Abstract
Objectives
To determine the longer-term efficacy and safety of initiating treatment for urgency-predominant urinary incontinence (UUI) in women diagnosed using a simple questionnaire rather than an extensive evaluation.
Study Design
Women completing a 12-week randomized controlled trial of fesoterodine therapy for UUI diagnosed by questionnaire were invited to participate in a 9-month open label continuation study. UUI and voiding episodes were collected using voiding diaries. Participant satisfaction was measured by questionnaire. Safety was assessed by measurement of post void residual volume and adverse event monitoring; if necessary, women underwent specialist evaluation. Longitudinal changes in UUI and voiding episodes were evaluated using linear mixed models adjusting for baseline.
Results
Of the 567 women completing the randomized trial, 498 (87.8%) took at least one dose of medication during this open label study. Compared to the enrollment visit in the randomized trial, fesoterodine was associated with a reduction in total incontinence episodes/day and urgency incontinence episodes/day at the end of the open label study [adjusted mean (standard error (SE)) 4.6 (0.12) to 1.2 (0.13) and 3.9 (0.11) to 0.9 (0.11) respectively, p-value<.0001 for both]. Most women were satisfied with treatment (89%, 92%, and 93% at 3, 6, and 9 months). Twenty-six women experienced 28 serious adverse events, one of which was considered possibly treatment-related. Twenty-two women had specialist evaluation: 5 women’s incontinence was misclassified by the 3IQ; none experienced harm due to misclassification.
Conclusions
Using a simple validated questionnaire to diagnose and initiate treatment for UUI in community dwelling women is safe and effective, allowing timely treatment by primary care practitioners.
Keywords: Primary Care, Treatment, Urgency Urinary Incontinence
Introduction
Urinary incontinence affects up to a third of women in the United States, resulting in over $20 billion in health care costs annually.1 In addition to its direct costs, incontinence is associated with falls, fractures, increased caregiver burden, and increased nursing-home care, making the actual costs likely much higher.2-5 Many women with incontinence fail to receive appropriate treatment, both because primary care providers do not routinely ask about incontinence and because patients do not volunteer the information.6-8 Women reporting incontinence tend to be referred to specialists prior to being offered treatment,9,10 a model that increases the cost of care delivery and delays therapy.
In 1996, the Agency for Healthcare Research and Quality recommended that primary care providers take a larger role in diagnosing and treating incontinence. In response, streamlined diagnostic measures such as the 3 Incontinence Questions (3IQ) have been developed to help classify women’s incontinence in primary care practice. The 3IQ is a brief validated, reproducible questionnaire with good sensitivity and specificity in distinguishing between urgency and stress incontinence.11 However, the longer-term efficacy and safety of treating incontinence based on this streamlined questionnaire are not known.
To address this issue, we conducted the BRinging simple urge Incontinence DiaGnosis & treatment to providerS (BRIDGES) study, a 12-week randomized, double blind, placebo-controlled clinical trial (RCT) of antimuscarinic therapy in ambulatory women who self-diagnosed as having urgency-predominant urinary incontinence (UUI) using the 3IQ.12 In this trial, women who flexibly dosed 4 to 8 mg of fesoterodine daily reported fewer urgency incontinence episodes over 12 weeks compared to placebo.12 In this manuscript, we report the results of a 9-month open label study, in which women completing the 12-week trial were invited to take fesoterodine daily for an additional 9 months. The purpose of this open label study was to determine the longer-term efficacy and safety of initiating treatment for UUI in women diagnosed using a simple questionnaire rather than a more extensive evaluation.
Materials and Methods
Study Population
Eligibility criteria for BRIDGES have been described previously.12 Briefly, ambulatory women ages 18 years and older with self-reported UUI were recruited from the general communities surrounding 13 clinical sites in the United States. During the initial in-person visit, potential participants reporting at least weekly incontinence completed the 3IQ on paper, without assistance from the research staff. Those whose 3IQ indicated UUI (versus stress-predominant, equally mixed, or other incontinence) were eligible to continue. Consistent with proposed use of the 3IQ in clinical practice,11 women had dipstick urinalysis testing to rule out urinary tract infection and hematuria before enrollment.
Other eligibility criteria were selected to define a community-dwelling sample of women who would be considered appropriate for evaluation and treatment in a primary care practice. Women were excluded if they self-reported complex medical histories (regardless of severity), including major neurologic conditions (stroke, Parkinson’s disease, spinal cord lesion, or multiple sclerosis), recent urologic surgeries (anti-incontinence surgery in the past 5 years or other pelvic surgeries in the past 6 months), more than 3 urinary tract infections in the past year, lower urinary tract or rectal fistula, interstitial cystitis, symptomatic pelvic prolapse, pelvic radiation, congenital abnormality leading to incontinence, or pelvic cancer, that would require a specialist evaluation for incontinence, or if they had known contraindications to anti-muscarinic therapy.
All women who completed the 12-week RCT were offered participation in the pre-planned 9-month open-label study of fesoterodine, in which participants were seen in person at open-label baseline and at 1, 3, 6, and 9 months and participated in a telephone visit at 2-weeks. The timing of study procedures and measurements is shown in Appendix Table 1. Institutional review boards at each site approved the study, all participants provided written informed consent before enrollment, and the study was registered with clinicaltrial.gov (NCT00862745).
