Abstract
Alterations in the quality, quantity and architecture of baseline and recovery sleep have been shown to occur during aging. Sleep deprivation induces endoplasmic reticular (ER) stress and upregulates a protective signaling pathway termed the unfolded protein response (UPR). The effectiveness of the adaptive UPR is diminished by age. Previously, we showed that endogenous chaperone levels altered recovery sleep in Drosophila melanogaster. We now report that acute administration of the chemical chaperone sodium 4-phenylbutyrate (PBA) reduces ER stress and ameliorates age-associated sleep changes in Drosophila. PBA consolidates both baseline and recovery sleep in aging flies. The behavioral modifications of PBA are linked to its suppression of ER stress. PBA decreased splicing of x-box binding protein 1 (XBP1) and upregulation of phosphorylated elongation initiation factor 2 α (p-eIF2α), in flies that were subjected to sleep deprivation. We also demonstrate that directly activating ER stress in young flies fragments baseline sleep and alters recovery sleep. Alleviating prolonged/sustained ER stress during aging contributes to sleep consolidation and improves recovery sleep/ sleep debt discharge.
Keywords: Aging, unfolded protein response, sleep, chaperone, sleep loss/ deprivation, 4-phenylbuturate
1. Introduction
Aging involves progressive deterioration of many physiological functions over time. Many age-associated diseases such as Alzheimer’s disease, Parkinson’s disease and type II diabetes are partially characterized by accumulation and aggregation of misfolded proteins, indicating a decline in quality control and chaperoning systems (Naidoo 2009a). One such quality control system, the endoplasmic reticulum (ER) stress response also known as the unfolded protein response (UPR), is responsible for maintaining protein homeostasis in the ER, the location for synthesis, processing, folding, and post-translational modifications of all secretory and integral membrane proteins. Alterations in ER homeostasis disrupt proper folding and lead to accumulation of misfolded proteins, which are deleterious to cell survival. A variety of physiological conditions can provoke ER stress, including glucose/energy deprivation, redox changes and alterations in calcium signaling. The UPR (Schroder & Kaufman 2005, Zhang & Kaufman 2006) has three distinct cellular responses: 1) upregulation of distinct molecular chaperones such as immunoglobulin binding protein/glucose-regulated protein 78(BiP/GRP78) through the inositol-requiring enzyme-1 (IRE1) pathway, 2) attenuation of protein translation mediated by the serine–threonine kinase PKR-like ER kinase (PERK), which phosphorylates the eukaryotic initiation factor 2α (eIF2α), subsequently reducing translation; and 3) degradation of misfolded proteins by ER-associated degradation (ERAD) (Harding et al. 1999). Excessive or extended ER stress leads to a maladaptive response and apoptosis, through activation of caspases and/or JNK signaling pathways (Szegezdi et al. 2006, Wu & Kaufman 2006).
Prolonged wakefulness/ sleep deprivation activates the UPR in mice (Naidoo et al. 2005) and the fruitfly Drosophila melanogaster (Shaw et al. 2000, Naidoo et al. 2007). Additionally, the UPR influences recovery sleep following sleep loss. Overexpression of BiP, also known as heat shock cognate 70 (HSC70-3) in Drosophila, results in increased recovery sleep when compared to sleep deprived wild type controls (Naidoo et al. 2007). Further, animals that had reduced levels of functional BiP recovered less sleep after deprivation. These results are particularly pertinent in the context of baseline sleep and recovery sleep in the aged/elderly. Impairments in sleep architecture and sleep consolidation, including an increase in excessive daytime sleepiness (EDS), nighttime awakenings and reductions in recovery sleep, are well documented in aging populations (Wolkove et al. 2007, Pandi-Perumal et al. 2002, Mendelson & Bergmann 2000). EDS is associated with significant negative health consequences including increased incidence of functional impairments (Lee et al. 2007) and mortality (Empana et al. 2009). EDS is also one of the most prevalent features of neurodegenerative diseases (Kato et al. 2012). Basal expression of BiP as well as other UPR components decreases with age (Naidoo 2009b). Collectively, these results suggest that the amount of chaperone present influences the amount of sleep recovered after sleep loss (Naidoo et al. 2007).
In this study, we examined the role of ER stress in sleep and sleep homeostasis. First, we wanted to determine if supplementing basal levels of endogenous molecular chaperones with a chemical chaperone would alleviate ER stress and alter baseline and recovery sleep in aged flies. Secondly, we sought to ascertain whether inducing ER stress in young flies would confer an aged phenotype. Lastly, we examined the effect of the chemical chaperone on sleep behavior in a short-sleeping mutant. The chemical chaperone we chose is sodium 4-phenylbutyrate (PBA), which is a non-selective chaperone that binds to the exposed hydrophobic regions of misfolded proteins. It has been shown to stabilize protein conformation, improve the folding capacity of the ER and facilitate the trafficking of mutant proteins (Ozcan et al. 2006). We wanted to establish whether acute administration of PBA would alter the UPR response and/or modify sleep behavior. We assessed sleep in aging populations of Drosophila and demonstrated consolidation of baseline sleep in aging flies by application of a clinically relevant dose of PBA. We also show that recovery sleep is altered in aged populations of flies and that PBA ameliorates some of these age-related sleep changes. We found that tunicamycin treatment, which induces ER stress, fragments baseline sleep and alters recovery sleep, demonstrating a direct link between ER stress and sleep. We also illustrate that PBA treatment consolidates sleep in a short sleeping mutant. These results demonstrate a correlation between the improvements in sleep by PBA application and attenuation of the IRE1 and PERK pathways of the UPR.
2. Methods
2.1 Fly stocks and maintenance
The D. melanogaster strain white Canton-Special (wCS10), a gift from Ronald Davis, Baylor College of Medicine (Houston, TX), w1118ex from Bloomington Stock Center (Indiana) and Sleepless (sssp1) and its background control strain iso31, a gift from Amita Sehgal/ Kyunghee Koh (University of Pennsylvania/ Thomas Jefferson University, Philadelphia, PA) were used in these studies. All flies were maintained at room temperature on standard food that contained molasses or dextrose, cornmeal and yeast on a 12 h: 12 h light: dark cycle. Flies were transferred onto new food every 2–3 days. wCS10 maximum lifespan = 93 days, w1118ex = 73 days, ctrl sss, isogenic wild-type strain, iso31 = 101 days and sssp1 = 50 days. Flies were divided into age groups as follows: wCS10 young (9–12 days) and aged (8 weeks). At 8 weeks >40% of these animals were still alive. For the w1118ex, iso31and sssp1 strains, flies were young (7 days) and aged (5–6 weeks).
2.2 Drug administration
4 sodium phenylbutyrate (PBA) and tunicamycin were purchased from Calbiochem, EMD Chemicals Inc. (Gibbstown, NJ). The purity of PBA was 99.6%. PBA was diluted in deionized distilled water. 5mM PBA was chosen as the preferred dose from a survival curve using 1mM, 5mM and 10mM. An acute treatment of PBA was shown to be more beneficial than a continuous dose on life span (Zhao et al. 2005). Tunicamycin was prepared in 95% ethanol for a stock solution of 1.19mM. For both PBA and tunicamycin treatment, flies were placed into locomotor tubes containing the sucrose/agar media and drug (5mM PBA or 12µM tunicamycin) or vehicle (distilled water or 0.01% ethanol).
2.3 Circadian and behavioral sleep assays
Flies were collected one day after eclosion and housed in groups until behavior was recorded using video (see 2.5). For all behavioral experiments, D. melanogaster virgin females were placed in individual locomotor activity tubes onto plates that hold 28 animals. Each locomotor activity tube contained a minimal media food mixture that consisted of 5% sucrose 1% agar media with or without 5mM of PBA. Flies were allowed to acclimate to the tubes at least 24 h before the recordings began. Baseline sleep was recorded for 2 days. Sleep was defined as a 5-minute bin without activity (Andretic & Shaw 2005). Sleep/ wake behavior was monitored throughout the study. We performed 15 baseline experiments of 28 flies each with young wCS10 flies. For the aged wCS10 flies, we performed 10 baseline experiments of 28 flies each. For the w1118ex strain, we performed 4 behavioral baseline experiments in the young flies and 4 experiments in the aged flies. The tunicamycin baseline experiments consisted of 4 experiments. The flies were treated with tunicamycin 24 h prior to recording sleep behavior. We performed 5 experiments for the sssp1 mutants and their background controls iso31.
2.4 Sleep deprivation (SD) and recovery
Baseline behavior was recorded for 2 days before SD. Flies were sleep deprived for 6 hours during the consolidated rest period from ZT 18 to ZT 24 (Naidoo et al. 2007). This task was accomplished manually by gently tapping the plates as necessary to keep the flies moving as determined by visual observation of fly behavior under red light conditions. We estimated that flies lost between 90–100% of their sleep based on previously published studies where sleep loss during SD was measured using the same method (Naidoo et al, 2007). Time and aged matched control flies were maintained in the monitors without intervention until sacrificed.
