects, the TSH level was above normal (range 5.23 to 60.98µU/mL, mean 20.64 ± 27 µU/mL). No individuals had evidence for clinical hypo- or hyperthyroidism. Abnormal TSH values had no effect on the analysis of the data (described below).
Table 1.
Characteristics of Hypoparathyroid Cohort at Baseline
| Hypoparathyroid cohort (n = 64) | Cohort range | Normal range | |
|---|---|---|---|
| Age (years) | 46 ± 13 | 18–71 | |
| Sex | Male: 16 | ||
| Female: 48 | |||
| (premenopause 28, postmenopause 20) | |||
| Etiology of hypoparathyroidism | Postoperative = 32 | ||
| Autoimmune = 30 | |||
| DiGeorge = 2 | |||
| Duration (years) | 15 ± 13 | 3–46 | |
| Calcium supplements (mg/d) | 2534 ± 1800 | 0–9,000 | |
| Calcitriol supplements (µg/d) | 0.75 ± 0.5 | 0–3 | |
| Parent vitamin D supplements (IU/d) (n = 28) | 5794 ± 15,568 | 50–75,000 | |
| Thiazide dose (mg) (n = 21) | 27.8 ± 21 | 6.25–100 | |
| Serum calcium (mmol/L) | 2.18 ± 0.3 | 1.58–2.53 | 2.13–2.55 |
| PTH (ng/L) | 4.4 ± 5 | 1–22 | 10–65 |
| Phosphate (mmol/L) | 1.42 ± 0.3 | 0.84–2.16 | 0.81–1.45 |
| Total alkaline phosphatase (U/L) | 63 ± 16 | 37–116 | 33–96 |
| 24-Hour urinary calcium excretion (mmol/L) | 63 ± 31 | 9–141 | |
| 25-Hydroxyvitamin D (nmol/L) | 142 ± 207 | 22–1425 | 22–130 |
| 1,25-Dihydroxyvitamin D3 (pg/mL) | 94 ± 55 | 31–377 | 39–156 |
| TSH (µU/mL) | 2.8 ± 8 | <0.3–61 | 0.4–4.7 |
| P1NP (ng/mL)a | 36.7 ± 32 | 9.5–228.0 | 16–96 |
| BAP (U/L)a | 25.5 ± 12.1 | 13.0–82.7 | 11–43 |
| Osteocalcin (ng/mL)a | 17.6 ± 47 | 2.3–321.8 | 8.8–55 |
| TRACP-5b (U/L)a | 2.5 ± 0.8 | 1.5–5.4 | 2.6–4.0 |
| s-CTX (ng/mL)a | 0.3 ± 0.3 | 0.1–1.6 | 0.1–1.4 |
Note: To convert serum calcium to mg/dL, divide by 0.25; PTH to pg/mL, divide by 1.0; phosphate to mg/dL, divide by 0.323; urinary calcium to mg/d, divide by 0.25; 25-hydroxyvitamin D to ng/mL, divide by 2.496; 1,25-dihydroxyvitamin D3 to pg/mL, divide by 2.6.
Measured three times.
Biochemical markers of bone turnover
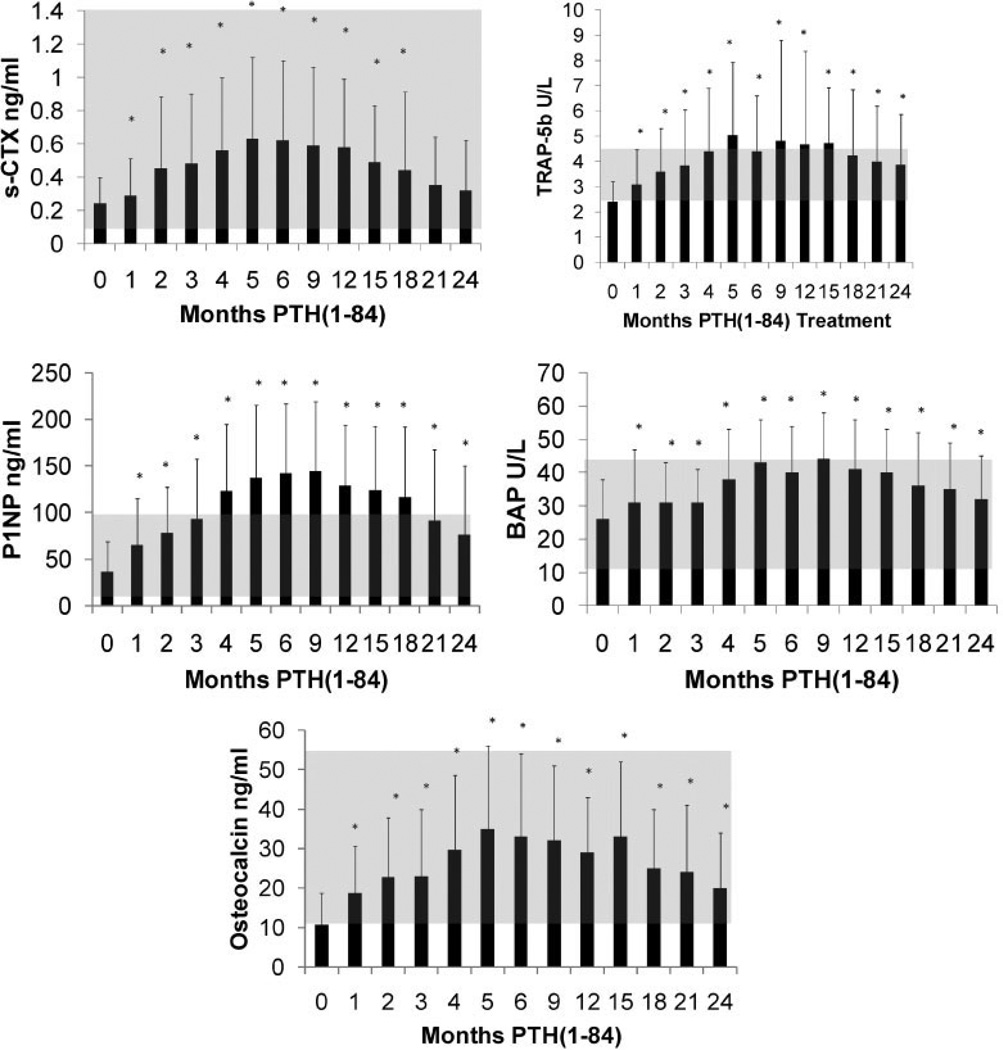
Baseline measurements of biochemical markers were in the lower half of the normal range (Table 1). With PTH(1–84) treatment, biochemical markers of bone formation and bone resorption (Fig. 1) increased significantly, reaching peak values at 5 to 9 months. All biochemical markers decreased from their peak levels by 24 months. With the exception of serum CTX, all bone turnover markers were still higher than baseline at month 24.
