Abstract
Background
Although common practice, evidence to support treatment of croup with prednisolone is scant.
Methods
We conducted a community-based randomized trial to compare the effectiveness of prednisolone (2mg/kg/day for 3 days, n=41) versus one dose of dexamethasone (0.6 mg/kg) and two doses of placebo (n=46). Participants were children 1–8 years of age with croup symptoms ≤ 48hr, categorized as mild (42%) or moderate (58%).
Results
There were no differences for those treated with dexamethasone or prednisolone for additional healthcare for croup (2% vs. 7%, p=0.34), duration of croup symptoms (2.8 days vs. 2.2 days, p=0.63), non-barky cough (6.1 days vs. 5.9 days, p=0.81), nights with disturbed sleep for the parent (0.68 nights vs. 1.21 nights, p=0.55), and days with stress (1.39 days vs. 1.56 days, p=0.51).
Conclusion
There were no detected differences in outcomes between the two croup treatments for either child or parent.
Keywords: croup, randomized trial, dexamethasone, prednisolone
INTRODUCTION
Emergency department (ED) treatment of laryngotracheobronchitis, or croup with corticosteroids is supported by more than twenty randomized placebo-controlled trials that demonstrate reduction of croup symptoms, the need for epinephrine treatment, time in the emergency department (ED), hospitalizations and return to healthcare, and parental sleep loss and stress.1–3 One oral dose of dexamethasone 0.6mg/kg is recommended for the ED management of mild and moderate croup.1,3–5
Children with croup are commonly cared for in the pediatricians’ office,6 where diagnosis and treatment is usually based on a history of nighttime croup symptoms rather than office presentation. To understand usual practice we conducted a survey of local primary care pediatricians (PCP) (n=116, 50% response) that confirmed widespread use of oral corticosteroids, most commonly prednisolone (63% prednisolone; 11% dexamethasone; 27% either drug). Although common practice, the evidence to support use of prednisolone for croup is scant. Two ED-based studies that compared single-dose regimens of prednisolone (1 mg/kg) and dexamethasone (0.15 mg/kg) for children with mild-to-moderate croup had disparate findings, with one finding no difference in outcomes7 and the other finding dexamethasone to be superior.8 While dexamethasone and prednisolone have similar anti-inflammatory actions, single-dose treatment regimens of these two medications fail to account for the longer duration of action of dexamethasone (36–72 hours compared to 12–36 hours) and its increased potency (5–6 times more potent than prednisolone).9 Treatment of croup with multiple doses of prednisolone has not been evaluated and no studies have compared the effectiveness of dexamethasone and prednisolone for the treatment of croup in the community setting.
Our objective was to compare the effectiveness of prednisolone 2mg/kg for 3 days, a treatment regimen already commonly prescribed by pediatricians in our community; with one dose of dexamethasone 0.6mg/kg, a treatment regimen known to be effective in the ED setting, for children with mild or moderate croup diagnosed at an office visit.
METHODS
We conducted a randomized trial in ten offices of PCPs in St Louis, MO. Each practice was a member of the Washington University Pediatric and Adolescent Ambulatory Research Consortium (WU PAARC), a practice-based research network of community pediatricians and pediatric nurse practitioners in St. Louis. Each participating practice had an active Federal Wide Assurance for the Protection of Human Subjects (FWA) and was trained in the ethical conduct of research. The parent or legal guardian provided written consent. The study was approved by the Human Research Protection Office at Washington University School of Medicine.
Study Treatments
Study treatments were prednisolone 2 mg/kg (maximum 60mg/d) once a day for 3 days or one dose of dexamethasone 0.6 mg/kg (maximum18mg) followed by 2 days of placebo comparable in appearance, smell, and taste. All active study treatments were supplied by Gallipot Inc, MN, and formulated as elixirs by a licensed pharmacist. Study Drug packages were prepared offsite by the pharmacist. Each package contained two bottles, the Day 0 supply (Office dose) and the Days 1 and 2 supply bottle (Home dose). The pharmacist labeled each bottle “Croup Study Drug,” and the drug ID number, identified the bottle as the Office or Home dose, and packaged the two bottles in a sealed opaque envelope labeled with the drug ID number and expiration date. The package also included two syringes for drug administration. For allocation concealment, the drug formulation ensured the volume of the weight-based dose was equivalent for each medication. Using these strategies, patients, parents, PCPs, and study team members were blinded to treatment assignment.