Recovery sleep was recorded for 24 h immediately following sleep deprivation beginning at ZT0. There were 12 recovery experiments performed for young wCS10 flies and 8 experiments for the aged wCS10 flies (n=28/ experiment). For w1118ex, we performed 4 recovery experiments for the young and 4 experiments in the aged flies (n=28/experiment).
The tunicamycin studies consisted of 4 experiments (n=28/experiment). For these sets of experiments, flies were placed on tunicamycin 18 h before SD. Each experiment consisted of 28 flies each recorded in a separate locomotor tube. Flies that died before the end of the experiments were not included in the behavioral analysis.
2.5 Video recording and analysis
Flies were recorded using the video system previously described (Zimmerman et al 2008). Images were acquired at 5 second intervals using a Retiga 2000R camera (Qimaging, Surrey BC) and custom software written using MatLab (Mathworks, Natick MA). Infrared LED lamps (Lilin Corp., Arcadia CA) at a peak wavelength of 850nm were used for camera illumination during the dark period. Video analysis: Custom software written with Matlab and C computer languages was used to analyze the video images using subtraction analysis. Corresponding pixels from two temporally adjacent images are subtracted and each pixel in the DIFFERENCE image has the value: GS (XiYj)=[(GS2(XiYj)-GS1(XiYj))/2]+127,where GS(XiYj) is the DIFFERENCE image grayscale value centered around a value of 127 at pixels X position i and Y position j and GS2 and GS1 are the grayscale values at that same pixel for the second and first video frames, respectively, in a pair of temporally adjacent frames.
2.6 Fly head preparation and western blotting
Flies were either sacrificed at the end of the undisturbed rest period or after sleep deprivation. Protein was extracted using a standard cell lysis protocol as described previously (Naidoo et al. 2007) (groups of 10 pooled fly heads, n=4). Protein concentrations were determined using the Pierce micro-BCA assay kit. Protein samples from pooled head homogenate (15µg/well) were run on SDS-PAGE gels (Bio-Rad, 4–20% Tris-HCl) and then transferred to nitrocellulose membranes (Bio-Rad) and incubated with primary antibodies. Primary antibodies and dilutions used were: Hsc 3 (Heat shock cognate 3)-BiP/GRP78 (Brabaham, UK) 1:2500, anti-phospho-eIF2α (Ser51) polyclonal antibody and β-actin (Cell Signaling Technology, Tokyo, Japan), 1:1000. This was followed by a 1 h incubation with secondary antibodies (anti-rat 1:20 000, Sigma, for BiP/GRP78 and anti-rabbit 1:10 000 for all other antibodies, except β-actin [mouse, 1:10 000]). Protein bands were detected and analyzed by the Odyssey Infrared Scanner (Li-Cor). We first detected the phospho-protein and then used NewBlot™ Nitro stripping buffer (Li-Cor, 30 min @ room temperature) and re-probed the membranes with an antibody recognizing the non-phosphorylated form.
2.7 Reverse transcriptase PCR and product quantification
Total RNA from flies was isolated using TRIzol reagent (Invitrogen) in conjunction with the RNeasy Mini Kit (Qiagen). We synthesized cDNA from one microgram of total RNA samples using Superscript III (Invitrogen). XBP1 cDNA was amplified by RT-PCR using primers that flanked the unconventional splice site (Sidrauski & Walter 1997) in xbp1 mRNA (Xbp1_F, 5’-CGCCAGCGCAGGCGCTGAGG-3’ and Xbp1_R, 5’-CTGCTCCGCCAGCAGACGCGC-3’). The protocol was 25 PCR cycles long. PCR product was then ran on a 3% agarose gel and stained with Ethidium bromide (EtBr) to visualize the bands. The unspliced band was observed at 127 base pairs and the spliced variant was observed at 104 base pairs. For quantification of the unspliced and spliced variants of XBP1, we used the Agilent 2100 Bioanalyzer and ran the PCR product on a DNA 1000 chip. The chip comes with its own ladder and set of internal standards. The generated product was then run on the chip with a standard curve and the internal standards and quantified.
2.8 Statistical analysis
Mixed effects models were utilized to examine the interactive effects of age, drug treatment and rebound on sleep outcomes. Multiple linear regression was utilized to examine between group effects on primary sleep outcomes in Drosophila. Day and night time data were analyzed separately. Comparisons were made between groups of young and aged animals and age-matched individuals for baseline and recovery sleep analysis. Student’s t test was applied when appropriate.
3. Results
3.1 PBA consolidates/increases baseline sleep in aged animals
Daytime Baseline Sleep and Wake
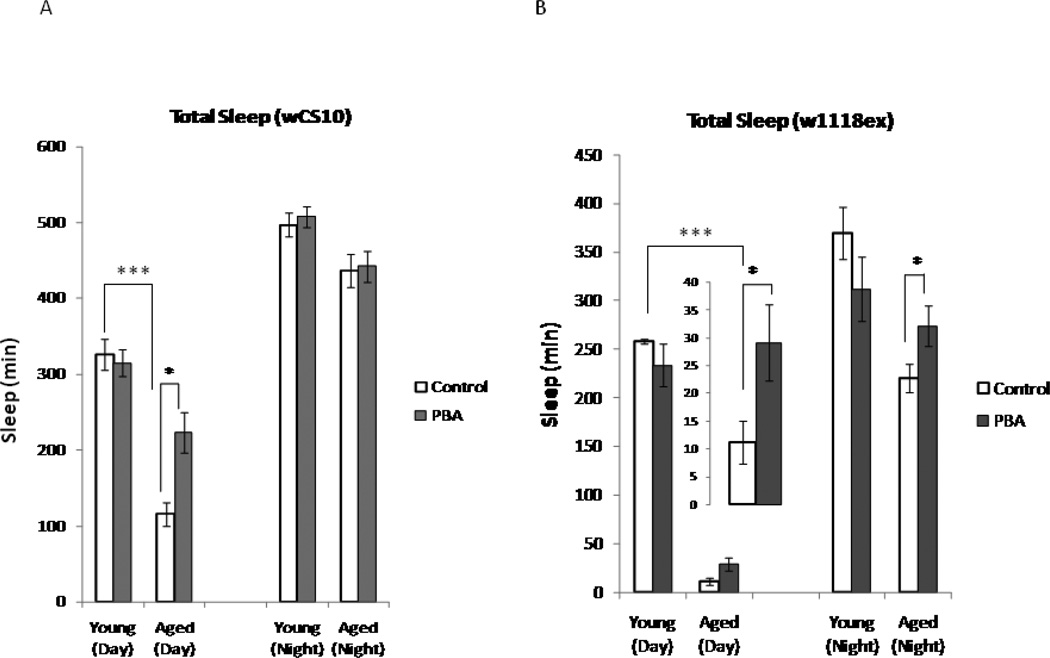
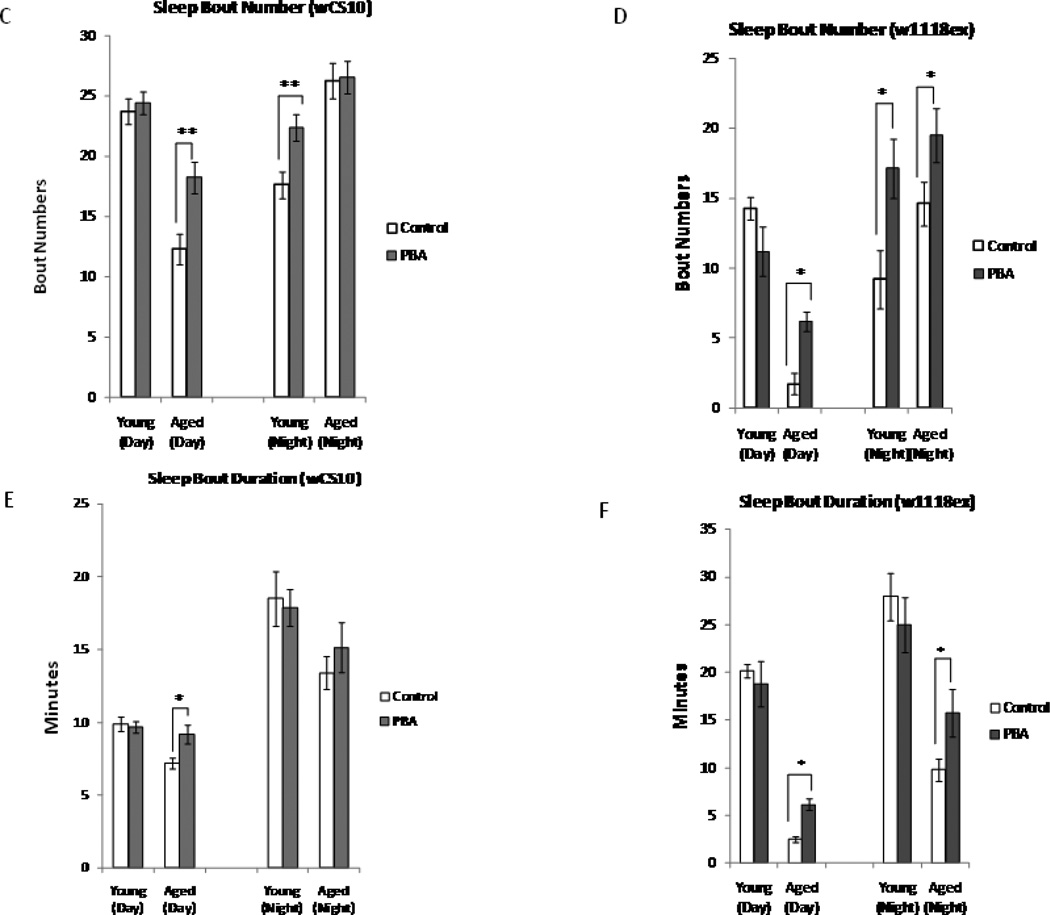
Sleep/ wake cycle strength in Drosophila diminishes with age and sleep becomes more fragmented, as demonstrated by increased sleep bout numbers and decreased sleep bout duration (Koh et al. 2006). In addition to fragmentation of sleep during the night, we found that aged flies sleep significantly less during the daytime as compared to their young counterparts, leading to an overall decrease in total sleep (Fig. 1A and Fig. 1B). We hypothesized that decreases in endogenous chaperone levels contribute to age-related changes in sleep. Therefore, we examined the effect of PBA treatment on baseline sleep at different ages. Flies were treated with PBA (5mM) for two days before recording sleep. We found that there was an age-dependent response to PBA treatment. Aged flies treated with PBA experienced a significant increase in total sleep time during the day in comparison to their untreated age-matched controls (wCS10, p<0.05, w1118ex, p<0.05 (Fig.1A and 1B). Sleep architecture was also modified with PBA treatment. Sleep bout numbers ([p<0.01], wCS10 and [p<0.05], w1118ex) (Fig. 1C and D) and sleep bout duration (p<0.05; wCS10 and w1118ex) (Fig. 1E and F) were both significantly increased in aged flies treated with PBA compared to their untreated age-matched controls.