Fesoterodine Distribution
At baseline of the open-label study, all participants were started on fesoterodine 4 mg daily; women in the active arm of the RCT had been taking 4mg or 8mg previously while those in the control arm had been taking placebo. Participants were invited to self-adjust their medication dose at 1-, 3-, 6-, and 9-month follow-up visits. Medication adherence was assessed through pill counts at each of these visits.
Urinary Symptom Assessment (Efficacy)
At baseline, 3-month, 6-month, and 9-month open-label visits, trained research study staff reviewed the participant-completed validated 3-day voiding diary, our primary efficacy outcome. In this diary, women recorded all voiding and incontinence episodes, classified their incontinence episodes by type (urgency, stress, or don’t know), and rated the severity of voiding episodes associated with a sensation of urgency as none, mild, moderate, or severe.13,14
Participants also completed validated questionnaires assessing the self-reported impact of their bladder symptoms, including: 1) the Overactive Bladder Questionnaire (OAB-q),15 a 33-item instrument assessing bladder symptom impact; 2) the Patient Perception of Bladder Condition (PPBC)15,16 a single-item assessing the patient’s current perception of her bladder problems; 3) and the Patient Perception of Urgency Scale (PPUS)17, a single-item measure assessing the patient’s perception of her voiding urgency.
Participant Satisfaction (Efficacy)
To assess participants’ subjective, personal evaluation of treatment effectiveness and willingness to continue therapy, a modified 4-item version of the Overactive Bladder Satisfaction (OAB-S) was administered at the 3-month, 6-month, and 9-month open label visits.18 Participants were asked to rate satisfaction with the fesoterodine medication and satisfaction with change in urine leakage using a 5-point scale ranging from very satisfied to very dissatisfied. At 9-months (or early termination), participants were also asked if they would continue using fesoterodine. If they said no, they were asked to select the reason for discontinuation; choices included 1) I don’t like taking pills, 2) my symptoms have not improved enough, 3) the side effects of the medication are too bothersome, and 4) other, with the opportunity to specify another reason.
Adverse Events (Safety)
At each study contact, participants were asked to report any negative changes in their health (i.e., adverse events). Adverse events involving dry mouth, constipation, drowsiness, tachycardia, or urinary hesitancy or retention were classified as “potentially associated with anti-muscarinic therapy.” Serious adverse events were defined as those that resulted in death, disability, or in-patient hospitalization. For all serious adverse events, site investigators rated the likelihood of relationship to treatment using a standardized attribution scale.
To objectively assess post-treatment urinary retention, measurement of post-void residual volume (PVR) was performed (based on site preference) by bladder ultrasound (8 sites) urinary catheterization (4 sites), or either (1 site) at the open label baseline, 3-month, and 9-month visits, or at early termination. If the initial PVR measurement was greater than 250mL, another PVR measurement was obtained on the same day.
Extended Evaluation (Safety)
Participants were offered extended evaluations with their site urologist or urogynecologist if they had two PVRs greater than 250 mL on the same day, if they reported lack of incontinence improvement, or otherwise expressed dissatisfaction with their treatment on the OAB-S questionnaire. Additionally, if site investigators had safety concerns, they could refer participants for evaluation at any time. Each participant could undergo only one extended evaluation during the study. During the extended evaluation, the site specialist collected and/or reviewed all data (medical, reproductive, surgical, and incontinence history; medication inventory; 3-day voiding diary; physical and pelvic exam; PVR; stress cough test) to diagnose the incontinence type (urgency-predominant, stress-predominant, or other-predominant) and assessed outcomes. If the resulting specialist diagnosis was not UUI, the specialist indicated whether the delay in the correct diagnosis resulted in harm to the participant and made alternative treatment recommendations.
A central independent specialist associated with the coordinating center and not employed by the sponsor also reviewed the extended evaluation data for each participant and made a diagnosis based on her own clinical judgment. If, after review, the diagnosis was not UUI, she also noted whether the delay in correct diagnosis caused harm, our primary safety outcome, and made alternate treatment recommendations. If the site and central independent specialists disagreed, they discussed the case and arrived at a consensus diagnosis and treatment plan, which was shared with the site investigator and participant.
Statistical Analysis
Summary statistics were evaluated from the initial visit of the RCT, open-label baseline, and open label follow-up visits. Continuous variables were summarized using mean, median, standard deviation, and inter quartile range. Categorical variables were described using absolute and relative frequency. Participant satisfaction, adverse events, and extended evaluations are described for all time points in the open label portion of the study. Voiding diary endpoints (urinary incontinence and voiding outcomes) were summarized at all of the above time points and evaluated compared to the initial RCT visit using linear mixed models adjusted for the baseline values were specified to account for repeated measures, site clusters, and transformed outcomes to approximate normal distribution.. All analyses were performed using SAS statistical software Version 9.2 (SAS Institute, NC).