For each child, the PCP calculated the Study Drug dose using a weight-based dosing chart. The first dose was given during the enrollment visit (Day 0). The PCP, or their trained designate, prepared the remaining two doses by filling two syringes for the parent to administer at home. The parent was also provided with the remaining elixir in bottle B in case of medication leakage. The PCP provided oral and written instructions for the use of the corticosteroid. Concurrent treatment with acetaminophen and ibuprofen for pain or fever was allowed.
Subject eligibility and enrollment
Children one to eight years old with croup symptoms for ≤ 48 hours with a clinical diagnosis of mild or moderate croup at a participating site were eligible for study participation. The level of severity of croup was determined by the pediatrician at the time of presentation and was based on the history of symptoms within the past 24–36 hours. The Westley score10 was assessed at the visit but was not used to determine eligibility due to the circadian change in symptoms. Patients were excluded if they were diagnosed with severe croup or impending respiratory failure by the PCP; or had prior treatment with epinephrine or oral corticosteroids for this croup episode; symptoms or signs suggesting another cause of stridor; chronic respiratory disease including asthma; or a known contraindication to systemic steroid use. Children were also excluded if the parent would not be in the same household as the child for the subsequent four days, could not participate in telephone follow-up interviews, or was not English speaking.
Randomization
Randomized blocks were used to assign subjects to treatment groups, with randomization stratified by site. Computer generated random numbers determined how the two treatments were allocated to the consecutively numbered Study Drug packages at each site. Randomization occurred when the next consecutively numbered Study Drug package was distributed by the PCP (or their designate).
Measurement
The primary outcome was additional health care for croup within 11 days of randomization assessed by self-report. This dichotomous variable was positive if any of the following occurred: office visit, ED visit or hospitalization for croup care.
Secondary outcomes included: duration of croup symptoms defined as the number of days from study enrollment to last day that the child had neither stridor nor a barky cough in the preceding 24 hours assessed using the Telephone Out patient (TOP) score, a validated score used to assess the clinical status of children with croup (0-no symptoms to 3-barky cough and stridor at rest);3,11 nights with disturbed sleep, defined as the sum of nights the parent’s ability to sleep was affected “extremely,” “very much,” “a lot,” or “somewhat,” by self-report.12 Parental stress due to the child’s illness was rated using a 4-point categorical scale (ranging from 3-very stressed to 0-not stressed).3 Adverse events were assessed with an open-ended question at day 11. In addition, specific questions, at each interview, asked if the child had experienced any sleep problems, mood changes, headache or dizziness, nausea, stomach pain, and secondary infections.3 Adverse events and additional healthcare for croup within 28 days of the index visit were assessed by chart review.
Adherence to the study drug treatment regimen was defined as missing no doses of the study drug and was measured by self-report.
Procedures and Data Collection
Eligible subjects were invited to participate in the study by their PCP at the end of the office visit. For families interested in participation, the PCP (or their trained designate) confirmed study eligibility, completed the consent process and completed the one-page provider form. This documentation included eligibility criteria, the child’s croup symptoms the night prior to and during the office visit, clinical assessment of croup severity, and the Westley Score.10 At study enrollment (day 0), the parent or legal guardian completed a brief self-administered questionnaire to assess the child’s current illness history, a TOP score, their medical history, and to gather patient and family demographic information. Contact information was faxed to study team members to initiate follow-up assessments.
Outcomes were assessed by five telephone interviews on days 1, 2, 3, 4, and 11 following randomization conducted by members of the study team. The study team attempted to interview the same respondent on each occasion and was blinded to study group assignment. The chart audit was completed at the end of the study.
Statistical Analysis
Our target sample size was 100 patients per group, based on the goal of estimating event rates and the width of the 95% confidence interval (CI). Adequate prior information to estimate the need for additional health care in the prednisolone group and, therefore, to calculate statistical power, was not available. Assuming 12% of subjects in the dexamethasone group would seek additional health care for croup after treatment,1 a sample of 100 would yield 95% CI from 7% to 20%.
All the analyses adhered to the intention-to-treat principle, and a probability of p < 0.05 (2-tailed) was used to establish statistical significance. Continuous variables are reported as mean (standard deviation, sd) or median (IQ range), and categorical data are reported as percentages. We used Fisher’s exact test to compare categorical variables and the Wilcoxon rank-sum test to compare continuous variables. All statistical analyses were done using SAS 9.2 (SAS Institute Inc., Cary N.C.).