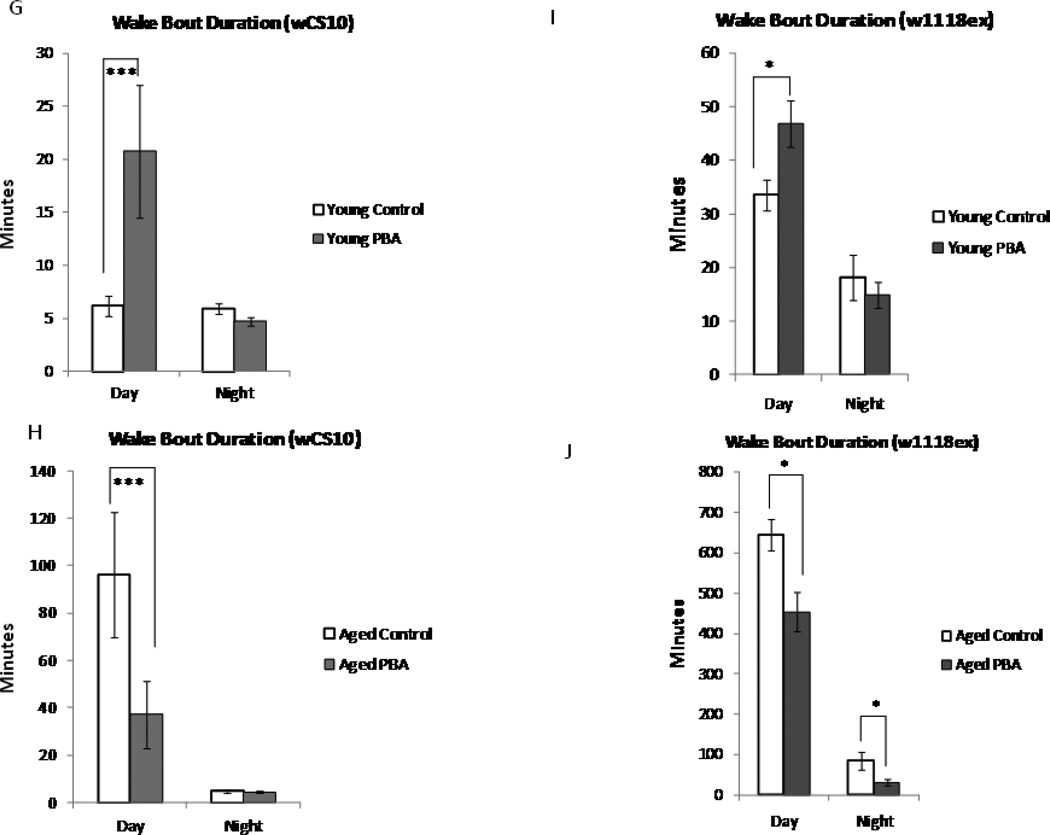
Fig. 1.
Total sleep time decreases with age. (A) Total sleep in minutes in young (n=143) and aged (n=106) wCS10 flies. Total sleep significantly decreases in the aged flies (p<0.001) in comparison to the young flies. Treatment with 5mM of PBA has little effect on young flies, but it significantly increases total daytime sleep in the aged flies (p<0.01). (B) Total sleep in minutes in young (n=54) and aged (n=40) w1118ex flies. PBA significantly increased total sleep in aged flies during both the day and night (p<0.05). (C, D) The number of sleep bouts significantly decreases during the daytime (p<0.01) in the aged flies in comparison to the young flies. Treatment with PBA significantly increased the number of sleep bouts (p<0.01) in the aged flies during the day and the young flies during the night. PBA significantly increased the sleep bout numbers in the young (night) and aged flies (day and night, p<0.05). (E, F) Sleep bout duration in the aged wCS10 during the day and in the aged w1118ex in both the day and night (p<0.05) was increased with PBA. (G-J) Mean wake bout duration is increased with PBA during the daytime in the young wCS10 (p<0.001) and the w1118ex flies (p<0.05). In the aged wCS10 and w1118ex flies, treatment with PBA significantly decreased wake bout duration during the daytime in comparison to their age-matched controls (wCS10, p<0.001; w1118ex p<-0.05). In the aged w1118ex flies, wake bout duration was also significantly decreased during the night (p<0.05). Mean + S.E.M. shown *P<0.05, **P<0.01, ***P<0.001
In young flies treated with PBA, wake bout duration was significantly increased in both strains (wCS10 [p<0.001]; w1118ex, [p<0.05] during the day (Fig.1G and 1I). Aged flies exhibit extremely long wake bout durations during the day in comparison to the young flies. PBA treatment significantly decreased wake bout duration in aged flies (wCS10 [p<0.001]; w1118ex [p<0.05]) (Fig.1H and Fig. 1J), during the day. In the wCS10 aged flies treated with PBA, the wake bout duration trended towards the amount observed in the young flies. These results suggest that PBA activity is different in young versus aged animals.
Nighttime Baseline Sleep and Wake
We found no significant changes in total sleep in young flies treated with PBA during the 12 h night time period. Sleep bout numbers however, (Fig. 1C and D) were increased in both strains when treated with PBA (wCS10; p<0.01, w1118ex (p<0.05). In aged w1118ex, PBA significantly increased total sleep (p<0.05; Fig. 1B), sleep bout number (p<0.05; Fig. 1D) and sleep bout duration (p<0.05; Fig. 1F) in these flies. Wake bout duration, on the other hand, decreased in aged w1118ex flies treated with PBA (p<0.05) (Fig. 1J). These results suggest that PBA treatment consolidates waking in young flies during the day and sleep both during the day and night in aged flies.
To determine whether the alterations observed in the aged flies under light-dark conditions had not occurred as a result of declining responsiveness to light, we also examined flies under constant darkness (DD). Flies were acclimated for 4 days in 12h light: 12h dark (LD) before being placed into DD for 7 days. Similar to other studies, we observed that the majority of the aged animals were arrhythmic and displayed a longer period (Rakshit et al. 2012). However, we found no significant differences in the sleep and wake of young or aged flies treated with PBA in DD compared to LD, indicating that the effects in LD are recapitulated in DD (data not shown).