Results
Participants and dosing
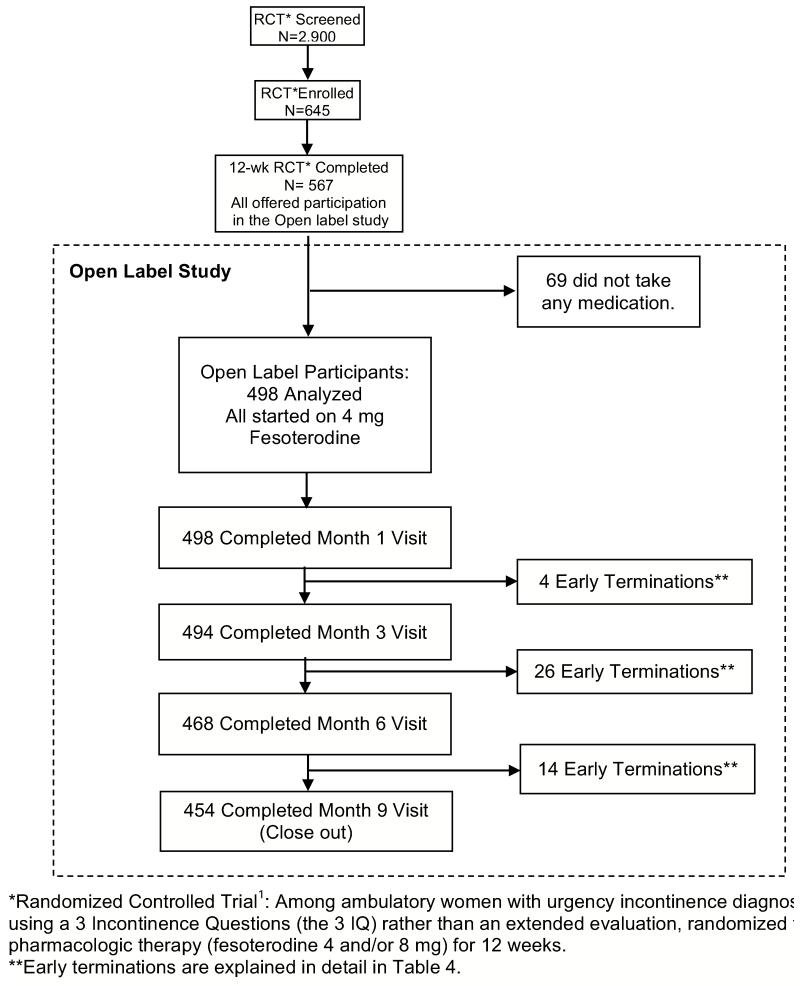
Between February 2009 and January 2010, 322 women were randomized to fesoterodine, and 323 to placebo; 567 women completed the randomized portion of the study. Of those women completing the RCT, 498 (87.8%) continued in the open label study (Figure 1). There were no significant differences with regard to age, race, health status, parity, smoking, alcohol use, number or type of incontinence episodes/day, response to treatment or side effects during the RCT, or bladder symptoms between women who did or did not participate in the open label study. Among these 498 women, 254 women had been on placebo and 244 on fesoterodine during the RCT.
Figure 1. Recruitment and retention of participants in the 9-month Open label extension study (N = 498).
RCT: Randomized Controlled Trial
On average, these 498 participants were 56.9 years old [standard deviation (sd) 13.8, range 21-90]. Eighty percent self-rated their health as excellent or very good (Table 1). Four hundred fifty-four women completed the 9-month open label study; there were no significant differences in baseline characteristics between women with and without follow up data in the open label study (data not shown).
Table 1. Characteristics of women enrolled in the 9-month open label (N= 498)*.
| Demographic | |
|---|---|
| Mean (SD) age, y | 56.9 (13.8) |
| Race/ethnicity, | No. (%)** |
| White | 340 (68.3) |
| Black | 103 (20.7) |
| Latina | 11 ( 2.2) |
| Asian/Pacific Islander | 32 ( 6.4) |
| Multiethnic/Other | 12 ( 2.4) |
| Married, No. (%) | 223 (44.8) |
| Clinical | |
| Excellent or very good overall health, No. (%)† | 399 (80.1) |
| Previous childbirth (parity ≥ 1), No. (%) | 400 (80.3) |
| No current menstrual periods, No. (%) | 363 (73.2) |
| History of hysterectomy, No. (%) | 155 (31.1) |
| Current cigarette smoking, No. (%) | 66 (13.3) |
| Current weekly alcohol consumption, No. (%) | 156 (31.3) |
| Current systemic hormone therapy, No. (%) | 40 ( 8.0) |
| Current stable diuretic therapy, No. (%) | 79 (15.9) |
All data obtained at the pre-randomization baseline of the 12-week randomized controlled trial.
Participants self-reported their primary racial/ethnic group as White/Caucasian, Black/African-American, Latina/Hispanic, Asian, Pacific Islander, Native American/American Indian, or multiethnic.
Overall health was assessed by asking women to rate their overall health as excellent, very good, good, fair, or poor.