Fig. 1.
Changes in biochemical markers of bone turnover with PTH(1–84) treatment. The shaded areas represent the normal ranges. Values are mean ± SD. *p < 0.05 as compared with baseline value.
Bone histomorphometry: untreated hypoparathyroid subjects versus controls
Baseline biopsies were obtained on 48 hypoparathyroid subjects. These were compared with data from 45 age- and sex-matched normal subjects. As we reported previously in a smaller number of subjects,(6) there was more bone in the cancellous and cortical compartments in the hypoparathyroid subjects. They had greater cancellous bone volume (Table 2), as well as greater trabecular width, and a trend for cortical width also to be increased. Histomorphometric indices of bone turnover also confirmed our previous data(6) (Table 3), showing significantly reduced osteoid width, osteoid surface, mineral apposition rate, mineralizing surface, and bone-formation rate on the cancellous, endocortical, and intracortical surfaces. The adjusted apposition rate was lower in the hypoparathyroid subjects in the cancellous envelope. Eroded surface did not differ in any of the envelopes, whereas the bone-resorption rate was lower in the cancellous and endocortical surfaces.
Table 2.
Histomorphometric Variables of Bone Structure
| Subjects | BV/TV (%) | Tb.W (µm) | Tb.N (/mm) |
Tb.Sp (µm) |
Ct.W (µm) |
Cortical porosity (%) |
|
|---|---|---|---|---|---|---|---|
| Baseline | Hypo (n = 48) | 23.4 ± 7.3 | 129.2 ± 36.3 | 1.83 ± 0.4 | 447.30 ± 144.6 | 872.2 ± 92.7 | 6.97 ± 3.4 |
| Control (n = 45) | 19.8 ± 4.5 | 114.8 ± 20.2 | 1.74 ± 0.3 | 484.71 ± 131.2 | 748.36 ± 247.3 | 7.42 ± 3.9 | |
| p Value | 0.007 | 0.02 | NS | NS | 0.08 | NS | |
| Quadruple label (n = 16) | Baseline | N/A | N/A | N/A | N/A | N/A | N/A |
| 3 Months | 26.7 ± 6.5 | 123.6 ± 30.8 | 2.19 ± 0.4 | 348.2 ± 80.5 | 1036.0 ± 413.6 | 8.57 ± 3.3 | |
| 1-Year hypo pairs (n = 14) | Baseline | 24.7 ± 7.6 | 143.8 ± 34.4 | 1.71 ± 0.4 | 472.69 ± 185.9 | 992.0 ± 550 | 7.01 ± 4.1 |
| 1 Year | 26.4 ± 8.0 | 127.5 ± 33.8 | 2.12 ± 0.6 | 389.35 ± 161.7 | 1091 ± 264 | 10.2 ± 4.1 | |
| p Value | NS | 0.03 | 0.02 | NS | NS | 0.01 | |
| 2-Year hypo pairs (n = 16) | Baseline | 22.8 ± 6.9 | 133.0 ± 38.3 | 1.74 ± 0.3 | 464.0 ± 110 | 922.57 ± 327.8 | 7.40 ± 3.2 |
| 2 Years | 24.2 ± 5.6 | 120.1 ± 23.1 | 2.07 ± 0.5 | 399.27 ± 126.2 | 922.03 ± 403.9 | 9.22 ± 2.4 | |
| p Value | NS | NS | 0.02 | 0.06 | NS | 0.03 |
BV/TV = trabecular bone volume; Tb.W = trabecular width; Tb.N = trabecular number; Tb.Sp = trabecular separation; Ct.W = cortical width. N/A = not available because only dynamic parameters can be evaluated at baseline with the quadruple labeling protocol.
Table 3.
