Abstract.
Gap junction-mediated intercellular communication influences a variety of cellular activities. In tendons, gap junctions modulate collagen production, are involved in strain-induced cell death, and are involved in the response to mechanical stimulation. The aim of the present study was to investigate gap junction-mediated intercellular communication in healthy human tendon-derived cells using fluorescence recovery after photobleaching (FRAP). The FRAP is a noninvasive technique that allows quantitative measurement of gap junction function in living cells. It is based on diffusion-dependent redistribution of a gap junction-permeable fluorescent dye. Using FRAP, we showed that human tenocytes form functional gap junctions in monolayer and three-dimensional (3-D) collagen I culture. Fluorescently labeled tenocytes following photobleaching rapidly reacquired the fluorescent dye from neighboring cells, while HeLa cells, which do not communicate by gap junctions, remained bleached. Furthermore, both -glycyrrhetinic acid and carbenoxolone, standard inhibitors of gap junction activity, impaired fluorescence recovery in tendon cells. In both monolayer and 3-D cultures, intercellular communication in isolated cells was significantly decreased when compared with cells forming many cell-to-cell contacts. In this study, we used FRAP as a tool to quantify and experimentally manipulate the function of gap junctions in human tenocytes in both two-dimensional (2-D) and 3-D cultures.
Keywords: fluorescence recovery after photobleaching, human tenocytes, gap junctions, three dimensional
1. Introduction
The mechanisms by which human tendon cells (tenocytes) sense and adapt to mechanical load are central to the ability of tendons to function as force transmitters between muscle and bone. Under physiological conditions, tendons are exposed to large tensile mechanical loads as well as compression and shear stress; thus, tenocytes must be able to effectively respond to changes in mechanical forces. Tenocytes are responsible for the secretion, assembly, and turnover of collagen, the major structural component resisting tension in tendons. Tendon injuries are common and can be highly debilitating.1,2 However, there is no agreement regarding the best management of tendon damage. Although operative treatment of Achilles tendon ruptures results in lower rerupture rates in comparison to nonoperative treatment, it is associated with complications such as infections and nerve dysfunction.3 Furthermore, it has been shown that symptomatic recovery after tendon injuries does not ensure the full recovery of muscle–tendon function.4 Current techniques for management of tendon injury are insufficient, and the development of new approaches to accelerate tendon healing is essential.5
Tendons are made up of bundles of collagen fibers with tenocytes aligned in rows forming a network of cell processes linked by gap junctions.6 Gap junctions play an essential role in intercellular communication, as they enable the exchange of ions and small metabolites between the cells by connecting the cytoplasm of adjacent cells. Each gap junction channel is composed of two half-channels, which in turn are made up of six protein subunits called connexins (Cx). In humans, the family of connexins consists of 21 members.7 Several members of the connexin family are present in tendons including Cx43.6,8,9 The regional distribution of connexins varies and is associated with changes in tenocyte phenotype.10
The role of gap junctions in human tenocytes is unclear, since the majority of work has been performed using nonhuman cells. Nevertheless, gap junctions have been suggested to regulate collagen production in both unstrained and mechanically loaded tenocytes. Treatment with various gap junction inhibitors, such as octanol, mimetic peptides, and antisense oligonucleotides, was shown to either decrease or increase the collagen production in tendons and tendon-derived cells in chicken depending on the inhibitor used.9,11 It has also been demonstrated that mechanical loading can directly regulate gap junction function. Prolonged static tensile load was shown to decrease gap junction permeability in rat tendons. This effect was accompanied by changes in Cx43 protein distribution and Cx43 mRNA expression.8 At the same time, human tenocytes subjected to cyclic strain in the presence of heptanol, a further gap junction inhibitor, were demonstrated to undergo strain-induced cell death.12 Furthermore, work in patients and knockout mice models with oculodentodigital dysplasia, a disease caused by mutations in the gene coding for Cx43, has shown that reduced Cx43 expression leads to impaired wound closure.13 Although the function of gap junction in wound healing is controversial with some other studies showing reduced inflammatory response and enhanced wound healing following downregulation of Cx43,14,15 together these data suggest a putative role of Cx43-mediated intercellular communication in tendon repair.