RESULTS
Participants
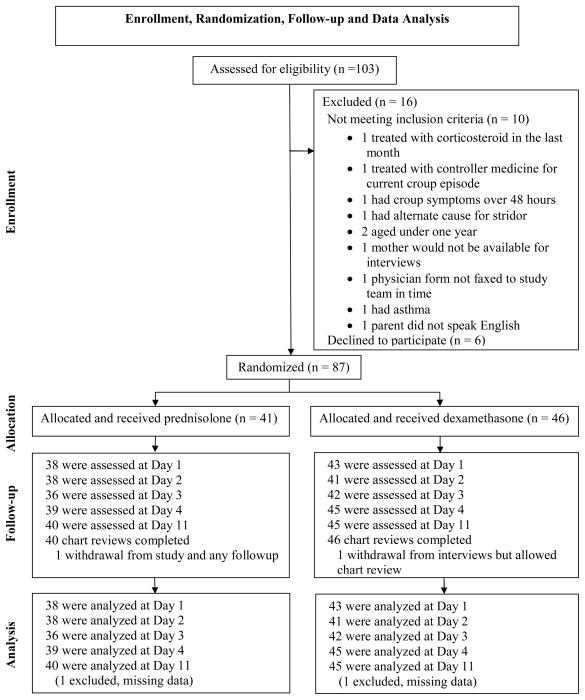
One hundred and three children from ten practices were screened between October 26, 2009 and April 16, 2010; and September 6, 2010 to April 29, 2011; of these, 87 were randomized (Figure 1). Follow-up interviews at days 1, 2, 3, 4 and 11 were completed by 93%, 91%, 91%, 97% and 98% participants respectively, with no difference by study group.
Figure 1.
Socio-demographic and disease characteristics were similar in both groups (Table 1). The majority of children included in the study were male (64%), white (94%), and from a two-parent household (87%); 49% of parents had graduated from college and 23% had completed postgraduate education. Subjects were diagnosed with mild (42%) or moderate (58%) croup severity: 53 (61%) children had stridor either the evening before (n=28), at the office visit (n=11), or on both occasions (n=14), and the median Westley score at baseline was 0 (range 0 to 3). Forty percent reported a prior episode of croup (6% > 3 episodes).
Table 1.
Baseline Patient and Disease Characteristics by Treatment Group
| Dexamethasone N=46 |
Prednisolone N=41 |
P Value | |
|---|---|---|---|
|
| |||
| Sociodemographic characteristics of child | |||
| Child’s age in years (mean, sd) | 3.11 (1.58) | 2.67 (1.43) | 0.18 |
| ≤3 years old | 34 (74%) | 32 (78%) | 0.65 |
| Male gender (N, %) | 28 (68%) | 28 (61%) | 0.47 |
| White race (N, %) | 43 (93%) | 39 (95%) | 1.00 |
| Hispanic (N, %) | 2 (4%) | 4 (10%) | 0.41 |
| Home environment (N, %) | |||
| Two-parent home | 39 (85%) | 37 (90%) | 0.44 |
| More than 1 child at home | 31 (67%) | 31 (78%) | 0.30 |
| Parent’s educational level (N, %) | |||
| High School Graduate | 5 (11%) | 1 (2%) | 0.31 |
| Some College | 9 (20%) | 9 (22%) | |
| College Graduate | 23 (50%) | 20 (49%) | |
| Post Graduate | 9 (20%) | 11 (27%) | |
| Prior episode of croup (N, %) | 21 (47%) | 13 (33%) | 0.27 |
| 1 prior episode | 14 | 7 | |
| 2 prior episodes | 6 | 2 | |
| >3 prior episodes | 1 | 4 | |
| History of Croup Episode | |||
| Symptoms: night before office visit (N, %) | |||
| No cough or stridor | 3 (7%) | 0 (0) | 0.06** |
| Barky cough and slept well | 1 (2%) | 5 (12%) | |
| Barky cough and slept poorly | 23 (50%) | 13 (32%) | |
| Stridor when upset, active, agitated | 16 (35%) | 17 (41%) | |
| Stridor at rest | 3 (7%) | 6 (15%) | |
| Symptoms: day of office visit (N, %) | |||
| No cough or stridor | 4 (9%) | 4 (10%) | 0.94** |
| Barky cough only | 30 (65%) | 24 (59%) | |
| Stridor when upset, active, agitated | 10 (22%) | 11 (27%) | |
| Stridor at rest | 2 (4%) | 2 (5%) | |
| Westley score= (mean, sd) | 0.6 (0.8) | 0.4 (0.7) | 0.38 |
| TOP score == (mean, sd) | 2.0 (0.9) | 2.2 (0.9) | 0.32 |
sd=standard deviation
Calculated with Fisher’s Exact Test