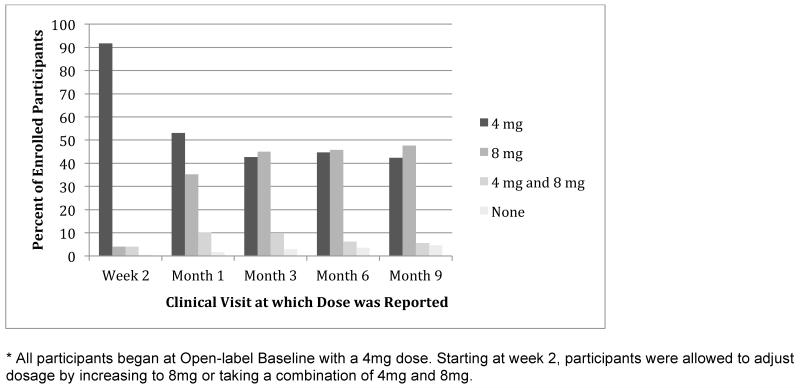
Although all women started the open label study on 4 mg/day of fesoterodine, 221 (44.3%) had changed to 8 mg/daily by 3 months. After 3 months, only 28.2% of women changed medication doses before the end of the open label study. (Figure 2). With participant-directed flexible dosing, 264 participants (53.0%), increased their dose of fesoterodine to 8mg and remained at this dose throughout the study, 162 (32.5%) remained at 4mg for the entire study, and 72 (14.4%) participants increased to 8mg but returned to 4mg before the end of the study.
Figure 2. Percent of participants taking 4mg, 8mg, a combination of 4mg and 8mg, and no medication at each time-point during the open label follow-up study.
Of the women originally enrolled in the open label study, 44 (8.8%) withdrew before the end of the study. A slightly higher proportion of women assigned to placebo in the RCT lead-in withdrew during the open label study compared to those assigned to fesoterodine in the RCT (11.4% vs. 6.2%, p=.04). Among these 44 women, the most common reasons for withdrawal were adverse events (n=16, including two serious adverse events), loss to follow-up (n=11), lack of improvement (n=5), and other patient circumstances (n=5) (e.g., family illness, relocation). At their final visits, 6 indicated that they would continue using study drug even though they withdrew for other reasons, 26 stopped using study drug, and 12 withdrew without indicating a preference.
Urinary Symptom Assessment (Efficacy)
Over the 9 months of the open-label study, participants reported a decrease in total incontinence episodes and in UUI episodes, daytime and nighttime micturitions, and moderate and severe urge sensations, as well as an improvement in self-report of overactive bladder symptoms and perception of bladder condition compared to the initial RCT visit (Table 2). Nearly all women (94%) who had improved incontinence (defined as any decrease in UUI episodes) exhibited improvement by two weeks.
| Open Label | |||||
|---|---|---|---|---|---|
|
| |||||
|
RCT Baseline* (n=498) |
Open Label Baseline** (n=497)† |
Month 3 (n=491) |
Month 6 (n=464) |
Month 9 (n=454) |
|
|
| |||||
| From 3-day voiding diaries ∞ | |||||
|
| |||||
| Urgency incontinence episodes per day† | |||||
| Adjusted means [standard errors (SE)] | 3.90 (0.11) | 1.75 (0.11) | 1.08 (0.11) | 0.97 (0.11) | 0.95 (0.11) |
| Median (IQR)F̄ | 3.0 (2.0-5.0) | 1.0 (0.0-2.3) | 0.3 (0.0-1.3) | 0.3 (0.0-1.0) | 0.3 (0.0-1.0) |
|
| |||||
| Total incontinence episodes per day | |||||
| Adjusted means (SE) | 4.64 (0.12) | 2.11 (0.13) | 1.36 (0.13) | 1.22 (0.13) | 1.16 (0.13) |
| Median (IQR) | 3.7 (2.3-6.0) | 1.0 (0.3-2.7) | 0.7 (0.0-1.7) | 0.3 (0.0-1.3) | 0.3 (0.0-1.3) |
|
| |||||
|
| |||||
| Daytime incontinence episodes per day | |||||
| Adjusted means (SE) | 4.03 (0.11) | 1.84 (0.11) | 1.19 (0.11) | 1.09 (0.11) | 1.06 (0.11) |
| Median (IQR) | 3.2 (2.0-5.0) | 1.0 (0.3-2.3) | 0.3 (0.0-1.7) | 0.3 (0.0-1.3) | 0.3 (0.0-1.3) |
|
| |||||
| Daytime voiding episodes per day | |||||
| Adjusted means (SE) | 8.67 (0.14) | 7.99 (0.14) | 7.59 (0.14) | 7.67 (0.14) | 7.70 (0.14) |
| Median (IQR) | 8.3 (6.7-10.0) | 7.7 (6.3-9.3) | 7.3 (6.0-9.0) | 7.3 (6.0-8.7) | 7.3 (5.8-9.2) |
|
| |||||
| Nocturnal voiding episodes per night¥ | |||||
| Adjusted means (SE) | 1.24 (0.05) | 0.89 (0.05) | 0.75 (0.05) | 0.75 (0.05) | 0.74 (0.05) |
| Median (IQR) | 1.0 (0.3-2.0) | 0.7 (0.0-1.3) | 0.3 (0.0-1.3) | 0.3 (0.0-1.3) | 0.3 (0.0-1.2) |
|
| |||||
| Moderate urgency-associated voids per day‡ | |||||
| Adjusted means (SE) | 7.65 (0.20) | 5.76 (0.20) | 5.07 (0.20) | 4.87 (0.20) | 4.96 (0.20) |
| Median (IQR) | 6.7 (4.7-9.7) | 5.0 (2.3-8.0) | 4.7 (1.7-7.3) | 4.3 (1.7-6.7) | 4.3 (2.0-7.0) |
|
| |||||