Histomorphometric Variables of Bone Remodeling
| A. Cancellous Envelope | |||||||||
| Subjects | O.W, lamellar, no. |
OS, % | MS, % | MAR, µm/d |
BFR/BS, mm/µm2/d |
Aj.AR, µm/d |
ES, % | BRs.R, µm/d |
|
| Baseline | Hypo (n = 48) | 2.00 ± 1.0 | 2.78 ± 3.0 | 1.00 ± 2.0 | 0.49 ± 0.3 | 0.008 ± 0.02 | 0.37 ± 0.4 | 4.47 ± 2.7 | 0.0018 ± 0.003 |
| Control (n = 45) | 4.00 ± 1.0 | 7.18 ± 4.2 | 4.33 ± 3.2 | 0.70 ± 0.2 | 0.033 ± 0.03 | 0.51 ± 0.4 | 4.16 ± 2.1 | 0.0100 ± 0.012 | |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.09 | NS | <0.0001 | |
| Quadruple label (n = 16) | Baseline | N/A | N/A | 0.39 ± 0.6 | 0.32 ± 0.2 | 0.002 ± 0.01 | N/A | N/A | N/A |
| 3 Months | 2.94 ± 0.9 | 8.51 ± 9.0 | 5.47 ± 6.0 | 0.67 ± 0.3 | 0.043 ± 0.05 | 0.53 ± 0.4 | 5.59 ± 2.7 | 0.0070 ± 0.006 | |
| p Value | N/A | N/A | 0.004 | <0.0001 | 0.004 | N/A | N/A | N/A | |
| 1-Year hypo pairs (n = 14) | Baseline | 2.00 ± 1.5 | 2.24 ± 1.8 | 0.65 ± 0.6 | 0.53 ± 0.3 | 0.005 ± 0.01 | 0.26 ± 0.3 | 5.15 ± 2.5 | 0.001 ± 0.01 |
| 1 Year | 3.93 ± 1.3 | 10.8 ± 5.1 | 7.05 ± 6.0 | 0.82 ± 0.2 | 0.067 ± 0.06 | 0.69 ± 0.6 | 5.56 ± 3.3 | 0.018 ± 0.02 | |
| p Value | 0.001 | 0.0001 | 0.001 | 0.006 | 0.001 | 0.02 | NS | 0.005 | |
| 2-Year hypo pairs (n = 16) | Baseline | 2.69 ± 1.5 | 2.80 ± 3.4 | 1.18 ± 2.2 | 0.44 ± 0.4 | 0.010 ± 0.02 | 0.38 ± 0.5 | 3.86 ± 2.5 | 0.002 ± 0.01 |
| 2 Years | 3.44 ± 0.8 | 12.04 ± 10.8 | 3.34 ± 0.8 | 0.74 ± 0.2 | 0.029 ± 0.04 | 0.23 ± 0.2 | 5.55 ± 2.5 | 0.005 ± 0.01 | |
| p Value | NS | 0.003 | 0.04 | 0.009 | 0.06 | NS | 0.07 | 0.05 | |
| B. Endocortical Envelope | |||||||||
| Subjects | O.W, lamellar, no. |
OS, % | MS, % | MAR, µm/d |
BFR/BS, mm/µm2/d |
Aj.AR, µm/d |
ES, % | BRs.R, µm/d |
|
| Baseline (n = 48) | Hypo | 2.00 ± 1.0 | 5.47 ± 6.9 | 2.20 ± 3.9 | 0.28 ± 0.3 | 0.016 ± 0.03 | 0.80 ± 1.5 | 5.36 ± 3.4 | 6.35 ± 4.0 |
| Control | 3.00 ± 1.0 | 12.56 ± 7.1 | 9.89 ± 10.0 | 0.68 ± 0.3 | 0.077 ± 0.08 | 0.34 ± 0.6 | 5.65 ± 3.1 | 0.0247 ± 0.033 | |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.08 | NS | 0.002 | |
| Quadruple label (n = 16) | Baseline | N/A | N/A | 1.19 ± 1.7 | 0.14 ± 0.2 | 0.006 ± 0.01 | N/A | N/A | N/A |
| 3 Month | 2.13 ± 1.2 | 12.67 ± 10.1 | 9.03 ± 8.3 | 0.57 ± 0.3 | 0.073 ± 0.07 | 0.65 ± 0.5 | 4.39 ± 3.1 | 0.0234 ± 0.020 | |
| p Value | N/A | N/A | 0.001 | <0.0001 | 0.001 | N/A | N/A | N/A | |