Calculated for the 5 × 2 or 4 × 2 table. The highest level of symptoms was recorded for each patient at each time-point (stridor at rest>stridor with activity>barky cough slept poorly>barky cough and slept well)
The Westley score is a validated symptom score that includes five components: stridor, chest retractions, cyanosis, level of consciousness, and air entry. The score ranges from 0 to 17, with higher scores indicating more severe symptoms.10
The Telephone Outpatient Score (TOP score) is a validated score used to determine the clinical status of children with croup by telephone. To determine this score, the interviewer asked whether the child had had either a seal-like barking cough or stridor in the preceding 24 hours. A barking cough was scored as 1 point, stridor scored 1 point if present when upset or agitated and an additional point if present at rest. The TOP score ranged from 0 to 3.3
Treatment Use
Of the 87 patients included in the analyses, 46 were randomized to receive dexamethasone and 41 to receive prednisolone. The difference in study group size was due to the use of stratified randomization by site. No drug leakage was reported. Most parents reported they experienced no difficulty administering the medication (63% dexamethasone, 59% prednisolone, p=0.82), although some reported their child did not like the taste of the study drug (33% dexamethasone, 32% prednisolone, p=1.0). One parent from the prednisolone study group reported their child vomited the study drug on day 2. No additional study drug was given to that child on day 2, but medication was given as planned on day 3. Duration of use and self-reported adherence did not differ between groups. Concurrent use of symptomatic treatments was common, decreased over time, and did not vary by study group (Table 2). The most commonly used medications were acetaminophen or ibuprofen for pain or fever.
Table 2.
Reported use of any symptomatic treatment over time (N, %)
| Dexamethasone N=46 |
Prednisolone N-41 |
P-Value | |
|---|---|---|---|
|
| |||
| Day 1 | 22 (48%) | 23 (56%) | 0.44 |
| Day 2 | 20 (43%) | 18 (44%) | 0.97 |
| Day 3 | 16 (35%) | 16 (39%) | 0.68 |
| Day 4 | 10 (22%) | 7 (17%) | 0.58 |
| Days 5–11 | 6 (13%) | 7 (17%) | 0.60 |
Effectiveness of Treatment
There was no difference in the two groups in reported additional healthcare for croup in the 11 days following the index visit (dexamethasone, 2%, 95%CI, 0.0% to 11.5%; prednisolone, 7%, 95%CI, 1.5% to 19.9%, p=0.34) or for any of the other study outcomes (Table 3). One child from the dexamethasone group was diagnosed with RSV pneumonia and hospitalized on day 2. Three children in the prednisolone group had an office visit for additional croup care verified by chart review (2 on day 3, 1 on day 11).
Table 3.