3.2 Recovery sleep is altered with age
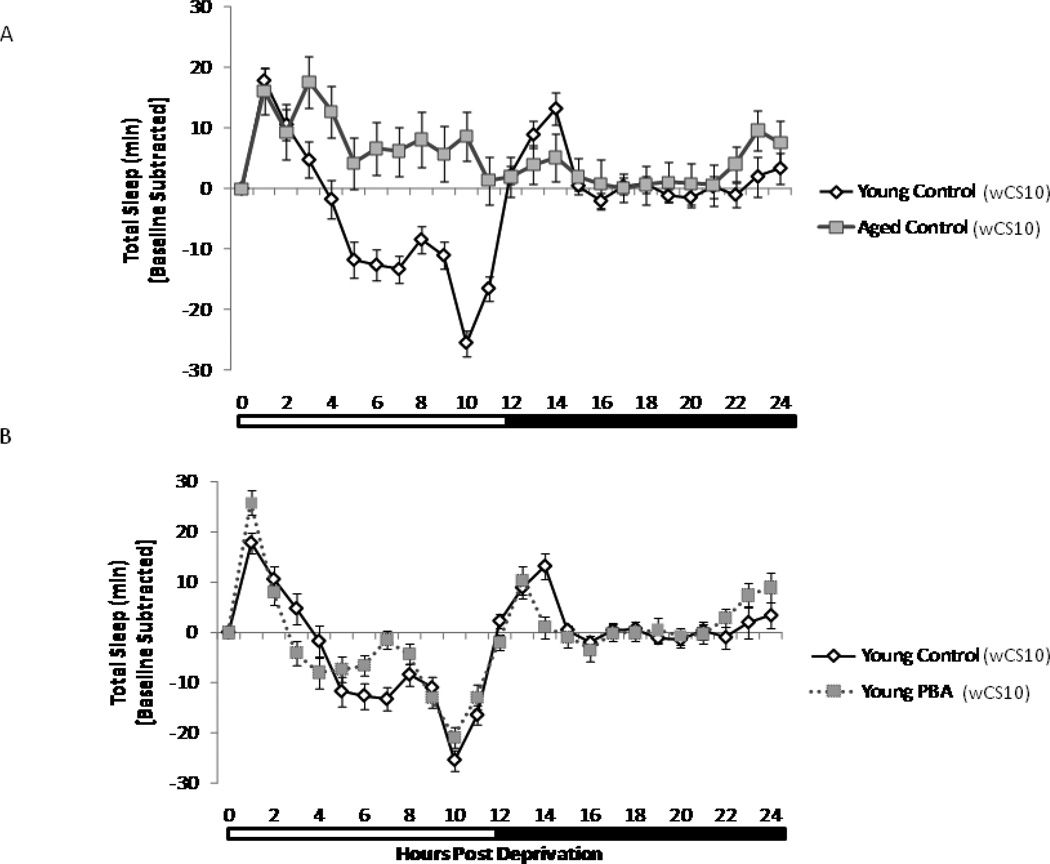
The homeostatic component of sleep governs the aspect involving recovery sleep, i.e. the longer animals are in a state of wakefulness, either normal or forced, the more profound the ensuing sleep period will be. Flies were sleep deprived for 6 h between ZT18 to ZT24; the sleep period where they display the most consolidated sleep (Naidoo et al. 2007) and unpublished observations). Recovery sleep was assessed immediately after lights on at ZT0 for 24 h. The mechanism(s) governing recovery sleep are intact in young wCS10 flies, so 4 h of recovery sleep is sufficient (Fig. 2A and Fig. 2B).
Fig. 2.
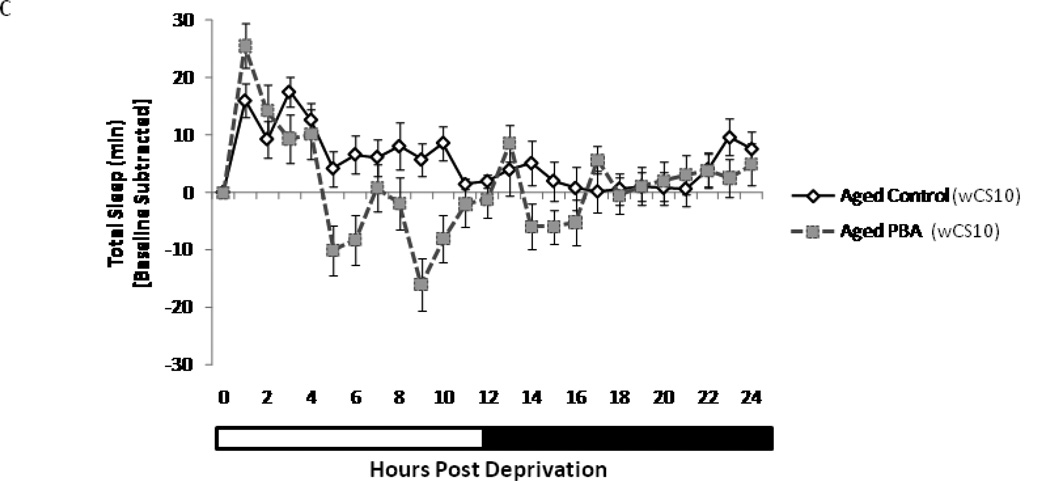
Sleep homeostasis is impaired during aging. Aging alters the homeostatic response after 6 h of sleep deprivation. Flies were sleep deprived (SD) for 6 h from (Zeitgeber times, ZT) ZT18 to ZT24 and allowed to recover sleep for 24 h after deprivation. Young flies efficiently recover lost sleep whereas the aged flies do not. Recovery sleep per hour 24 hours post sleep deprivation in (A) young (n=47) and aged untreated control (n=43) flies. Data is shown as the difference in sleep per hour post deprivation to that over same time interval on preceding day for each fly averaged across all flies with standard error of the mean. PBA has minimal effects in the (B) young (n=47) flies, however, it increased the amount and efficiency of recovery sleep in the (C) aged wCS10 flies (n=37). Data is displayed as the average amount of total minutes of sleep after baseline subtraction and S.E.M.
Aged flies also display a homeostatic response to sleep deprivation however, the pattern of recovery is altered; they recover sleep over an extended period (>12 h) (Fig. 2C). Since the aging process diminishes the efficiency of sleep debt discharge following sleep deprivation, we wanted to determine if this defect could be modified. Based on the rationale that endogenous chaperones can regulate the homeostatic component of sleep (Naidoo et al. 2007) and that these chaperones decrease with age (Naidoo et al. 2008); we asked whether application of a chemical chaperone would alter recovery sleep in aged flies.
PBA treated young wCS10 flies recover sleep in the first 3 h post deprivation, which is an hour earlier than their untreated age-matched controls (Fig. 2B). Aged wCS10 flies that were treated with PBA, like the young control flies (both treated and untreated), recover sleep during the first 4–5 hours post deprivation. This period is shorter than the age-matched untreated control flies which sleep throughout the day (Fig.2A and 2C). In aged flies treated with PBA, the recovery sleep peak is left shifted. Aged w1118ex flies demonstrated a similar recovery profile as the aged wCS10 animals in that PBA altered the recovery sleep pattern in a comparable manner in these flies (Supplemental Fig. 1).
3.3 Induction of ER stress fragments baseline sleep and alters recovery sleep
Aged animals display sleep fragmentation and also exhibit basal ER stress (Naidoo et al. 2008). To directly demonstrate that ER stress and sleep fragmentation in aged flies affects the recovery sleep response to sleep deprivation; we treated young flies with the drug tunicamycin to induce sleep fragmentation and simulate an aging phenotype. Tunicamycin, an inhibitor of protein glycosylation which leads to protein misfolding, was used to induce ER stress (Ozcan et al. 2006) in young flies. Flies were treated with tunicamycin 24 h prior to behavioral recordings. We found that tunicamycin fragmented daytime and nighttime sleep in a dose dependent manner in concentrations ranging from 1 to 24µM (data not shown).