| 1-Year hypo pairs (n = 14) | Baseline | 1.79 ± 1.4 | 6.21 ± 6.8 | 1.81 ± 3.3 | 0.27 ± 0.3 | 0.012 ± 0.02 | 0.23 ± 0.3 | 5.81 ± 3.1 | 0.003 ± 0.01 |
| 1 Year | 3.36 ± 1.5 | 13.66 ± 6.4 | 12.05 ± 7.7 | 0.71 ± 0.3 | 0.108 ± 0.09 | 0.87 ± 0.7 | 5.76 ± 2.9 | 0.032 ± 0.03 | |
| p Value | 0.006 | 0.02 | 0.0004 | 0.009 | 0.001 | 0.008 | NS | 0.003 | |
| 2-Year hypo pairs (n = 16) | Baseline | 2.00 ± 1.4 | 5.32 ± 6.0 | 2.62 ± 3.3 | 0.32 ± 0.3 | 0.021 ± 0.03 | 0.54 ± 1.0 | 4.96 ± 2.7 | 0.004 ± 0.01 |
| 2 Years | 2.63 ± 1.0 | 15.55 ± 10.9 | 4.21 ± 6.3 | 0.40 ± 0.3 | 0.032 ± 0.06 | 0.25 ± 0.4 | 6.90 ± 3.2 | 0.008 ± 0.02 | |
| p Value | NS | 0.004 | NS | NS | NS | 0.08 | 0.06 | NS | |
| C. Intracortical Envelope | |||||||||
| Subjects | O.W, lamellar, no. |
OS, % | MS, % | MAR, µm/d |
BFR/BS, mm/µm2/d |
Aj.AR, µm/d |
ES, % | BRs.R, µm/d |
|
| Baseline (n = 48) | Hypo | 2.50 ± 1.5 | 5.25 ± 5.4 | 4.42 ± 5.3 | 0.57 ± 0.4 | 0.041 ± 0.06 | 0.88 ± 1.2 | 3.24 ± 2.8 | 0.0228 ± 0.056 |
| Control | 3.40 ± 1.3 | 10.19 ± 7.3 | 9.39 ± 7.7 | 0.81 ± 0.4 | 0.087 ± 0.08 | 1.12 ± 1.2 | 4.59 ± 3.8 | 0.0318 ± 0.036 | |
| p Value | 0.002 | <0.0001 | <0.0001 | 0.004 | 0.002 | NS | 0.05 | NS | |
| Quadruple label (n = 16) | Baseline | N/A | N/A | 4.38 ± 4.3 | 0.73 ± 0.5 | 0.046 ± 0.06 | N/A | N/A | N/A |
| 3 Months | 2.31 ± 1.0 | 7.33 ± 5.1 | 8.56 ± 7.0 | 0.84 ± 0.4 | 0.093 ± 0.09 | 1.37 ± 1.1 | 4.39 ± 3.1 | 0.0234 ± 0.020 | |
| p Value | N/A | N/A | 0.001 | NS | 0.001 | N/A | N/A | N/A | |
| 1-Year hypo pairs (n = 14) | Baseline | 2.79 ± 1.5 | 5.42 ± 5.2 | 4.25 ± 5.0 | 0.57 ± 0.3 | 0.034 ± 0.04 | 0.95 ± 1.2 | 2.62 ± 1.7 | 0.018 ± 0.02 |
| 1 Year | 4.36 ± 1.0 | 15.42 ± 7.1 | 18.64 ± 9.4 | 1.02 ± 0.2 | 0.200 ± 0.12 | 1.48 ± 1.0 | 4.53 ± 2.1 | 0.074 ± 0.06 | |
| p Value | 0.002 | 0.003 | 0.004 | 0.0001 | 0.0002 | NS | 0.05 | 0.006 | |
| 2-Year hypo pairs (n = 16) | Baseline | 2.40 ± 1.5 | 4.64 ± 5.3 | 3.50 ± 5.0 | 0.59 ± 0.5 | 0.038 ± 0.06 | 0.84 ± 1.5 | 2.83 ± 3.3 | 0.036 ± 0.09 |
| 2 Years | 3.56 ± 1.0 | 9.84 ± 8.8 | 5.26 ± 4.5 | 0.77 ± 0.3 | 0.049 ± 0.05 | 0.76 ± 0.7 | 3.37 ± 1.9 | 0.020 ± 0.02 | |
| p Value | 0.006 | NS | NS | NS | NS | NS | NS | NS | |
O.W = osteoid width; OS = osteoid surface; MS = mineralizing surface; MAR = mineral apposition rate; BFR/BS = bone-formation rate; AjAR = adjusted apposition rate; ES = eroded surface; BRs.R = bone-resorption rate.
Bone histomorphometry: longitudinal treatment of hypoparathyroid subjects with PTH(1–84)