Patient Outcomes by Treatment Group
| Dexamethasone N=46 |
Prednisolone N=41 |
p-value | |
|---|---|---|---|
|
| |||
| Any additional health care for croup reported within 11 days of index visit* (N, %) | 1 (2%) | 3 (7%) | 0.34 |
| Office or Clinic Visit | 0 (0%) | 3 (7%) | 0.10 |
| Urgent Care Visit | 0 | 0 | |
| Emergency Department Visit | 0 | 0 | |
| Hospital Admission | 1 (2%) | 0 | 0 |
| Calls to Pediatrician During Office Hours | 5 (11%) | 3 (7%) | 0.72 |
| Calls to Pediatrician After Office Hours | 2 (4%) | 1 (2%) | 1.00 |
| Days with croup symptoms (mean, sd) | |||
| Any croup symptoms | 2.8 (2.7) | 2.2 (1.3) | 0.63 |
| Stridor | 1.26 (1.73) | 1.27 (1.20) | 0.68 |
| Barking cough | 2.30 (2.22) | 1.73 (0.92) | 0.35 |
| Non-barking cough | 6.09 (4.13) | 5.90 (4.06) | 0.81 |
| Any cough (median, IQ range) | 9 (5 to 12) | 8 (5 to 12) | 0.63 |
| TOP score= (mean, sd) | |||
| Day 1 | 0.9 (1.1) | 1.0 (1.0) | 0.42 |
| Day 2 | 0.5 (0.8) | 0.4 (0.8) | 0.66 |
| Day 3 | 0.2 (0.7) | 0.1 (0.5) | 0.61 |
| Day 4 | 0.1 (0.4) | 0.1 (0.3) | 0.85 |
| Impact on Parents | |||
| Nights with disturbed sleep (mean, sd) | 0.68 (1.55) | 1.21 (2.69) | 0.55 |
| Days with stress (mean, sd) | 1.39 (1.08) | 1.56 (2.69) | 0.51 |
| Hours missed from work (mean, sd) | 4.06 (5.54) | 3.79 (7.40) | 0.24 |
sd=standard deviation
= The Telephone Outpatient Score (TOP score) is used to determine the clinical status of children with croup by telephone. To determine this score, the interviewer asked whether the child had had either a seal-like barking cough or stridor in the preceding 24 hours. A barking cough was scored as 1 point, stridor scored 1 point if present when upset or agitated and an additional point if present at rest. The TOP score ranged from 0 to 3.3
Croup symptoms persisted an average of 2.8 days with dexamethasone and 2.2 days with prednisolone (p=0.63), with a general cough being present for a median of 8 days (IQ range, 5 to 12 days) across both groups. The child’s croup affected the parent, but with no difference by study group. Seventy-seven percent of parents reported being stressed by their child’s illness (mean duration 1.5 days, sd 1.2), 43% missed some work (mean 3.9 hours, sd 6.5) and 29% had at least one night with disturbed sleep (mean 0.9 nights, sd 2.1).
In the chart review, one child in the dexamethasone group had an office visit on day 11 where croup symptoms were recorded. As the mother reported this visit was for sinus infection/cold (confirmed by chart review) and not croup, it was not included in the primary analysis. Three children (2 dexamethasone, 1 prednisolone) had an additional office visit for croup after the completion of study interviews i.e., 12 to 28 days.
Thirty-seven percent of parents reported their child developed a new infection during the 11 days of follow-up interviews (42% dexamethasone, 33% prednisolone, p=0.38), most commonly colds (23%), ear (7%) and sinus (6%) infections, but few of these reported infections could be verified by chart review (43% of ear infections, 40% of colds and 20% of sinus infections).
Adverse events
No serious adverse events occurred. Study groups did not differ in reporting side effects from the study medications (24% dexamethasone, 26% prednisolone, p=1.0). The most common side effects identified with specific questioning were mood changes (57%), sleep problems (36%), stomach pain (19%), and headache (13%).
DISCUSSION
Prednisolone offers a convenient and familiar treatment for the primary care management of croup, as it is commonly used for in-office treatment of asthma exacerbations. Our study is the first to evaluate three days of treatment with prednisolone (2mg/kg) for croup, a commonly used treatment regimen by general pediatricians in our community. We found this approach to be equivalent to a single oral dose of dexamethasone (0.6mg/kg), a treatment already established as effective for children with severe, moderate and mild croup,1,2 but often not available in the pediatrician’s office. Two prior ED-based studies comparing single doses of prednisone and dexamethasone provided conflicting results but suggested superiority of dexamethasone.1,7,8 In our study, the dose of prednisolone represented about half the strength of the dose of dexamethasone for its anti-inflammatory effect,9 yet no differences in effect were found for any patient outcomes. This supports evidence from other studies that a lower dose of dexamethasone may be effective (0.3 mg/kg and 0.15 mg/kg).13–15 Further study will help determine if a lower dose of prednisolone is equally effective to the higher, more commonly used dose studied here.