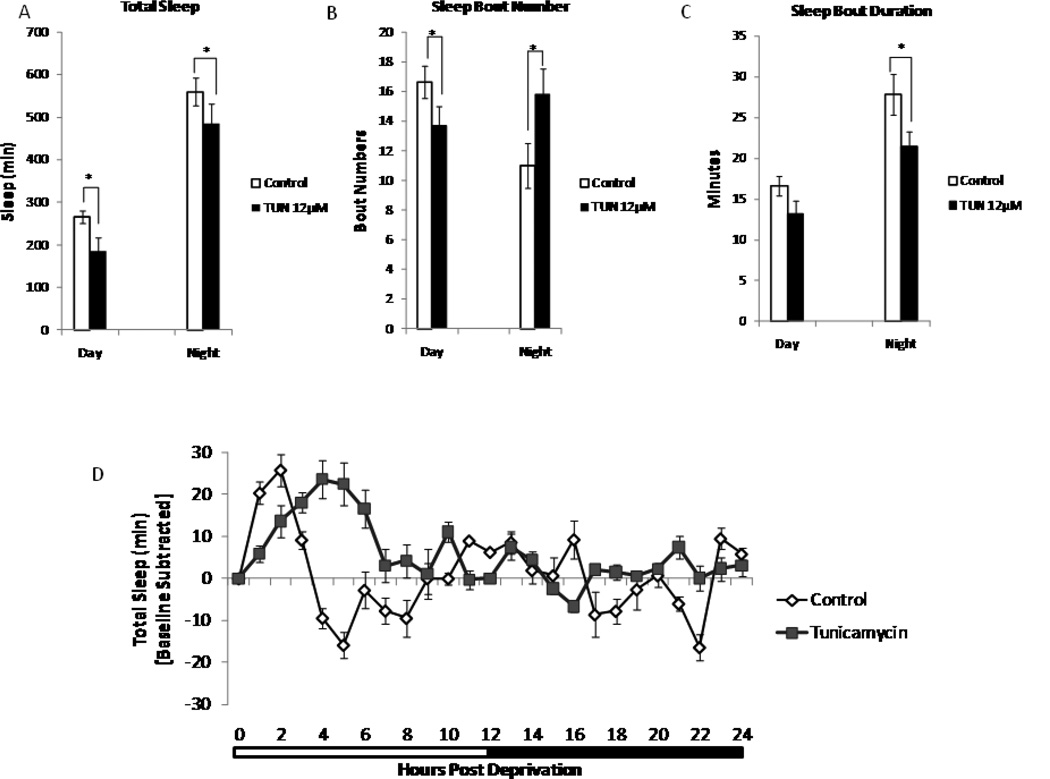
Total sleep was significantly decreased in wCS10 flies that were treated with tunicamycin (12µM) (day and night p<0.05). Tunicamycin also significantly decreased sleep bout numbers during the day (p<0.05) and increased sleep bout numbers during the night (p<0.05). Flies treated with tunicamycin also displayed significant decreases in sleep bout duration (p<0.05) during the night (Fig. 3A–C). We then subjected tunicamycin treated flies to 6 h of acute sleep deprivation from ZT18 to ZT24. We found that recovery sleep in young flies treated with tunicamycin (12µM) phenocopied recovery sleep in the aged flies (Fig. 3D); as flies continued to get recover sleep over a longer period.
Fig. 3.
Tunicamycin fragments baseline sleep. (A) Total sleep at baseline during both the day and the night (12µM [p<0.05]) is decreased. (B) Sleep bout numbers are increased (p<0.05) and (C) sleep bout duration is decreased (p<0.05) during the night in young wCS10 flies treated with tunicamycin (n=36). (D) Recovery sleep is altered in young flies treated with 12µM tunicamycin after 6h of sleep deprivation in comparison to the young untreated control flies (n=20) subjected to SD. Mean +S.E.M. shown, *P<0.05, **P<0.01
3.4 PBA increases daytime sleep and consolidates nighttime sleep in a short sleeping mutant remember to underline all changes in this section
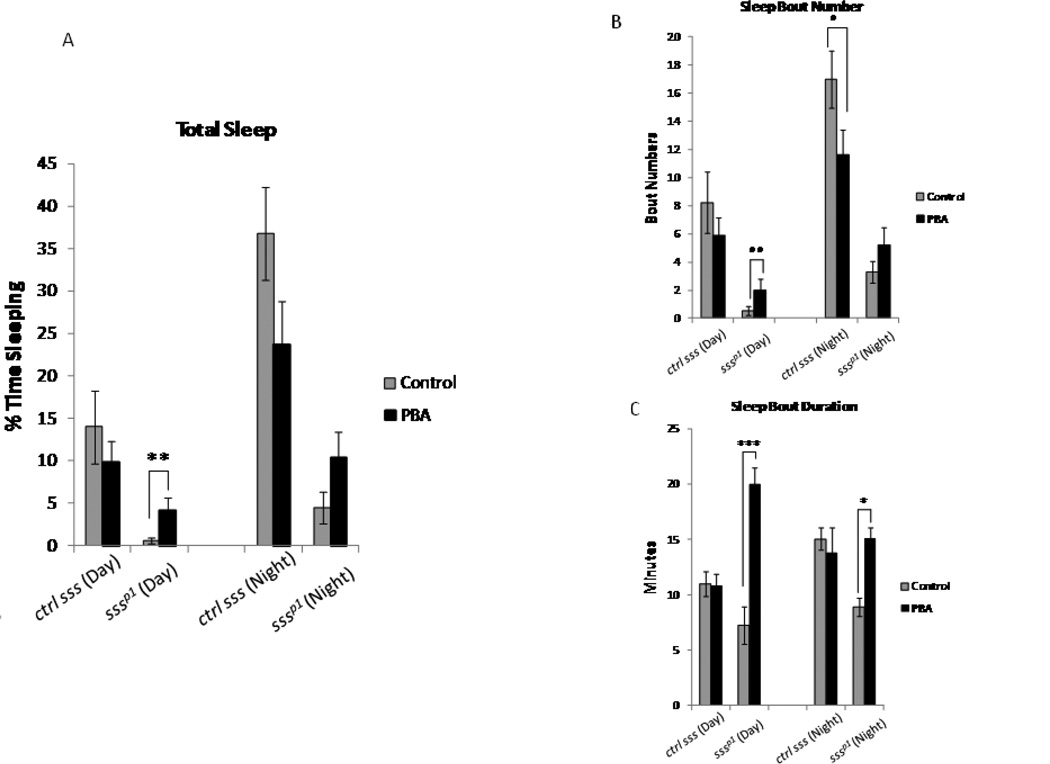
The major effect of PBA on sleep was observed largely in aged flies; total sleep and sleep bout duration were increased. That these effects were not observed in young flies could be due in part to the significant amount of sleep that young flies experience, which could potentially mask PBA effects. To determine the effect of PBA on sleep in young flies, we examined the short sleeping fly sleepless (sssp1). The mutant sssp1 displays a drastic decrease in total sleep with severe reductions in both sleep bout numbers and sleep bout duration and reduced longevity (Koh et al. 2008). Similar to what was observed in the aged wild type flies (wCS10 and w1118ex); PBA increased and consolidated sleep in the mutant sssp1. We found that PBA significantly increased total sleep (p<0.001) (Fig. 4A), the numbers of sleep bouts (p<0.001) (Fig. 4B) and sleep bout duration (Fig. 4C) (p<0.0001) during the day in the sssp1 flies. Sleep bout duration was also significantly increased during the night (p=0.03) in the sssp1 flies, although there were no significant effects on total sleep. Interestingly, PBA treatment actually decreased sleep bout numbers (p=0.013) and total sleep during the night in the background strain (Fig. 4A), although the decrease in total sleep was not significant.
Fig. 4.
PBA consolidates sleep in the short sleeping Sleepless (sssp1) mutant. (A) Total sleep in sssp1 (n=20) and its background control strain iso31 (n=32). PBA increased total sleep (p<0.01) during the day in the sssp1 mutant flies (n=28). (B) PBA also significantly increased both sleep bout number (p<0.01) and (C) sleep bout duration during the day (p<0.001) in the sssp1 mutants. Total sleep (NS) and sleep bout duration, (p<0.05) are decreased during the night in the control strain (iso31), treated with PBA (n=32). Mean + S.E.M. shown,*P<0.05, **P<0.01, ***P<0.001
3.5 Molecular characterization of the UPR and recovery sleep
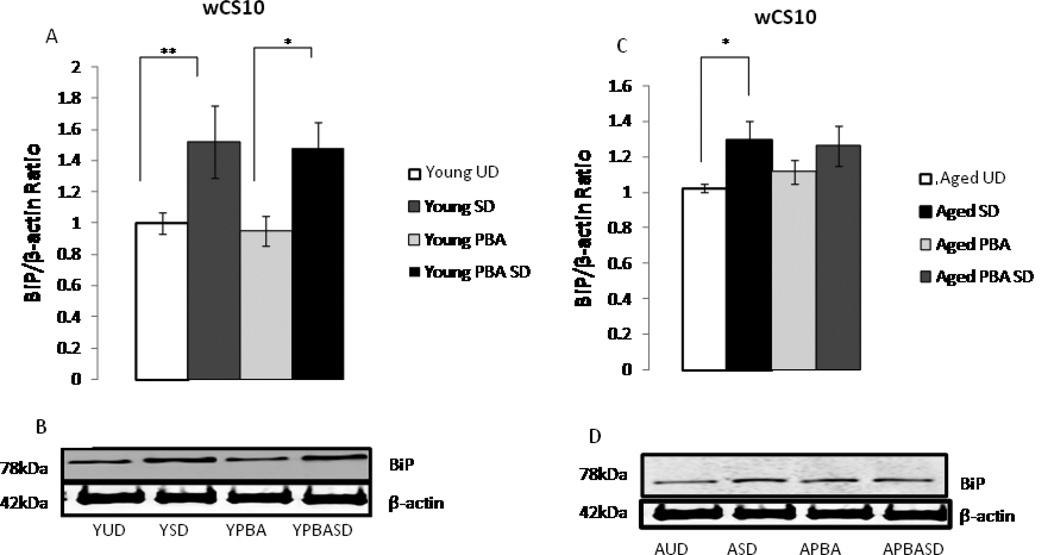
To establish whether the behavioral changes following the administration of PBA were mediated by the UPR, we assessed the following UPR markers: BiP, x-box binding protein 1 (XBP1) and phosphorylated elongation initiation factor 2 α (p-eIF2α). Since similar results for these molecular markers were obtained in both the wCS10 and w1118ex strains, only the results for wCS10 flies are presented. Sleep deprivation increased BiP expression levels in both the young and aged flies. Young sleep deprived (YSD) flies display a greater than 1.5 -fold increase in BiP levels when compared their young undisturbed counterparts (YUD) (Fig. 5A and Fig. 5B) (p=0.007). Young flies treated with PBA (YPBA) responded similarly to 6 h of SD (p<0.05) as the young sleep deprived (YSD) flies without PBA (p<0.05). Aged sleep deprived flies (ASD) displayed a 1.2 fold increase in BiP expression versus aged undisturbed flies (AUD) (p<0.05). However, aged flies treated with PBA showed no significant induction of BiP after sleep deprivation (Fig. 5C and Fig. 5D).