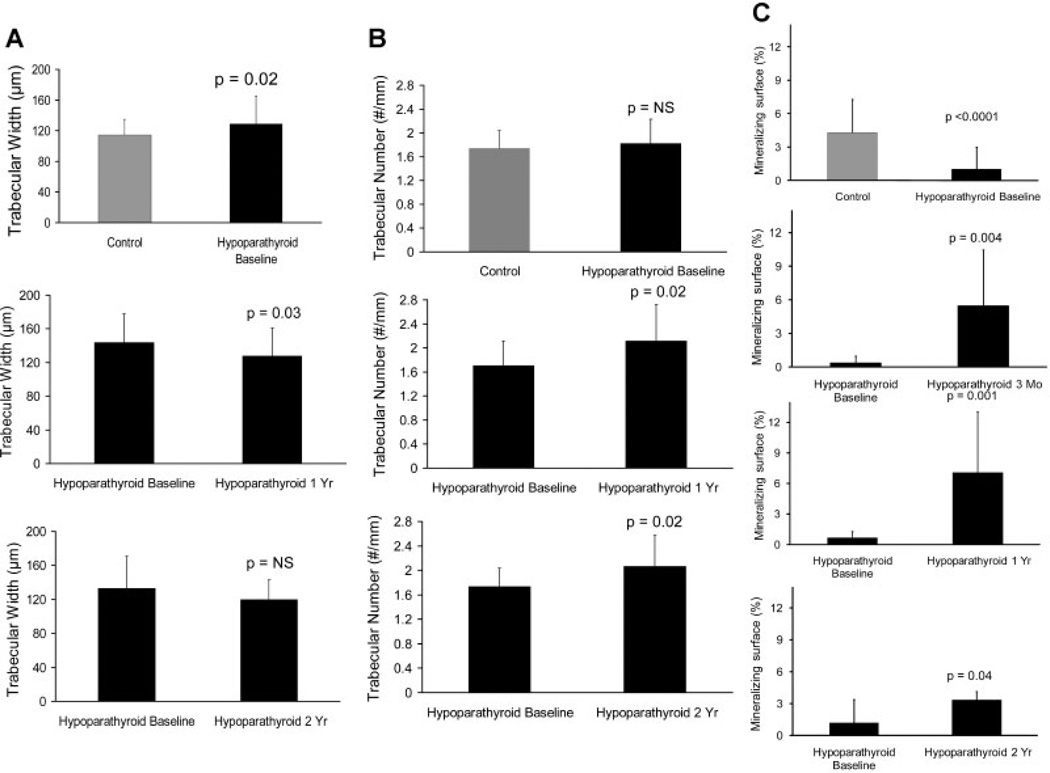
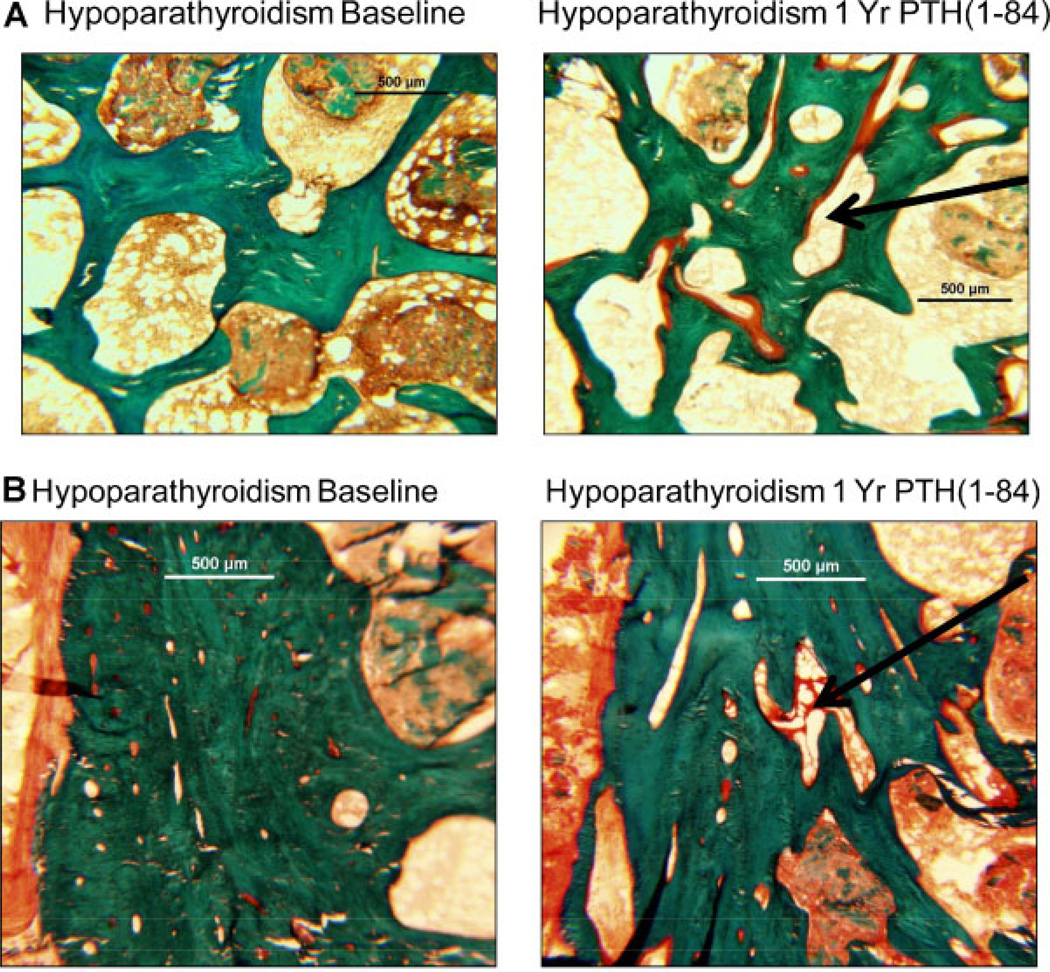
Requirements for supplemental calcium and calcitriol decreased with PTH treatment, whereas biochemistries remained stable.(15) A comparison of structural parameters was performed in 14 biopsy pairs from 0 to 12 months of PTH treatment and in 16 other biopsy pairs from 0 to 24 months of PTH treatment. While cancellous bone volume did not change, trabecular width decreased from baseline at 12 months (Table 2 and Fig. 2A), and trabecular number increased from baseline at both 12 and 24 months (Table 2 and Fig. 2B). At 24 months of PTH treatment, Tb.W was no longer different in the hypoparathyroid subjects and the controls. A representative depiction of these changes in trabecular indices in one subject, before and after 1 year of PTH is shown in Fig. 3A. The trabecular tunneling evident at 1 year also was seen in the 2-year biopsies (data not shown). Cortical width did not change, but cortical porosity increased at both 1 and 2 years (Table 2). A representative depiction of these changes in cortical indices in one subject before and after 1 year of PTH is shown in Fig. 3B.
Fig. 2.
Changes in trabecular width (A), trabecular number (B), and mineralizing surface (C) with PTH(1–84) treatment. Measurements are shown in the cancellous envelope. Values are mean ± SD.
Fig. 3.
Iliac crest biopsy illustrating changes in the trabecular (A) and cortical (B) structure before and after 1 year of PTH(1–84) treatment in a hypoparathyroid subject. Note the increases in trabecular tunneling and cortical porosity (arrows) in the posttreatment biopsy.
In addition to the 1- and 2-year paired histomorphometric analyses, 16 subjects underwent quadruple-label assessment at 3 months, permitting the calculation of early changes in bone turnover indices, including mineralizing surface, mineral apposition rate, and bone-formation rate. The 3-month data also could be assessed in relationship to the 12- and 24-month time points after PTH(1–84) treatment. All three dynamic parameters increased at 3 and 12 months in all three bone envelopes (Table 3 and Fig. 2C). By 24 months, mineralizing surface and mineral apposition rate returned to baseline levels at the endocortical and intracortical envelopes, whereas the bone-formation rate returned to baseline values in all envelopes.