| Severe urgency-associated voids per day§ | |||||
| Adjusted means (SE) | 3.44 (0.12) | 1.90 (0.12) | 1.29 (0.12) | 1.30 (0.13) | 1.38 (0.13) |
| Median (IQR) | 2.3 (1.0-4.7) | 0.7 (0.0-2.7) | 0.7 (0.0-1.7) | 0.3 (0.0-1.7) | 0.7 (0.0-1.7) |
|
| |||||
| Self-report Bladder-specific questionnaires | |||||
| Overactive Bladder Questionnaire score¶ | |||||
| Adjusted means (SE) | 36.70 (0.85) | 21.65 (0.85) | 15.39 (0.85) | 14.90 (0.86) | 14.86 (0.86) |
| Median (IQR) | 33.6 (21.8-47.3) | 15.8 (6.7-31.5) | 9.7 (3.6-20.0) | 9.1 (3.6-18.8) | 8.5 (3.6-18.8) |
|
| |||||
| Patient Perception of Bladder Condition score∥ | |||||
| Adjusted means (SE) | 3.04 (0.06) | 2.12 (0.06) | 1.52 (0.06) | 1.49 (0.06) | 1.44 (0.06) |
| Median (IQR) | 3.0 (2.0-4.0) | 2.0 (1.0-3.0) | 1.0 (1.0-2.0) | 1.0 (0.0-2.0) | 1.0 (0.0-2.0) |
|
| |||||
| Patient Perception of Urgency Scale score± | |||||
| Adjusted means (SE) | 1.38 (0.03) | 1.01 (0.03) | 0.86 (0.03) | 0.80 (0.03) | 0.80 (0.03) |
| Median (IQR) | 1.0 (1.0-2.0) | 1.0 (1.0-1.0) | 1.0 (0.0-1.0) | 1.0 (0.0-1.0) | 1.0 (0.0-1.0) |
Randomized Control Trial (RCT): the pre-randomization visit of the 12-week RCT.
Baseline Open Label bladder diaries included data on RCT participants that had been on fesoterodine or placebo, all comparisons are significant at p<0.001 compared to the initial RCT visit.
One participant failed show for the Baseline Open Label visit, but was enrolled in open label at the subsequent visit.
Values reflect events per day based on participant completion of a 3-day voiding diary.
Self report on diary as an urgency or stress incontinence episode.
Interquartile range (IQR), Medians are unadjusted
Self report on the diary as to the time the participant went to bed for the evening and when the participant woke in the morning.
Moderate urgency-associated voids were defined as voiding episodes associated with at least a “moderate” sensation of urgency on voiding diary.
Severe urgency-associated voids were defined as voiding episodes associated with a “severe” sensation of urgency on voiding diary.
Overactive Bladder Questionnaire scores range from 0 to 100, with higher scores indicating more severe or bothersome overactive bladder symptoms.13,14
Patient Perception of Bladder Condition scores range from 1 to 6, with higher scores indicating more severe bladder-related problems.14
Patient Perception of Urgency Scale scores range from 1 to 3, with higher scores indicating greater urgency.15
We examined the difference in efficacy based on assignment (fesoterodine or placebo) in the RCT-phase of the study. While there were differences between groups at open label baseline and 3 months, these differences disappeared at 6 and 9 months (data not shown).
Satisfaction with treatment (Efficacy)
Nearly all participants who continued in the open label self-dosing study reported being very satisfied or somewhat satisfied with the study medication at each follow-up visit [89%, 92%, and 93% at 3, 6, and 9 month, respectively] as well as the change in their urine leakage at each follow-up visit [93%, 92%, and 92% at 3, 6, and 9 month, respectively]. The vast majority of women at each visit [98%, 98%, and 87% at 3, 6, and 9 month, respectively] reported that they would continue using fesoterodine.
Adverse Events (Safety)
Two hundred forty-one women (48%) experienced at least one moderate or severe adverse event (Table 3). Of these, 59 women experienced an anticholinergic adverse event that was plausibly related to the study medication; 15 reduced their medication dose, and 17 discontinued medication as a result of an adverse event. Twenty-six women experienced one or more serious adverse events; one of these (hospitalization for intestinal blockage) was identified as related to study treatment by the site principal investigator.