This is the first community-based study of the management of croup. Community pediatricians seeking evidence to support care for this common illness were actively engaged in development of the study question, protocol and study implementation. Practical considerations guided study selection. Although prednisolone has not been directly compared to placebo for the treatment of mild croup, multiple randomized controlled trials have established corticosteroid treatment as the standard of care, with a number needed to treat of five for short-term improvement.1 Thus, a placebo-controlled trial was neither feasible or warranted,1 and we chose instead to conduct a comparative effectiveness study. Study inclusion criteria were different to prior treatment trials for croup, but nevertheless defined a population that is representative of patients treated for croup by PCPs. We were surprised that forty percent of patients reported a prior croup episode; although we think it unlikely these patients had spasmodic croup, as those with persistent asthma were excluded by their PCP. Also, recurrent croup is usually defined as at least two or three prior episodes with an estimated incidence of ~ 6%,16,17 similar to the study cohort. Outcome assessment differed from ED-based studies by focusing on outcomes that are relevant to primary care management of this common condition, such as additional healthcare, symptom duration and parental impact.
The child’s croup had a significant impact on the parents: many reported stress, loss of sleep and missed work. This study provides data to inform parents about what to expect with corticosteroid treatment for croup, which may be useful in reducing their anxiety and number of calls to the pediatrician. Croup symptoms quickly improved but persisted for about two days after treatment with either corticosteroid, and a non-barky cough persisted for about a week. Despite concern about the palatability of these medications,6 both drugs were well tolerated and easy to administer, with few reported side effects. However, over half reported mood changes and sleep problems on specific questioning. Without a placebo arm, we cannot tell if these symptoms were due to the medication or the illness, but parental anxiety may be allayed if they are aware these changes may occur. Many parents reported a new upper respiratory illness following their child’s croup, most commonly colds, that likely were a continuation of the croup infection.
Several limitations to our study should be noted. We were unable to recruit our target sample of 200 patients, and our failure to demonstrate a significant difference between the two study drugs may be due to inadequate power associated with the small sample size. However, our study sample is comparable to other trials to evaluate croup treatments (median sample size 73).1 Our results may not be generalizable to other communities as we recruited patients from one geographic area. Additionally, we were unable to gather data to determine how representative the study patients were of all patients with croup cared for at the study sites.
CONCLUSION
Study findings suggest that for children clinically diagnosed with mild or moderate croup during an office visit, there are no differences in patient or parent outcomes between one oral dose of dexamethasone 0.6mg/kg and a 3-day course of oral prednisolone 2mg/kg once per day.
Table 4.
Adverse Events
| Dexamethasone N=46 |
Prednisolone N=41 |
p-value | |
|---|---|---|---|
|
| |||
| A side-effect reported at day 11 | 11/45 (24%) | 10/39 (26%) | 1.00 |
|
| |||
| On specific questioning on days 1–4, symptom was reported to be present at least once (N, %) | |||
| Mood changes | 25 (56%) | 24 (59%) | 0.78 |
| New sleep problems | 18 (40%) | 13 (32%) | 0.42 |
| Stomach pain | 9 (20%) | 7 (17%) | 0.73 |
| Headache | 7 (16%) | 4 (10%) | 0.45 |
| Vomiting | 3 (7%) | 7 (17%) | 0.18 |
| Nausea | 3 (7%) | 4 (10%) | 0.70 |
| Dizziness | 3 (7%) | 2 (5%) | 1.00 |
| Tremor | 1 (2%) | 0 | 1.00 |
Acknowledgments
We thank all the patients who participated in this study; the physicians, nurses, and staff at Children’s Clinic, Esse Health – Creve Coeur Pediatrics, Esse Health – Florissant Pediatrics, Esse Health – Pediatric and Adolescent Medicine at Watson Road, Fenton Pediatrics, Health Care for Kids, Heartland Pediatrics – Edwardsville, Heartland Pediatrics – Granite City, Pediatric Healthcare Unlimited, Southwest Pediatrics, Strashun Pediatrics, Tots thru Teens Pediatrics, Way to Grow Pediatrics who recruited patients for the study; and members of the data and safety monitoring board (Drs. David Jaffe, Thomas Ferkol and Kenneth Schechtman, PhD)
Study data were collected and managed using REDCap electronic data capture tools hosted at Washington University.18 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
This study was funded by grant UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Abbreviations
- CI
confidence interval
- ED
Emergency department
- FWA
Federal Wide Assurance
- PCP
primary care pediatrician
- RT
randomized trial
- RSV
respiratory syncytial virus
- SAS
Statistical Analysis System
- sd
standard deviation
- TOP
Telephone Outpatient Score
- WU PAARC
Washington University Pediatric and Adolescent Ambulatory Research Consortium
Footnotes
Financial Disclosures: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: None of the authors report any relationships, conditions or circumstances that present a potential conflict of interest.