Fluorescence recovery after photobleaching (FRAP) permits measurements of gap junction function without disrupting the cell integrity. It gives the possibility to continuously monitor intercellular communication performing multiple measurements of the same cells. In the FRAP assay, diffusion-dependent redistribution of a gap junction-permeable fluorescent dye following photobleaching is measured. In a fluorescently labeled cell that is in contact with other labeled cells, photobleaching results in a rapid decrease in fluorescence intensity followed by fluorescence increase over a period of time due to gap junctional activity.16 The FRAP method has been successfully used to investigate intercellular communication in various tissues and cells types.17–19 So far, this technique has been used to study cells isolated from horse tendons.20 In the present study, we have used FRAP to characterize gap junction-mediated communication in healthy human tendon cells.
2. Materials and Methods
2.1. Cell Isolation and Culture
Human hamstring tendon samples were obtained from the Oxford Musculoskeletal Biobank with informed donor consent in full compliance with National and Institutional ethical requirements, the United Kingdom Human Tissue Act, and the Declaration of Helsinki. They were collected from healthy male and female donors aged 24 to 55 years undergoing an allograft repair of anterior cruciate ligament rupture. Tenocytes were isolated by explant cultures of tendon tissue, as described previously.21 In brief, tendon tissue was cut into 1-mm2 pieces and cultured in 50% fetal bovine serum (FBS)/Dulbecco's Modified Eagle Medium (DMEM)-F12 for 7 to 10 days until the cells begun to migrate out. Next, the tissue pieces were removed, and the cells further cultured until they reached 70% to 80% confluency. Lastly, the cells were frozen in 10% dimethyl sulfoxide (DMSO) and 90% FBS for later use. In order to avoid a phenotypic drift,22 primary tenocytes were used in subsequent experiments until passage 4. The cells were passaged at 70% confluence. Standard culture medium used for the cells was DMEM-F12 supplemented with 10% FBS. The culture medium was changed every 3 to 4 days. Before a FRAP experiment, cells were seeded onto 35-mm Petri dishes with glass bottom (Ibidi, Martinsried, Germany) and cultured between 4 and 16 days to reach confluency. For low-density culture condition, the cells were subjected to FRAP protocol after at least 4 days of culture when they reached about 20% confluency.
HeLa cells were maintained in OptiMEM with 0.5% FBS and 1% antibiotics. Before the FRAP experiment, the cells were seeded onto 35-mm Petri dishes with a glass bottom and cultured until they reached confluency.
2.2. Three-Dimensional Culture
In order to perform three-dimensional (3-D) FRAP, human tenocytes were seeded in collagen gels. The cells were trypsinized, counted, and subsequently mixed with rat tail collagen I (BD Biosciences, Plymouth, UK) in phosphate buffered saline (PBS) at . Collagen I was neutralized beforehand with 1-N NaOH according to manufacturer’s instructions. Drops containing 20,000 cells suspended in 100 μl of collagen I were pipetted directly onto the glass bottom culture dishes and allowed to set for 30 min at 37°C. Lastly, the dishes were filled with DMEM-F12 supplemented with 10% FBS to cover the formed gels. The cells in collagen gels were cultured for at least 4 days before beginning FRAP experiments.
2.3. Fluorescence Recovery After Photobleaching
The cells were loaded for 15 min with 4-μM calcein acetoxymethylester (AM) solution (Sigma-Aldrich, Poole, UK), diluted in serum-free culture medium, then washed several times with prewarmed colorless medium, and subjected to the FRAP-modified method used by Young et al.20 Gap junction communication was blocked by pretreating the cells with 100-μM 18β-glycyrrhetinic acid (GA) (Acros Organics, Geel, Belgium) or 100-μM carbenoxolone disodium (CBX) (Tocris Bioscience, Bristol, UK) for 5 min. The stock solutions of GA and CBX were prepared in DMSO and water, respectively. The cells were grown to confluency, stained with calcein AM (Sigma, Poole, UK), and subsequently preincubated either with CBX or GA diluted in culture medium. When treated with GA, 10% serum was present throughout the experiment and the inhibitor was always effective. For CBX inhibition, the cells were tested in 10% serum and also the serum was starved for 2 days before the experiment. The photobleaching was performed in the absence of serum. The CBX appeared to be more reliable without serum, so data for serum-starved conditions are shown. Both inhibitors were present in the medium during the entire FRAP experiment. All experiments have been carried out on cells from at least three different tissue donors. For each condition, between 4 and 10 cells were photobleached and imaged.