Osteoid width increased in all envelopes at 12 months, although it did not remain increased at 24 months in the cancellous and endocortical envelopes (Table 3), consistent with the observation that bone-formation rate peaked at 12 months. Osteoid surface similarly increased at 12 months, remaining increased at 24 months in the cancellous and endocortical envelopes but not at the intracortical site. Adjusted apposition rate increased at 12 months in the cancellous and endocortical envelopes, with no difference from baseline at 24 months (Table 3). With regard to parameters of bone resorption, the bone-resorption rate increased at all envelopes at 12 months but remained above baseline at 24 months only in the cancellous envelope. Eroded surface did not change in any envelope. Exclusion of the subjects with abnormal TSH values from the analysis did not alter any of these findings.
Relationship between biochemical markers of bone turnover and histomorphometry
Changes in the biochemical markers of bone turnover significantly predicted changes in histomorphometric parameters at 3, 12, and 24 months (Table 4). The relationship between changes in bone turnover markers and changes in dynamic histomorphometry was observed most frequently at the cancellous envelope. Increases in the bone-formation marker P1NP were particularly predictive of increases in the dynamic histomorphometric parameters of bone formation.
Table 4.
Relationship Between Changes in Biochemical Markers of Bone Turnover and Histomorphometric Indices With PTH(1–84) Treatment
| Increase in histomorphometric parameter |
Predicted by increase in turnover marker |
R2 | Slope (β) | p Value | |
|---|---|---|---|---|---|
| Quadruple label (3 months) | Cancellous Md.Pm | P1NP | 0.51 | 0.102 ± 0.03 | < 0.01 |
| TRAP | 0.50 | 3.04 ± 0.96 | 0.01 | ||
| Cancellous BFR | P1NP | 0.36 | 0.001 ± 0.01 | 0.04 | |
| TRAP | 0.45 | 0.024 ± 0.01 | 0.02 | ||
| 12-Month repeat biopsy | Cancellous Md.Pm | P1NP | 0.54 | 0.056 ± 0.02 | 0.01 |
| BAP | 0.34 | 0.192 ± 0.08 | 0.05 | ||
| CTX | 0.41 | 6.75 ± 2.53 | 0.02 | ||
| TRAP | 0.50 | 0.689 ± 0.22 | 0.01 | ||
| Cancellous MAR | BAP | 0.36 | 0.007 ± 0.01 | 0.04 | |
| Cancellous BFR | P1NP | 0.52 | 0.001 ± 0.01 | 0.01 | |
| BAP | 0.33 | 0.002 ± 0.01 | 0.05 | ||
| CTX | 0.41 | 0.066 ± 0.03 | 0.03 | ||
| TRAP | 0.46 | 0.007 ± 0.01 | 0.02 | ||
| Endocortical MAR | OCN | 0.39 | 0.002 ± 0.01 | 0.03 | |
| 24-Month repeat biopsy | BV/TV | P1NP | 0.41 | 0.071 ± 0.02 | 0.01 |
| BAP | 0.36 | 0.326 ± 0.12 | 0.02 | ||
| Cancellous Md.Pm | P1NP | 0.37 | 0.04 ± 0.01 | 0.02 | |
| OCN | 0.37 | 0.219 ± 0.08 | 0.02 | ||
| CTX | 0.44 | 4.302 ± 1.35 | <0.01 | ||
| TRAP | 0.50 | 2.197 ± 0.61 | <0.01 | ||
| Cancellous BFR | P1NP | 0.40 | 0.001 ± 0.01 | 0.01 | |
| OCN | 0.37 | 0.002 ± 0.01 | 0.02 | ||
| CTX | 0.42 | 0.039 ± 0.01 | <0.01 | ||
| TRAP | 0.53 | 0.021 ± 0.01 | <0.01 | ||
| Endocortical Md.Pm | CTX | 0.83 | 7.884 ± 0.98 | <0.0001 | |
| Endocortical BFR | CTX | 0.87 | 0.078 ± 0.01 | <0.0001 |
Discussion
This study demonstrates that PTH(1–84) treatment of hypoparathyroidism influences the abnormal dynamic and structural skeletal properties characteristic of this disorder. Biochemical evidence of dynamic changes was reflected by marked increases in biochemical bone turnover markers. These increases persisted for the study duration, peaking at 5 to 9 months of therapy and declining, in part, by 24 months. Corresponding histomorphometric parameters also increased dramatically, with a similar pattern of an early increase at 3 months, a peak at 1 year, and a partial decrease at 24 months. These dynamic changes suggest that the initial response to PTH is an exuberant one with subsequent tempering over time. Structural changes were seen as early as 1 year after PTH(1–84) treatment, with reduced trabecular thickness and increased trabecular number and cortical porosity. These structural changes are consistent with an increase in bone-remodeling rate in both the trabecular and cortical compartments with tunneling resorption in the trabecular compartment. Overall, these changes suggest that administration of PTH(1–84) ameliorates the abnormal dynamic and structural properties seen in untreated hypoparathyroidism and restores bone metabolism to levels more typical of euparathyroid individuals.