Fig. 5.
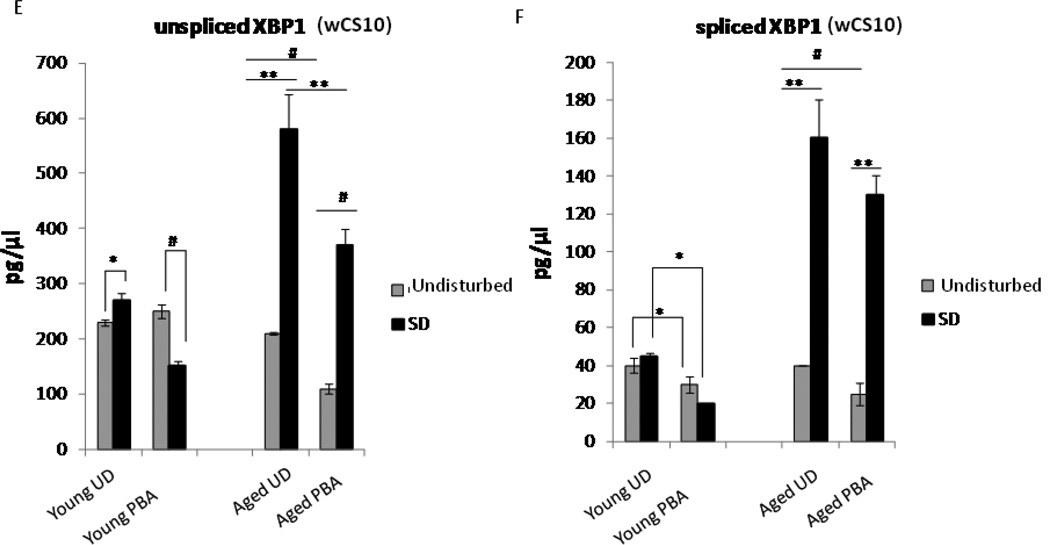
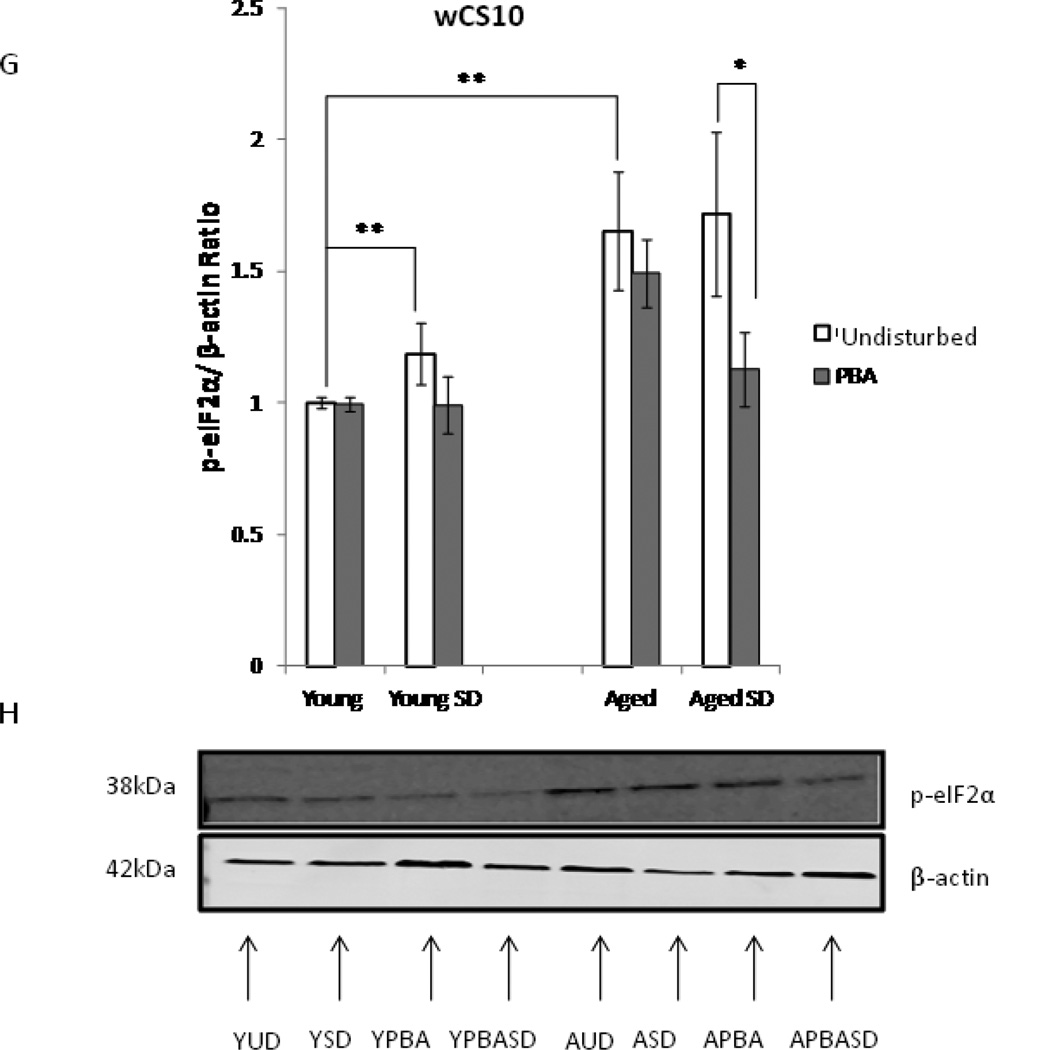
Upregulation of ER stress markers with sleep deprivation (SD). (A) Densitometric quantification of BiP expression in young undisturbed (YUD), young sleep deprived (YSD), young PBA (YPBA) and young PBA sleep deprived (YPBASD) wCS10 fly heads (n=4 trials, 10 heads/pool). 6 h of SD increased BiP levels in young flies in comparison to their non-SD counterparts (p=0.007). Young flies treated with PBA responded similarly to young untreated flies when sleep deprived (p<0.05). (B) Representative western blots. 15µg of protein were loaded per well from undisturbed and sleep deprived flies. (C) Quantification of BiP expression in aged undisturbed (AUD); aged sleep deprived (ASD), aged PBA (APBA) and aged PBA sleep deprived (APBASD) fly heads. (D) Representative western blot shows 15µg of protein loaded per well from undisturbed and SD flies. BiP expression increased in aged sleep deprived flies (ASD) versus aged undisturbed flies (AUD) (p<0.05) (n=4 trials, 10 heads/pool). Aged flies treated with PBA showed no significant induction of BiP after sleep deprivation. All gels were stripped and reprobed with β-actin as the loading control. (E-F) ER stress triggers Drosophila XBP1 mRNA splicing, which is characterized by the elimination of a 23 base pair intron from Drosophila XBP1 mRNA. PBA reduced both the unspliced and spliced variant of XBP1 in the YPBASD and APBA flies. In the APBASD flies XBP1 splicing is decreased. Mean +S.E.M. shown for XBP1 *P<0.05, **P<0.01, #P<0.001 (G) Densitometric quantification of phosphoeIF2α levels in fly heads (n=4 trials, 10 heads/pool). 6 h of sleep deprivation (SD) increased peIF2α expression in YSD flies in comparison to their undisturbed counterparts (YUD) (p=0.032). Aged flies (AUD) have increased basal levels of p-eIF2α that are significantly higher than the YUD flies (p=0.006). There was no significant difference between the AUD and aged ASD flies; however, p-eIF2α levels are markedly reduced in aged SD flies with acute application of PBA (ASDPBA) (p<0.05). Mean +S.E.M. shown, *P<0.05, **P<0.01. (H) Representative Western blots of p-eIF2α shows 15µg of protein loaded per well from undisturbed and SD flies. All gels were stripped and reprobed with β-actin as the loading control.