The FRAP and imaging system consisted of a Zeiss LSM 710 scanhead (Zeiss GmbH, Jena, Germany) coupled to an inverted Zeiss Axio Observer.Z1 microscope equipped with a and a objectives (Zeiss GmbH). Samples were placed in an incubator chamber capable of maintaining temperature at 37°C. All experiments were performed using the objective, except when HeLa cells were used as they were analyzed using objective. Both photobleaching and fluorescence imaging of calcein used an argon-ion laser (488 nm) and a piezomultiplier tube for detection of fluorescence between 500 and 550 nm. The following acquisition mode was used: speed 9 (pixel dwell 1.58 μs), averaging number 1, and 8 bit depth. The following bleaching parameters were applied: scan speed 3 (pixel dwell 50.42 μs), 50 scans, and 4 iterations. In order to perform precise measurements in both two-dimensional (2-D) and 3-D cultures in each experiment, three regions of interest (ROI) were measured. A ROI was manually drawn around cells selected for bleaching. To monitor the changes in fluorescence associated with acquisition bleaching, a reference ROI within an unbleached cell and base ROI, a region outside the cells to measure the background, were drawn. Experiments in which considerable fluctuations in fluorescence intensity in reference ROI were observed were not included in the analysis. Furthermore, throughout the experiments, the cells were monitored for abnormal morphological or physiological features caused by photobleaching. Cells selected for bleaching after one prescan were bleached at 100% power. A time-lapse series was then taken to record calcein recovery using 1% of the power used for bleaching every 5 s up to 4 min. The recovery was fitted with a mono-exponential function. The following fit formula was used: , where is the average intensity of the bleached region at time (s), IE is the final signal intensity in the analyzed bleach region following recovery, is the amplitude of the fitted curve, and is a constant that is associated with the rate of recovery. The mobile fraction percentage was calculated by measuring the fluorescence intensity in the bleached region at the times indicated in the equation: mobile fraction , where is the fluorescence intensity in the bleached region after full recovery, just after bleaching, and before bleaching. The image datasets and fluorescence recovery data including the mobile fraction percentage were exported from ZEN 2009, the microscope control software, to GraphPad Prism for plotting.
2.4. Immunocytochemistry
Primary human tenocytes were grown on 1-cm diameter cover glasses in DMEM-F12 media supplemented with 10% FBS until confluent. Subsequently, the cells were fixed in 10% formalin for 30 min, washed with PBS, permeabilized with 0.5% Triton X-100 for 5 min, and blocked with 1% horse serum for 30 min. The cells were stained with 1:100 anti-Cx43 antibody (Ab-367, Sigma-Aldrich) overnight at 4°C. After washing with PBS, the cells were incubated with 1:100 anti-rabbit secondary antibody DY594 for 1 h at room temperature. The cells were washed with PBS, and mounted and viewed with an inverted Zeiss Axio Observer.Z1 microscope.
2.5. Statistical Analysis
All experiments were repeated at least three times using different tissue donors. Results were analyzed using two-tailed, unpaired Student’s -test using GraphPad Prism software. Results are expressed as . A -value lower than 0.05 was considered statistically significant.
3. Results
3.1. Cx43 Expression in Human Tenocytes
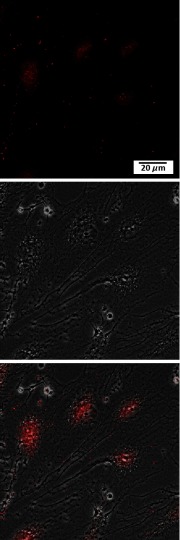
Cx43 is one of the major connexin types expressed in tendon and tendon-derived cells, playing a crucial part in gap junction communication.6,8,9 In order to study gap junctional communication in human tenocytes in vitro, the presence of Cx43 was first confirmed in the cells. Tenocytes were grown to confluence and immunolabeled for Cx43. Figure 1 shows the distribution of Cx43 protein in the cells. Cx43 expression was found around the nucleus and in large foci at cell–cell borders, corresponding to gap junctions between adjacent cells.
Fig. 1.
Localization of Cx43 protein in human tenocytes. Representative confocal images of cells immunostained for Cx43 are shown. Images showing Cx43 staining (red), corresponding phase contrast image, and merged image.