Its contents are solely the responsibility of the author and do not necessarily represent the official view of NCRR or NIH.
Dr. Garbutt and Dr. Schechtman had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Authorship contributions:
Conception and design: JMG, RS, RCS
Acquisition of data: BC, KM, EL, SG, JMG
Analysis and interpretation of data: JMG, JB, KS, KM, RS, RCS
Drafting manuscript: JMG
Critical revision of manuscript: JMG, BC, RS, JB, KS, KM, EL, SG, and RCS
Statistical analyses: JB, KS
Obtaining funding: JMG
Administrative, technical or material support: KM, SG
Supervision: JMG, RCS
References
- 1.Russell KF, Liang Y, O’Gorman K, Johnson DW, Klassen TP. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955. doi: 10.1002/14651858.CD001955.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Segal AO, Crighton EJ, Moineddin R, Mamdani M, Upshur RE. Croup hospitalizations in Ontario: a 14-year time-series analysis. Pediatrics. 2005 Jul;116(1):51–55. doi: 10.1542/peds.2004-1892. [DOI] [PubMed] [Google Scholar]
- 3.Bjornson CL, Klassen TP, Williamson J, et al. A randomized trial of a single dose of oral dexamethasone for mild croup. N Engl J Med. 2004 Sep 23;351(13):1306–1313. doi: 10.1056/NEJMoa033534. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe DM. The treatment of croup with glucocorticoids. N Engl J Med. 1998 Aug 20;339(8):553–555. doi: 10.1056/NEJM199808203390810. [DOI] [PubMed] [Google Scholar]
- 5.Ausejo M, Saenz A, Pham B, et al. The effectiveness of glucocorticoids in treating croup: meta-analysis. West J Med. 1999 Oct;171(4):227–232. [PMC free article] [PubMed] [Google Scholar]
- 6.Freid VM, Makuc DM, Rooks RN. Ambulatory health care visits by children: principal diagnosis and place of visit. Vital Health Stat. 1998 May;13(137):1–23. [PubMed] [Google Scholar]
- 7.Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup: a randomized, double-blinded clinical trial. Emerg Med Australas. 2007 Feb;19(1):51–58. doi: 10.1111/j.1742-6723.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 8.Sparrow A, Geelhoed G. Prednisolone versus dexamethasone in croup: a randomised equivalence trial. Arch Dis Child. 2006 Jul;91(7):580–583. doi: 10.1136/adc.2005.089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schimmer B. Goodman and Gilman’s the pharmacologocal basis of therapeutics. 9. New York: McGraw-Hill; 2005. Adrenocorticotropic hormone: adrenocortical steroids and their synthetic analogs: inhibitors of the synthesis and actions of adrenocortical hormones; pp. 1459–1485. [Google Scholar]
- 10.Westley CR, Cotton EK, Brooks JG. Nebulized racemic epinephrine by IPPB for the treatment of croup: a double-blind study. Am J Dis Child. 1978 May;132(5):484–487. doi: 10.1001/archpedi.1978.02120300044008. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D, Willaimason J. Telephone out patient score: the derivation of a telephone follow-up assessment tool for children with croup. Pediatr Res. 2003:185A. doi: 10.1097/PEC.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 12.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM., Jr Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007 Dec;161(12):1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 13.Chub-Uppakarn S, Sangsupawanich P. A randomized comparison of dexamethasone 0.15 mg/kg versus 0.6 mg/kg for the treatment of moderate to severe croup. Int J Pediatr Otorhinolaryngol. 2007 Mar;71(3):473–477. doi: 10.1016/j.ijporl.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995 Dec;20(6):362–368. doi: 10.1002/ppul.1950200605. [DOI] [PubMed] [Google Scholar]
- 15.Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ. 1996 Jul 20;313(7050):140–142. doi: 10.1136/bmj.313.7050.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hide DW, Guyer BM. Recurrent croup. Arch Dis Child. 1985 Jun;60(6):585–586. doi: 10.1136/adc.60.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Bever HP, Wieringa MH, Weyler JJ, Nelen VJ, Fortuin M, Vermeire PA. Croup and recurrent croup: their association with asthma and allergy. An epidemiological study on 5-8-year-old children. Eur J Pediatr. 1999 Mar;158(3):253–257. doi: 10.1007/s004310051062. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]