Table 3. Moderate and Severe Adverse Events and Postvoid Residual Volume (PVR) among women in the Open Label Study (N=498).
| Adverse Events† | |
|---|---|
| Reported at least one moderate or severe adverse event, N (%) | 241 (48.4) |
| Reported a potentially anticholinergic adverse event‡, N (%) | 59 (11.9) |
| Reported a serious adverse event§, N (%) | 26 (5.2) |
| Serious adverse event “possibly” related to treatment¶, N (%) | 1 (0.2) |
| Postvoid Residual Volume ∥ | |
| Mean (SD) PVR volume | 38.4 (48.3) |
| PVR volume ≥250 mL, N (%) | 3 (0.6) |
†Adverse events were assessed in the 498 women who took at least one dose of study drug and completed at least one follow-up visit. Common adverse events reported were (n): Dry mouth (30); Urinary tract infection (28); Cold/flu (25); Constipation (23); Dry throat (10); Back pain (9); Cough (8); Respiratory infection (8); Diarrhea (7); Headache (7); Runny/stuffy nose, sinus congestion (7); Bronchitis (5); Abdominal Pain (4); Allergy (4); Heartburn (4); Hematuria (4); Back strain (3); Chest pain (3); Dry eyes (3); Dyspepsia (3); Kidney infection (3); Pneumonia (3); Sciatica (3); Shortness of breath (3); Sinus infection (3); Vertigo (3); Weight gain (3)
Potentially anticholinergic adverse events were defined a priori as constipation, dry mouth, or self-report urinary hesitancy or retention.
Serious adverse events were defined as adverse events that resulted in death, disability, or hospitalization.
Serious adverse events “possibly” related to treatment were defined as serious adverse events that were rated by site investigators as having a possible, probable, or definite relationship to study medication.
PVR volume was measured at 3, 9 months or early termination among women taking at least one dose of study medication. PVR data was unavailable for 20 participants (17 refused, and 3 were lost to follow-up).
Thirty-five women (including 26 of the 44 women who withdrew) stopped taking medication during the open label study. Of these women, 20 reported discontinuing fesoterodine because side effects were too bothersome, 13 because of no improvement, and 9 for another, nonspecific, reason (women could select more than one reason). Six of these participants later restarted study medication.
Three participants had PVRs greater than 250 mL (1 at month 3 and 2 at month 9) and were scheduled for extended evaluations; one participant did not return for the evaluation. There was no difference in PVR based on measurement technique (ultrasound or catheter measurements, Kruskal-Wallis p=0.09).
Summary of Extended Evaluations (Safety)
Eighty-three women were offered extended evaluations during the open-label study; 22 completed extended evaluations and 61 did not. There was no difference in having an indication for extended evaluation or completing an extended evaluation based on treatment assignment in the RCT. The indications for the majority of the 61 declined extended evaluations were based on women’s decision to not continue fesoterodine (n=58) rather than serious adverse events or elevated PVR (Table 4). Five women had other health issues (e.g., elevated blood pressure, discontinued contraception). Several chose to see their own physician instead of the site specialist.
Table 4. Summary of indications for the Extended Evaluation (ExE, n (%))*.
| Offered ExE (n=83) |
Completed ExE (n=22) |
|
|---|---|---|
| Indication for ExE | ||
| Post Void Residual >250cc twice in one day | 3 (3) | 2 (9) |
| Side effects too bothersome | 39 (43) | 9 (40) |
| Symptoms did not improve | 32 (35) | 14 (64) |
| Urinary Tract Infection | 1 (1) | 1 (5) |
| Other** | 16 (19) | 0 (0) |
| No reason*** | 1 (1) | 1 (5) |
May sum to greater than 100% as participants could have more than one reason for ExE
Other reasons included: physician recommendation, other health reason, participant circumstances, “wait and see” what happens upon medication discontinuation, and other
One woman had an ExE with her private physician without having a study indication for ExE
In 17 of the 22 completed extended evaluations, the site and central specialists agreed that the diagnosis based on the 3IQ was correct (urgency-predominant incontinence). In only 5 of the cases, the specialists decided that the participant’s incontinence was misclassified by the 3IQ. In 4 of the 22 completed evaluations, the site specialist and central specialist disagreed about the diagnosis and reached a consensus diagnosis after discussion. For all of the 22 evaluations, the site specialist and central specialist agreed that none of the missed diagnoses resulted in participant harm.
Comment
We conducted a 9-month open-label follow-up study after a 12-week RCT to assess the longer-term safety and efficacy of anti-muscarinic therapy in community-dwelling women diagnosed with UUI using a simple 3-item questionnaire, the 3IQ. To our knowledge, this study is the first of its kind to evaluate longer-term clinical outcomes associated with using a simple, questionnaire-based screening instrument to diagnose and treat UUI in women without more extensive specialist evaluation.
These findings support the longer-term safety of using the 3IQ to guide treatment for UUI in women. Although 28 serious adverse events (in 26 women) were reported, only 1 case (0.2%) was considered possibly related to treatment. Based on review of the completed specialist-based extended evaluations, a minority of participants (1%) were misdiagnosed as having UUI. If we extrapolate that rate to all of 83 women offered extended evaluations, 4% of participants were possibly misdiagnosed. No participants experienced harm from the use of the 3IQ to make the initial diagnosis followed by treatment.
While 48% of women experienced an adverse event, only 53 women in this study discontinued treatment, similar to the adverse event rate19-21 and discontinuation rates19-22 of other long-term UUI treatment studies in which participants had more extensive evaluations before initiating therapy. These adverse events (Table 3) are symptoms that primary care practitioners are comfortable assessing and treating. Reduction in UUI frequency was also similar to that seen in other long-term studies with more extensive evaluations for eligibility.19,21 Overall, women in our study reported they were very satisfied with improvement in urine leakage, and the vast majority indicated that they would continue treatment.