3.2. Human Tendon Cells Form Functional Gap Junctions
Intercellular communication in human tenocytes was assessed using a FRAP assay. In brief, cells were labeled with a nontoxic concentration of calcein AM for 15 min. Next, a cell of interest was selected, bleached at 100% power, and subsequently a time-lapse series was taken to record calcein recovery. Calcein AM is a nonfluorescent dye, which is converted by intracellular esterases to green-fluorescent and membrane-impermeable calcein. It has been widely used to study intercellular communication in FRAP experiments, as it can be transported across gap junctions.8,17,20,23
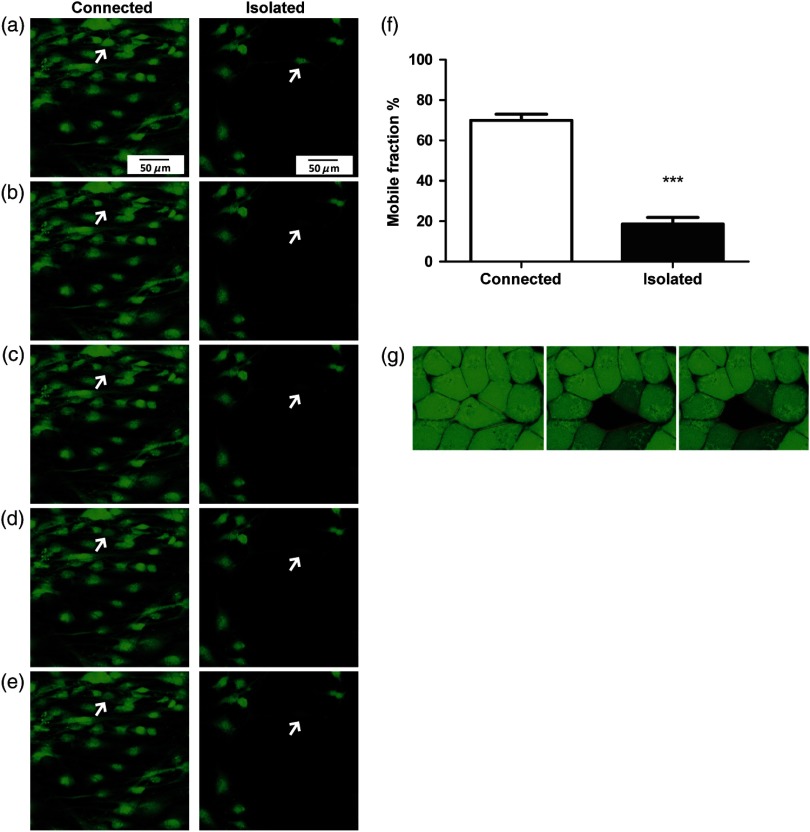
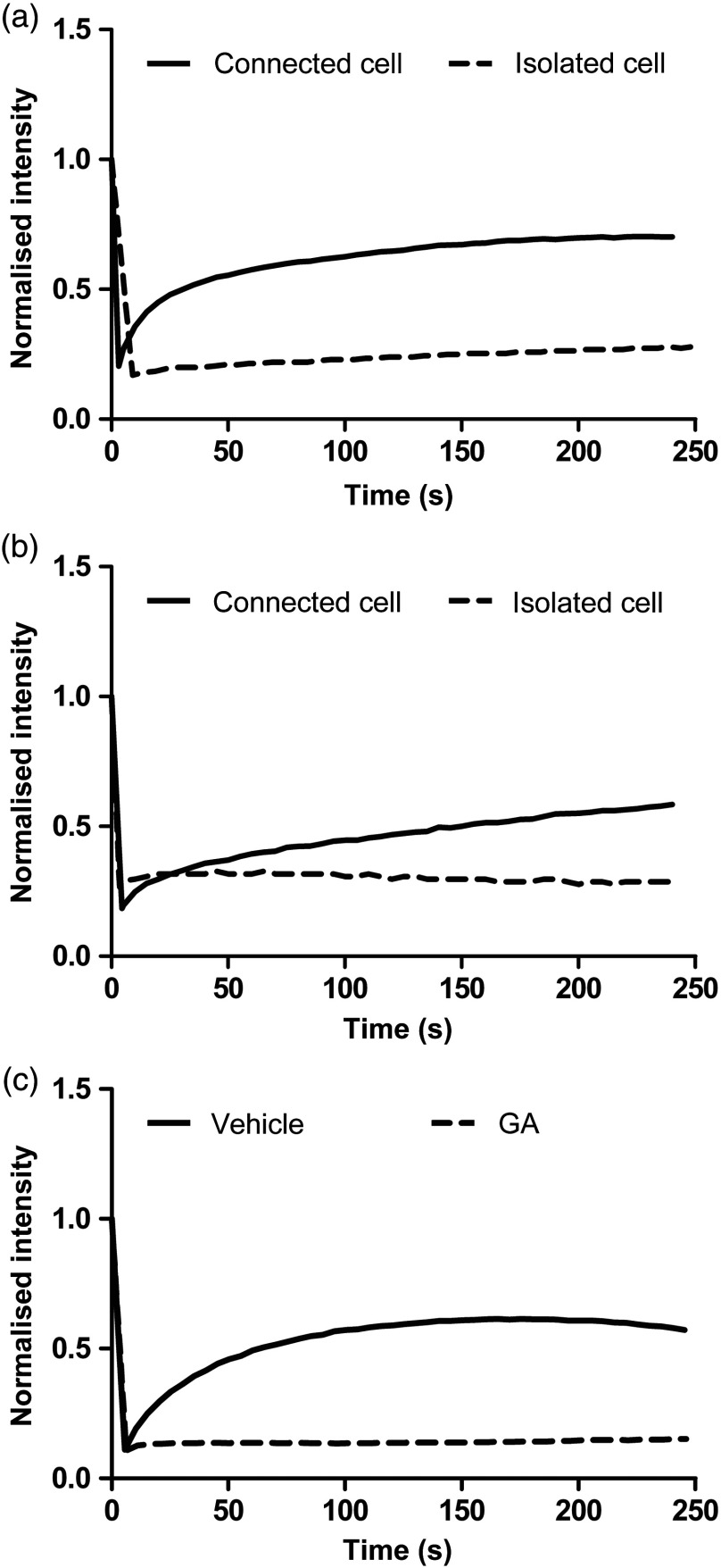
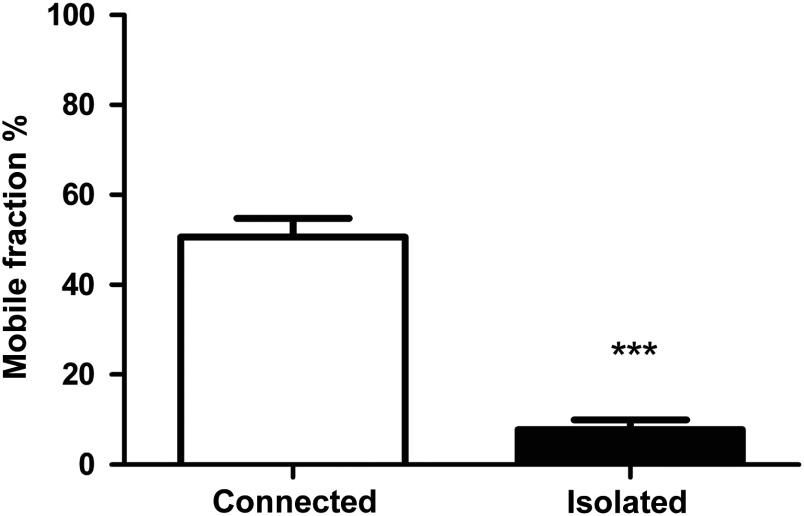
Initially, two culture conditions were compared to study the function of gap junctions in human tenocytes, namely a low- and a high-cell density. The recovery after photobleaching in tenocytes grown in a confluent monolayer, where cells formed many cell-to-cell contacts (connected), is demonstrated in the Figs. 2(a)–2(e), left. Tenocytes grown at low density rarely formed cell-to-cell contacts (isolated) and when subjected to photobleaching, their recovery was considerably impaired [Figs. 2(a)–2(e), right]. Representative fluorescence intensity curves acquired during a FRAP experiment, highlighting the different response of connected and isolated cells to photobleaching, are shown in Fig. 5(a). To quantify intercellular