As induction of BiP in young and aged flies did not seem to be markedly different, we examined an earlier event during the UPR, the activation of IRE1. IRE-1, a transmembrane protein with both cytoplasmic kinase and endoribonuclease activity, is very sensitive to protein misfolding (Lin et al. 2007) and small amounts of misfolded proteins can initiate its activity. Activation of the ribonuclease leads to the splicing of XBP1, a conserved UPR transcription factor (Pincus et al. 2010, Walter 2010) which in turn launches the transcription of ER chaperones, including BiP. We measured XBP1 mRNA splicing by reverse transcription polymerase chain reaction (RT-PCR) and quantified the amount of spliced to unspliced XBP1 by DNA chip analysis. The unspliced variant of XBP1 was significantly increased in young flies subjected to 6 h of sleep deprivation (YSD) ([p=0.017]), although the increase in the spliced variant was not significant (Fig. 5E–F). PBA, however, suppressed the increase in both the unspliced (p= 0.035) and spliced (p=0.044) variants of XBP1 after sleep deprivation (Fig. 5E–F) in the young flies. In the aged flies (ASD), both the unspliced (p<0.01) and spliced (p<0.05) variants of XBP1 were increased with sleep deprivation. Conversely, the unspliced (p=0.008) and spliced variants (p=0.047) of XBP1 were significantly decreased with PBA treatment in the aged sleep deprived flies (Fig. 5E and Fig. 5F). Our data demonstrates that PBA treatment reduces XBP1 splicing as well as the unspliced variant, which suggests a diminution in ER stress and in protein misfolding.
3.6 PBA treatment represses age-related activation of p-eIF2α
PERK, a transmembrane kinase, is another of the three transducers of the UPR. Upon activation, PERK homodimerizes, autophosphorylates, then phosphorylates the elongation initiation factor 2 α (eIF2α) to attenuate protein translation, thereby reducing cellular protein synthesis. Phospho-eIF2α levels are increased in young sleep deprived animals compared to undisturbed animals (Fig. 5G and Fig. 5H) (p=0.006). Undisturbed aged flies display a 1.65 fold increase in the basal expression of phospho-eIF2α (Fig. 5H) (p=0.03) when compared to the young undisturbed flies. These animals also demonstrate significant decreases in total eIF2α expression (data not shown), suggesting that the UPR is activated during aging without the stimulus of an external stressor. PBA treatment significantly reduced p-eIF2α levels (0.58 fold or 32%) in aged sleep deprived flies when compared to their untreated sleep deprived controls (p<0.001). These results are consistent with earlier in vitro studies that show PBA treatment suppresses activation of the UPR by inhibiting PERK phosphorylation (Kubota et al. 2006).
4. Discussion
With advancing age, humans demonstrate a number of sleep disruptions (Ancoli-Israel 1997, Wolkove et al. 2007), which contribute to poor health consequences and accelerated senescence. The mechanisms that lead to these negative health outcomes, however, remain unclear. In this study, we found that both baseline and recovery sleep are changed with age and that these alterations can be modulated by the chemical chaperone PBA. PBA is a low molecular weight fatty acid that has been shown to act as a chemical chaperone for misfolded or mislocalized proteins (Ozcan et al. 2006, Yam et al. 2007) (Ozcan et al. 2006, Perlmutter 2002, Yam et al. 2007). It has also been shown to suppress ER-stress induced cell death without the aid of endogenous ER chaperones (Kubota et al. 2006). We also demonstrate in this study, that directly inducing ER stress in young flies fragments baseline sleep and alters recovery sleep, phenocopying aged flies. Sleep fragmentation may contribute to accelerated aging and it has been suggested that an intervention that consolidates sleep may delay the aging process and increase life span (Koh et al. 2006).
Aged flies had less total baseline sleep and an altered homeostatic response in that they recovered sleep over an extended 12 h period, whereas young flies recoup lost sleep within a much shorter window. Similar sleep disturbances are seen in humans and mammals, where aged individuals lose the ability to initiate and maintain nighttime sleep (Wolkove et al. 2007) and recovery sleep after prolonged wakefulness is altered (Mendelson & Bergmann 2000, Tobler & Borbely 1990). Similar to Mendelson et al., (Mendelson & Bergmann 2000) we demonstrate a significant age by time interaction, where young flies recover sleep in the first 4–5 h following sleep deprivation. Sleep deprived aged flies however; recover sleep over much a longer period. Variations in our behavioral results compared with those existing in the literature are possibly due to our use of video analysis, which has been shown to be more accurate than DAMS in measurements of fly sleep, in particular daytime sleep and architecture (Zimmerman et al. 2008).
During the course of this study, we observed an increase in locomotor activity in both young and aged flies treated with PBA (Supplemental Fig.2 A–D). A previous study found that PBA maintained locomotor vigor (activity) during aging in Drosophila (Kang et al. 2002). These results suggest that PBA is in fact increasing sleep and not merely reducing movement in the aged flies. Although total wake did not change in the young flies treated with PBA, wake became more consolidated. Not observing changes in total wake is possibly due to a ceiling effect in the young healthy flies. These results indicate that there is an age by drug interaction in the modification of sleep by PBA that remains to be elucidated.
Aging also impairs quality control systems that are necessary for protein homeostasis. Earlier work from our laboratory demonstrated that aging decreased BiP levels and increased pro-apoptotic factors such as CCAAT/enhancer-binding protein-homologous protein (CHOP), in the cerebral cortex of mice (Naidoo et al. 2008). Young flies experience less ER stress and their adaptive UPR is fully functional. Aged flies, on the other hand, are under chronic ER stress conditions with a diminished adaptive UPR. Therefore, basal ER stresses for the two groups are vastly different and likely contributes to the disparate responses to sleep deprivation. The young flies are readily able to handle acute sleep deprivation, whereas aged flies, already under stress, are overwhelmed by the secondary stressor. Addition of PBA has little effect on recovery sleep in young flies; however, PBA treatment ameliorates existing ER stress and suppresses further UPR induction in aged flies. As a result, treated aged flies display more efficient recovery sleep or are able to discharge sleep debt more efficiently than untreated aged flies.
Although we found no significant differences in BiP expression in flies treated with PBA, XBP1 was significantly affected by PBA treatment. PBA not only reduces the amount of spliced XBP1, it also reduces the expression of the unspliced variant as well. PBA likely suppresses UPR induction by chaperoning unfolded proteins, so that BiP stays bound to IRE1 and PERK and delays or prevents its activation, even under the condition of sleep deprivation. Phosphoe-IF2α levels were significantly reduced in the PBA treated aged flies subjected to sleep deprivation. Changes in p-eIF2α can be directly linked to the behavioral changes in the aged flies given that increased p-eIF2α facilitates baseline (Methippara et al. 2009) and recovery sleep (Methippara et al. 2012). The reduced eIF2α phosphorylation in PBA treated animals may account for the improved homeostatic response in the aged SD flies.
In this study, we also chemically induced ER stress in young flies and then subjected them to sleep deprivation. This acute tunicamycin treatment decreased and fragmented baseline sleep and phenocopied the recovery sleep behavior that was seen in the aged flies (Fig. 6). There are two possible explanations for the tunicamycin results. First, tunicamycin activity leads to inhibition of glycosylation and general misfolding of proteins, which will ultimately result in ER stress. Secondly, tunicamycin could potentially prevent maturation of glycoproteins required for sleep maintenance. This data demonstrates that ER stress induces sleep fragmentation. Together with our previous findings that sleep deprivation caused ER stress and activated the UPR, these results suggests that both processes are intimately linked and feedback on one another.