By 3 months, most women saw improvement in their incontinence, became bothered by medication side effects, or felt the need to change their medication dose. These results suggest that 3 months may be a sufficient time frame for an initial empiric trial of therapy after 3IQ diagnosis. In clinical practice, the patient and clinician should discuss next steps (e.g., continued therapy, dose adjustment, more extensive evaluation) after a 3-month medication trial.
Our study has several limitations that deserve mention. While we used a simple diagnostic tool that can be implemented in primary care, women were diagnosed within the context of a research study rather than primary care practices. As such, women were actively queried for side effects at regular intervals. When implementing the 3IQ in primary care, it would be useful to provide primary care practitioners with common antimuscarinic side effects as well as the 3IQ questions. The study was conducted with only one antimuscarinic medication, fesoterodine, and therefore we cannot comment on safety, efficacy, or satisfaction with other medications in this class. In addition, what should be done next for women who do not improve on fesoterodine, such as trial of a second antimuscarinic medication or referral to a specialist, cannot be answered by this study. Also, this open label study was conducted only in women and it is not known if the results can be generalized to men, in whom the 3IQ has not been validated, or in women who have less frequent UUI. Finally, we do not have data on the 20 of 498 participants (4%) who did not attend an early termination or final study visit and cannot comment on safety, efficacy, or satisfaction with treatment in that group.
For over 15 years, recommendations have existed to increase diagnosis and treatment of incontinence in the primary care setting. Given new evidence of the safety and efficacy of using the 3IQ to diagnose incontinence type, there is reason to believe that a streamlined approach to diagnosing and treating UUI is likely to result in good clinical outcomes in community-dwelling women without major comorbidities. Increased access to treatment for women with UUI in primary care practices could have significant implications for women’s quality of life.
Acknowledgements
We would like to thank the BRIDGES participants.
Support.
Pfizer, Inc. provided funding for the study and the study medication, but did not provide other input into the design of the study; collection, analysis, or interpretation of data; writing of the report; or the decision to submit the paper for publication. Drs. Hess, Huang and Brown and Mr. Schembri had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study funders provided no manuscript preparation assistance.
APPENDIX
Appendix Table 1. Open Label Study Measures.
| Study Measure | Time Point* | |||||
|---|---|---|---|---|---|---|
| Baseline Open label |
2 week phone |
1 mo | 3 mo | 6 mo | 9 mo** | |
| 3-Day Voiding Diary | X | X | X | X | ||
| Patient Perception of Bladder Condition (PPBC)¶ | X | X | X | X | ||
| Patient Perception of Urgency Scale (PPUS)‡ | X | X | X | X | ||
| Overactive Bladder Questionnaire (OAB-Q)§ | X | X | X | X | ||
| Patient directed Dose Adjustment | X | X | X | X | ||
| Adverse events (self-report) | X | X | X | X | X | X |
| Post Void Residual (PVR) | X | X | X | |||
| OAB Satisfaction (OAB-S)† | X | X | X | |||
| Referral for Extended Evaluation (ExE)∥ | X | X | X | |||
| Medication Distribution | X | X | X | X | ||
SV: Screening visit, RCT: Randomized controlled trial, mo: month
Or Early Termination
The Patient Perception of Bladder Condition is a single-item measure scored on a 6-point Likert scale, with higher scores indicating more severe bladder-related problems.13,14
The Patient Perception of Urgency Scale is a single-item measure scored on a 3-point Likert scale, with higher scores indicating greater urgency15
The Overactive Bladder Questionnaire is a 31-item instrument in which scores range from 0 to 100, with higher scores indicating more severe or bothersome overactive bladder symptoms.14
The Overactive Bladder Satisfaction is a validated disease-specific satisfaction measure our team adapted to assess participant satisfaction with treatment.19
Extended Evaluations were offered if the participant had two post void residuals (PVR) greater than 250cc on the same day or the participant reported lack of clinical improvement as captured on the OAB-S form or a safety issue was identified by the site PI.