communication in both conditions, we used the mobile fraction percentage as previously described for rabbit ligaments.23 The mobile fraction percentage values were significantly higher in connected cells, reaching about 70% in connected and 20% isolated cells [Fig. 2(f)]. As a control, confluent HeLa cells, which do not form gap junctional connections,24 were also loaded with calcein AM and subjected to FRAP. The cells displayed no recovery, indicating that functional gap junctions are required for dye transfer [Fig. 2(g)].
Fig. 2.
Gap junctional communication in human tenocytes grown at low density (isolated) and in a confluent monolayer (connected). Tenocytes grown at low density and subjected to photobleaching recover slower than tenocytes in a confluent monolayer, where cells are forming many cell-to-cell contacts. An example of a time-lapse series recorded for isolated and connected cells is shown. Images were taken prior to photobleaching (a), just after bleaching (b), and at subsequent time intervals 25.6, 55.6, and 85.6 s and 29.7, 59.7, and 89.7 for connected and isolated cells, respectively (c–e). (f) The mobile fraction percentage values in isolated and connected tendon cells. Results shown are of three patients. Statistical differences are indicated as ***. (g) An example of a time-lapse series recorded for HeLa cells. Cells are shown before photobleaching (left) and subsequently 24 s (middle) and 56 s (right) after photobleaching. No recovery was observed.