Previous studies on the structural effects of PTH replacement in the hypoparathyroid skeleton have been limited to densitometric assessment. Using PTH(1–34) in 27 hypoparathyroid subjects for 3 years, Winer and colleagues found that BMD did not change at any site in comparison with calcitriol treatment.(23) We reported previously that 2 years of PTH(1–84) led to BMD changes, with an increase at the cancellous-enriched lumbar spine and a decrease at the predominantly cortical distal 1/3 radius.(15) The densitometric pattern that we observed is consistent with the known proclivity of PTH to be anabolic at cancellous sites and catabolic at cortical sites when used both as an osteoporosis treatment(24) and in a disease of PTH excess, namely, primary hyperparathyroidism.(25) Although this study does not fully explain the mechanisms for the PTH-induced densitometric changes that we reported previously, the additional data available in this report further suggest that the decrease in cortical BMD is likely attributable to an increase in cortical porosity. This change differs from that seen by histomorphometry in cortical bone with PTH treatment for osteoporosis, where cortical thickness increases with no change in cortical porosity.(26,27) The increase in spine BMD that we observed could be explained by an increase in trabecular tunneling, with more numerous, thinner trabeculae being detected as greater BMD by densitometry.(28) Such changes also have been seen in trabecular bone by histomorphometry with PTH treatment for osteoporosis.(26,27,29) Further studies using tools such as central quantitative computed tomography and high-resolution peripheral quantitative computed tomography will help to relate these changes to our previous densitometric findings as well as indices of bone strength.
The dynamic indices of bone turnover showed dramatic increases in response to PTH treatment, as measured both by multiple biochemical markers and by histomorphometric parameters. Moreover, changes in biochemical markers of bone turnover strongly predicted changes in histomorphometric assessment of bone remodeling. In the 3-year study of Winer and colleagues, biochemical markers of bone turnover increased with PTH(1–34) at a mean dose of 40 mcg twice daily, peaking at 2.5 years. Our data showed a similar pattern, with turnover markers also peaking and then falling, although with a more rapid time course. It is possible that the later peak of 2.5 years in the Winer study compared with 5 to 9 months in our study was due to differences in the dosing regimen of PTH, as well as in patient populations; the Winer study included subjects with renal insufficiency and calcium-sensing receptor mutations. When PTH is used in the treatment of osteoporosis, bone turnover markers show a pattern similar to that which we observed in hypoparathyroidism, with a peak in markers by 12 months and a variable decline thereafter.(30–32)
It could be argued that the decline in the dynamic changes that we observed, both biochemically and histomorphometrically, suggests that a “tachyphylaxis” to the skeletal effects of PTH is developing over time. Over the first months, the initial, exuberant increases in remodeling show a rise from the suppressed baseline levels to levels that are close to or above the upper limit of the normal range when compared with both normal ranges for turnover markers and control biopsies. Clinical data match this time course, with the lowest requirement for supplemental calcium and vitamin D being at 9 months, coincident with the peak levels of biochemical markers of bone turnover.(15) Rather than a tachyphylaxis, this initial exuberant response may reflect, alternatively, heightened sensitivity of hypoparathyroid subjects to PTH, an observation made many years ago by Chase and Aurbach using urinary cyclic AMP as a marker of PTH responsiveness.(33,34) Consistent with this idea, by 24 months, the biochemical and histomorphometric parameters of remodeling fall to levels within the normal range while still generally remaining higher than baseline levels. In our view, the decline is best viewed as a return toward normal from an initial state of heightened PTH responsiveness in hypoparathyroidism.
While it could be argued with justification that this study has a limitation of a relatively small biopsy sample size for each of the three biopsy groups, this experience still remains the largest histomorphometric study of PTH therapy in hypoparathyroidism. Given the rarity of hypoparathyroidism and the design of our protocol, the data do provide sufficient justification for the conclusions that we have reached. A related limitation is that, by necessity, the 3-month quadruple-label biopsies and the 1- and 2-year paired biopsies were each performed in three different groups of subjects because each subject can only undergo a maximum of two biopsies. The random assignment to the different biopsy groups was designed to address this point, and in fact, there were no apparent differences in the three cohorts that had sampling at different times after PTH administration. We did not have a placebo group that underwent biopsies over time. However, it appears highly unlikely that the observed changes could be attributable to factors other than PTH administration.
In conclusion, administration of PTH(1–84) improves abnormal dynamic and structural skeletal properties in hypoparathyroidism, restoring bone metabolism toward normal euparathyroid levels. Our findings provide new insight into the actions of PTH on skeletal kinetics, as well as into the fundamental importance of PTH in the maintenance of skeletal structure and function.
Acknowledgments
This work was funded by NIH Grants DK067619, DK069350, FD-R-02525, and a Columbia University Florence Irving Scholars Award.
MRR and JPB have received research funding from NPS Pharmaceuticals.
Footnotes
Disclosures
All the other authors state that they have no conflicts of interest.
Authors’ roles: All authors made substantial contributions to either the conception and design, acquisition of data or analysis and interpretation of data, participated in drafting the manuscript or revising it critically for important intellectual content, and approved the final version of the submitted manuscript.