Footnotes
Conflicts of Interest
Dr. Richter has received investigator-initiated grant funding from and was a consultant to Astellas. Dr Kraus serves as Course Director/faculty for Laborie Medical and has served as a consultant for Pfizer, Allergan, Merck and Astellas. Dr. Huang has received investigator-initiated grant funding from Pfizer, Inc. Dr. R Rogers is the Data Safety Monitoring Chair for the TRANSFORM trial sponsored by American Medical Systems. Dr. Bradley has served as a consultant to Astellas and GlaxoSmithKlein. Dr. Brown has received investigator-initiated grant funding from Astellas, and is a Scientific Advisor for Allergen
References
- 1.Subak LL, Brown JS, Kraus SR, et al. The “costs” of urinary incontinence for women. Obstet Gynecol. 2006 Apr;107(4):908–916. doi: 10.1097/01.AOG.0000206213.48334.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? J Am Geriatr Soc. 2000;48:1–5. doi: 10.1111/j.1532-5415.2000.tb04744.x. [DOI] [PubMed] [Google Scholar]
- 3.Fultz NH, Fisher GG, Jenkins KR. Does urinary incontinence affect middle-aged and older women’s time use and activity patterns? Obstet Gynecol. 2004 Dec;104(6):1327–1334. doi: 10.1097/01.AOG.0000143829.21758.3c. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard I, Turvey C, Burns TL, Crischilles E, Wallace R. Urinary incontinence and depression in middle-aged United States women. Obstet Gynecol. 2003 Jan;101(1):149–156. doi: 10.1016/s0029-7844(02)02519-x. [DOI] [PubMed] [Google Scholar]
- 5.Langa KM, Fultz NH, Saint S, Kabeto MU, Herzog AR. Informal caregiving time and costs for urinary incontinence in older individuals in the United States. J Am Geriatr Soc. 2002 Apr;50(4):733–737. doi: 10.1046/j.1532-5415.2002.50170.x. [DOI] [PubMed] [Google Scholar]
- 6.Shaw C, Tansey R, Jackson C, Hyde C, Allan R. Barriers to help seeking in people with urinary symptoms. Fam Pract. 2001 Feb;18(1):48–52. doi: 10.1093/fampra/18.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Shaw C, Atwell C, Wood F, Brittain K, Williams K. A qualitative study of the assessment and treatment of incontinence in primary care. Fam Pract. 2007 Oct;24(5):461–467. doi: 10.1093/fampra/cmm041. [DOI] [PubMed] [Google Scholar]
- 8.Williams KS, Assassa RP, Smith N, Rippin C, Shaw C, Mayne C. Good practice in continence care: development of nurse-led service. Br J Nurs. 2002 Apr;11(8):548–559. doi: 10.12968/bjon.2002.11.8.10164. 25-May 8. [DOI] [PubMed] [Google Scholar]
- 9.Managing acute and chronic urinary incontinence AHCPR Urinary Incontinence in Adults Guideline Update Panel. Am Fam Physician. 1996;54(5):1661–1672. [PubMed] [Google Scholar]
- 10.Assessment and treatment of urinary incontinence Scientific Committee of the First International Consultation on Incontinence. The Lancet. 2000;355:2153–2158. [PubMed] [Google Scholar]
- 11.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006 May 16;144(10):715–723. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang AJ, Hess R, Arya LA, et al. Pharmacologic treatment for urgency-predominant urinary incontinence in women diagnosed using a simplified algorithm: a randomized trial. Am J Obstet Gynecol. 2012 May;206(5):444 e441–444 e411. doi: 10.1016/j.ajog.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JS, McNaughton KS, Wyman JF, et al. Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology. 2003 Apr;61(4):802–809. doi: 10.1016/s0090-4295(02)02505-0. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS, OROS Oxybutynin Study Group Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol. 1999 Jun;161(6):1809–1812. [PubMed] [Google Scholar]
- 15.Matza LS, Thompson CL, Krasnow J, Brewster-Jordan J, Zyczynski T, Coyne KS. Test-retest reliability of four questionnaires for patients with overactive bladder: the overactive bladder questionnaire (OAB-q), patient perception of bladder condition (PPBC), urgency questionnaire (UQ), and the primary OAB symptom questionnaire (POSQ) Neurourol Urodyn. 2005;24(3):215–225. doi: 10.1002/nau.20110. [DOI] [PubMed] [Google Scholar]
- 16.Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol. 2006 Jun;49(6):1079–1086. doi: 10.1016/j.eururo.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Cardozo L, Coyne KS, Versi E. Validation of the urgency perception scale. BJU Int. 2005 Mar;95(4):591–596. doi: 10.1111/j.1464-410X.2005.05345.x. [DOI] [PubMed] [Google Scholar]
- 18.Piault E, Evans CJ, Espindle D, Kopp Z, Brubaker L, Abrams P. Development and validation of the Overactive Bladder Satisfaction (OAB-S) Questionnaire. Neurourol Urodyn. 2008;27(3):179–190. doi: 10.1002/nau.20455. [DOI] [PubMed] [Google Scholar]
- 19.Appell RA, Abrams P, Drutz HP, Van Kerrebroeck PE, Millard R, Wein A. Treatment of overactive bladder: long-term tolerability and efficacy of tolterodine. World J Urol. 2001 Apr;19(2):141–147. doi: 10.1007/pl00007094. [DOI] [PubMed] [Google Scholar]
- 20.Haab F, Cardozo L, Chapple C, Ridder AM, Solifenacin Study G Long-term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. Eur Urol. 2005 Mar;47(3):376–384. doi: 10.1016/j.eururo.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002 Jun;41(6):588–595. doi: 10.1016/s0302-2838(02)00177-x. [DOI] [PubMed] [Google Scholar]
- 22.Diokno A, Sand P, Labasky R, et al. Long-term safety of extended-release oxybutynin chloride in a community-dwelling population of participants with overactive bladder: a one-year study. Int Urol Nephrol. 2002;34(1):43–49. doi: 10.1023/a:1021372426421. [DOI] [PubMed] [Google Scholar]