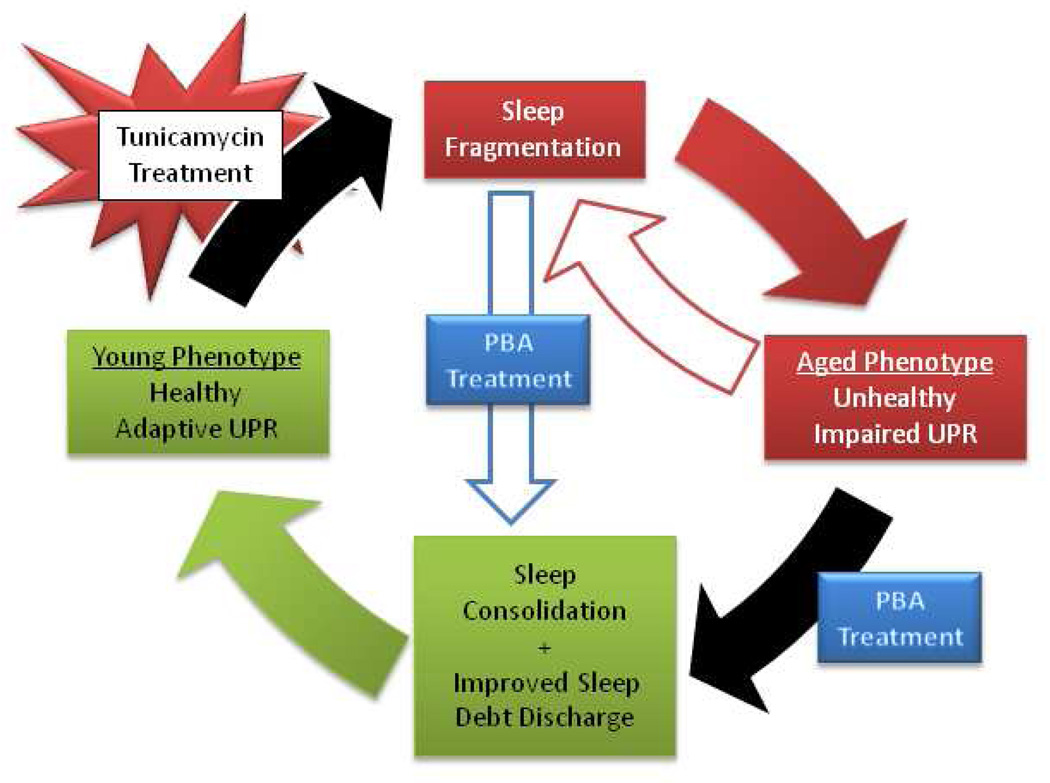
Fig. 6.
Model demonstrating the relationship between aging, UPR activation and sleep. Sleep fragmentation resulting from age-related impairments in the UPR can be ameliorated by administration of a chemical chaperone. Treatment with the chemical chaperone results in sleep consolidation and improved sleep debt discharge, recapitulating a young healthy phenotype. Tunicamycin treatment induces ER stress, which leads to sleep fragmentation and confers an aged phenotype.
PBA has been shown to significantly increase life span in Drosophila, purportedly through inhibition of HDAC activity (Kang et al. 2002). The moderate effects of PBA on sleep consolidation in young sssp1 mutant flies suggest that protein stabilization by PBA is only partially ameliorating defects in these animals. The decrease in sleep in the ctrl sss background strain treated with PBA supports the increase and consolidation of wake in the wild-type lines. It was previously shown that induction of ER stress impaired waking in mice (Naidoo et al. 2011). We speculate that application of PBA also promotes wake-active neuronal function and consolidates wake in the flies by reducing ER stress. The issue is not the accumulation of sleep debt, but the discharge of sleep debt that is impaired in the aged flies. By ameliorating ER stress and suppressing activation of the UPR, PBA allows for more efficient recovery sleep in the aged flies.
Since PBA ameliorates ER stress by directly reducing aberrant protein load (Kubota et al. 2006), treating Drosophila with PBA supplements chaperone activity and decreases the burden of misfolded proteins that occurs as a consequence of an external stressor, sleep deprivation, as well as normal aging.
Supplementary Material
Sleep homeostasis in aged w1118ex flies. Recovery sleep per hour 24 hours post sleep deprivation (SD) in aged control and aged PBA treated flies. Data is shown as the difference in sleep per hour post deprivation to that over same time interval on preceding day for each fly averaged across all flies with standard error of the mean (S.E.M.). Aged w1118ex flies (n=27) demonstrate a similar recovery profile as the aged wCS10 flies. PBA treated w1118ex flies (n=32) showed increased efficiency of recovery sleep.
24 hours of activity data in (A) young (n=20), (B) young treated with PBA (n=32), (C) aged (n=14) and (D) aged treated with PBA (n=30) wCS10 flies. Flies were acclimated to a 12: 12 light/ dark cycle. Activity data was recorded over a 2 day period.
Acknowledgments
This project was supported by funding from NHLBI T32 HL07713 NIA AG032500 and 3P01HL094307-04S1. The authors would like to thank Dr. Kristan Singletary for helpful suggestions and discussion. We would also like to thank Laura Shook, Micah Romer, Mangeet Kaur, Alice Yu and Shweta Dutta for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors state that there are no actual or potential conflicts of interest.
References
- Ancoli-Israel S. Sleep problems in older adults: putting myths to bed. Geriatrics. 1997;52:20–30. [PubMed] [Google Scholar]
- Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40:1219–1224. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci U S A. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Watanabe H, Senda J, et al. Widespread cortical and subcortical brain atrophy in Parkinson's disease with excessive daytime sleepiness. J Neurol. 2012;259:318–326. doi: 10.1007/s00415-011-6187-6. [DOI] [PubMed] [Google Scholar]
- Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Niinuma Y, Kaneko M, et al. Suppressive effects of 4-phenylbutyrate on the aggregation of Pael receptors and endoplasmic reticulum stress. J Neurochem. 2006;97:1259–1268. doi: 10.1111/j.1471-4159.2006.03782.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Bliwise DL, Ansari FP, Goldstein FC, Cellar JS, Lah JJ, Levey AI. Daytime sleepiness and functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:620–626. doi: 10.1097/JGP.0b013e3180381521. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB, Bergmann BM. Age-dependent changes in recovery sleep after 48 hours of sleep deprivation in rats. Neurobiol Aging. 2000;21:689–693. doi: 10.1016/s0197-4580(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Methippara M, Mitrani B, Schrader FX, Szymusiak R, McGinty D. Salubrinal, an endoplasmic reticulum stress blocker, modulates sleep homeostasis and activation of sleep- and wake-regulatory neurons. Neuroscience. 2012;209:108–118. doi: 10.1016/j.neuroscience.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Methippara MM, Bashir T, Kumar S, Alam N, Szymusiak R, McGinty D. Salubrinal, an inhibitor of protein synthesis, promotes deep slow wave sleep. Am J Physiol Regul Integr Comp Physiol. 2009;296:R178–R184. doi: 10.1152/ajpregu.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N. Cellular stress/the unfolded protein response: relevance to sleep and sleep disorders. Sleep Med Rev. 2009a;13:195–204. doi: 10.1016/j.smrv.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009b;8:150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI. A role for the molecular chaperone protein BiP/GRP78 in Drosophila sleep homeostasis. Sleep. 2007;30:557–565. doi: 10.1093/sleep/30.5.557. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Zhu J, Zhu Y, Fenik P, Lian J, Galante R, Veasey S. Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging Cell. 2011;10:640–649. doi: 10.1111/j.1474-9726.2011.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Seils LK, Kayumov L, Ralph MR, Lowe A, Moller H, Swaab DF. Senescence, sleep, and circadian rhythms. Ageing Res Rev. 2002;1:559–604. doi: 10.1016/s1568-1637(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Pincus D, Chevalier MW, Aragon T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit K, Krishnan N, Guzik EM, Pyza E, Giebultowicz JM. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int. 2012;29:5–14. doi: 10.3109/07420528.2011.635237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler I, Borbely AA. The effect of 3-h and 6-h sleep deprivation on sleep and EEG spectra of the rat. Behav Brain Res. 1990;36:73–78. doi: 10.1016/0166-4328(90)90161-7. [DOI] [PubMed] [Google Scholar]
- Walter P. Walking along the serendipitous path of discovery. Mol Biol Cell. 2010;21:15–17. doi: 10.1091/mbc.E09-08-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176:1299–1304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Ophthalmol Vis Sci. 2007;48:1683–1690. doi: 10.1167/iovs.06-0943. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun H, Lu J, Li X, Chen X, Tao D, Huang W, Huang B. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J Exp Biol. 2005;208:697–705. doi: 10.1242/jeb.01439. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Raizen DM, Maycock MH, Maislin G, Pack AI. A video method to study Drosophila sleep. Sleep. 2008;31:1587–1598. doi: 10.1093/sleep/31.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sleep homeostasis in aged w1118ex flies. Recovery sleep per hour 24 hours post sleep deprivation (SD) in aged control and aged PBA treated flies. Data is shown as the difference in sleep per hour post deprivation to that over same time interval on preceding day for each fly averaged across all flies with standard error of the mean (S.E.M.). Aged w1118ex flies (n=27) demonstrate a similar recovery profile as the aged wCS10 flies. PBA treated w1118ex flies (n=32) showed increased efficiency of recovery sleep.
24 hours of activity data in (A) young (n=20), (B) young treated with PBA (n=32), (C) aged (n=14) and (D) aged treated with PBA (n=30) wCS10 flies. Flies were acclimated to a 12: 12 light/ dark cycle. Activity data was recorded over a 2 day period.