References
- 1.Mayer A, Ploix C, Orgiazzi J, Desbos A, Moreira A, Vidal H, Monier JC, Bienvenu J, Fabien N. Calcium-sensing receptor autoantibodies are relevant markers of acquired hypoparathyroidism. J Clin Endocrinol Metab. 2004;89:4484–4488. doi: 10.1210/jc.2004-0021. [DOI] [PubMed] [Google Scholar]
- 2.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 3.Neufield R, Maclaren N, Blizzard R. Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune syndromes. Medicine. 1981;60:355. doi: 10.1097/00005792-198109000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kao PC, van Heerden JA, Grant CS, Klee GG, Khosla S. Clinical performance of parathyroid hormone immunometric assays. Mayo Clin Proc. 1992;67:637–645. doi: 10.1016/s0025-6196(12)60717-4. [DOI] [PubMed] [Google Scholar]
- 5.Michelangeli VP, Heyma P, Colman PG, Ebeling PR. Evaluation of a new, rapid and automated immunochemiluminometric assay for the measurement of serum intact parathyroid hormone. Ann Clin Biochem. 1997;34(Pt 1):97–103. doi: 10.1177/000456329703400115. [DOI] [PubMed] [Google Scholar]
- 6.Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J, Jr, Silverberg SJ, Bilezikian JP. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res. 2008;23:2018–2024. doi: 10.1359/JBMR.080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langdahl BL, Mortensen L, Vesterby A, Eriksen EF, Charles P. Bone histomorphometry in hypoparathyroid patients treated with vitamin D. Bone. 1996;18:103–108. doi: 10.1016/8756-3282(95)00443-2. [DOI] [PubMed] [Google Scholar]
- 8.Kruse K, Kracht U, Wohlfart K, Kruse U. Biochemical markers of bone turnover, intact serum parathyroid horn and renal calcium excretion in patients with pseudohypoparathyroidism and hypoparathyroidism before and during vitamin D treatment. Eur J Pediatr. 1989;148:535–539. doi: 10.1007/BF00441552. [DOI] [PubMed] [Google Scholar]
- 9.Mizunashi K, Furukawa Y, Miura R, Yumita S, Sohn HE, Yoshinaga K. Effects of active vitamin D3 and parathyroid hormone on the serum osteocalcin in idiopathic hypoparathyroidism and pseudohypoparathyroidism. J Clin Invest. 1988;82:861–865. doi: 10.1172/JCI113690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abugassa S, Nordenstrom J, Eriksson S, Sjoden G. Bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab. 1993;76:1617–1621. doi: 10.1210/jcem.76.6.8501170. [DOI] [PubMed] [Google Scholar]
- 11.Fujiyama K, Kiriyama T, Ito M, Nakata K, Yamashita S, Yokoyama N, Nagataki S. Attenuation of postmenopausal high turnover bone loss in patients with hypoparathyroidism. J Clin Endocrinol Metab. 1995;80:2135–2138. doi: 10.1210/jcem.80.7.7608266. [DOI] [PubMed] [Google Scholar]
- 12.Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL. Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Invest. 1982;69:1302–1309. doi: 10.1172/JCI110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touliatos JS, Sebes JI, Hinton A, McCommon D, Karas JG, Palmieri GM. Hypoparathyroidism counteracts risk factors for osteoporosis. Am J Med Sci. 1995;310:56–60. doi: 10.1097/00000441-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Rubin MR, Dempster DW, Kohler T, Stauber M, Zhou H, Shane E, Nickolas T, Stein E, Sliney J, Jr, Silverberg SJ, Bilezikian JP, Muller R. Three dimensional cancellous bone structure in hypoparathyroidism. Bone. 2010;46:190–195. doi: 10.1016/j.bone.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin MR, Sliney J, Jr, McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21:1927–1934. doi: 10.1007/s00198-009-1149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recker RR, Kimmel DB, Parfitt AM, Davies KM, Keshawarz N, Hinders S. Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res. 1988;3:133–144. doi: 10.1002/jbmr.5650030203. [DOI] [PubMed] [Google Scholar]
- 17.Parisien M, Cosman F, Morgan D, Schnitzer M, Liang X, Nieves J, Forese L, Luckey M, Meier D, Shen V, Lindsay R, Dempster DW. Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. J Bone Miner Res. 1997;12:948–957. doi: 10.1359/jbmr.1997.12.6.948. [DOI] [PubMed] [Google Scholar]
- 18.Clarke BL, Ebeling PR, Jones JD, Wahner HW, O’Fallon WM, Riggs BL, Fitzpatrick LA. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996;81:2264–2270. doi: 10.1210/jcem.81.6.8964862. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21:366–373. doi: 10.1359/JBMR.051109. [DOI] [PubMed] [Google Scholar]
- 20.Rosenquist C, Qvist P, Bjarnason N, Christiansen C. Measurement of a more stable region of osteocalcin in serum by ELISA with two monoclonal antibodies. Clin Chem. 1995;41:1439–1445. [PubMed] [Google Scholar]
- 21.Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 1999;84:1562–1566. doi: 10.1210/jcem.84.5.5652. [DOI] [PubMed] [Google Scholar]
- 22.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 23.Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, Gerber LH, McGarvey C, Cutler GB., Jr Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88:4214–4220. doi: 10.1210/jc.2002-021736. [DOI] [PubMed] [Google Scholar]
- 24.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, Bilezikian JP. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 26.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 28.Cosman F, Schnitzer MB, McCann PD, Parisien MV, Dempster DW, Lindsay R. Relationships between quantitative histological measurements and noninvasive assessments of bone mass. Bone. 1992;13:237–242. doi: 10.1016/8756-3282(92)90203-9. [DOI] [PubMed] [Google Scholar]
- 29.Recker RR, Bare SP, Smith SY, Varela A, Miller MA, Morris SA, Fox J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone. 2009;44:113–119. doi: 10.1016/j.bone.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618–1623. doi: 10.1056/NEJM199412153312404. [DOI] [PubMed] [Google Scholar]
- 31.Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34): a randomized controlled trial. JAMA. 1998;280:1067–1073. doi: 10.1001/jama.280.12.1067. [DOI] [PubMed] [Google Scholar]
- 32.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069–3076. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 33.Chase LR, Aurbach GD. Parathyroid function and the renal excretion of 3′5′-adenylic acid. Proc Natl Acad Sci U S A. 1967;58:518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law WM, Jr, Heath H., 3rd Increased renal responses to exogenous parathyroid hormone in postsurgical hypoparathyroidism. J Clin Endocrinol Metab. 1984;59:394–397. doi: 10.1210/jcem-59-3-394. [DOI] [PubMed] [Google Scholar]