Fig. 5.
Representative fluorescence recovery after photobleaching (FRAP) curves showing the changes in fluorescence intensities over time in cells from the same donor, subjected to photobleaching, and cultured in different conditions. (a) Connected versus isolated cells in 2-D culture. (b) Connected versus isolated cells in 3-D culture. (c) Gap junction blocking with GA.
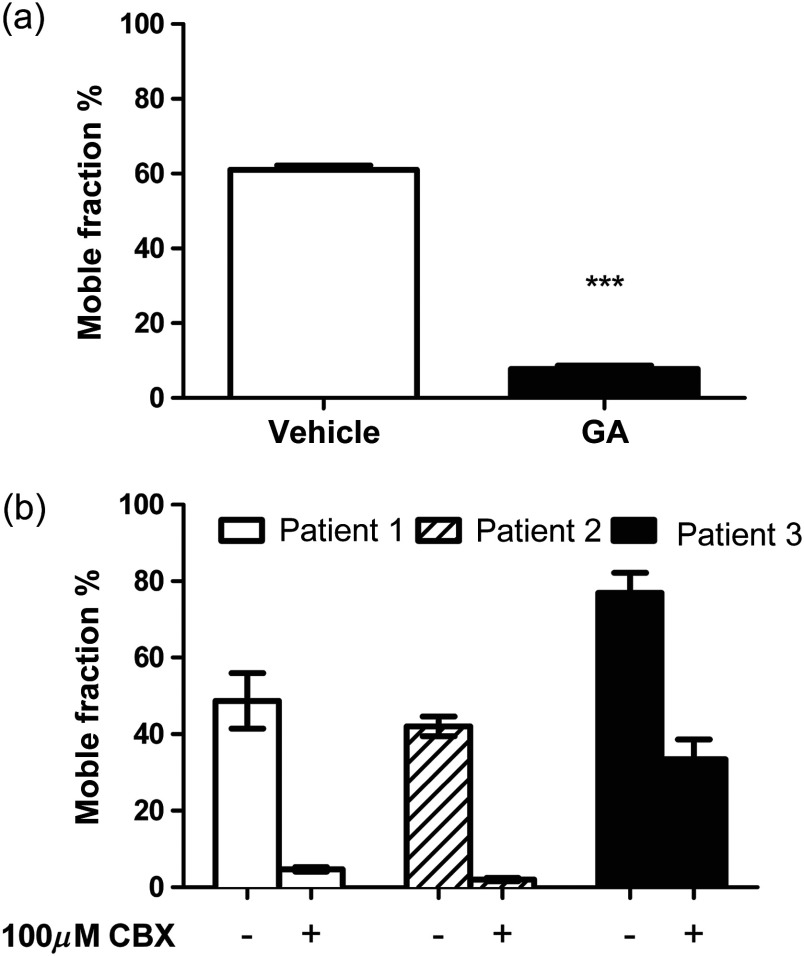
To further confirm our results and to explore druggability, we disrupted gap junctional communication in the cells by using two nonspecific gap junction blockers: 18β-GA and CBX. The latter has not been used in tendon before. As demonstrated in Fig. 3(a), the pretreatment with GA prevented fluorescent recovery in the cells, significantly reducing the mobile fraction percentage to 7.8%. A characteristic FRAP curve for a GA-treated cell also shows no fluorescent recovery after photobleaching [Fig. 5(c)]. Although CBX produced more variable results, pretreatment with CBX blocked the fluorescent recovery in cells from two donors and significantly decreased the recovery in a third [Fig. 3(b) and Videos 1 and 2]. Collectively, these results confirm the presence of functional gap junctions in human tendon cells.
Fig. 3.
Gap junctional communication in human tenocytes treated with nonspecific gap junction blockers glycyrrhetinic acid (GA) and carbenoxolone disodium (CBX). Both GA and CBX disrupt gap junctional communication in tendon cells. (a) The mobile fraction percentage values in cells treated with 100-μM GA and vehicle control. Results shown are of three patients. Statistical differences are indicated as ***. (b) The mobile fraction percentage values in tenocytes incubated with and without 100-μM CBX. Results shown are of each tissue donor. Significant statistical differences were noted for all three donors with for patients 1 and 2 and for patient 3.
Video 1.
Video 2.
3.3. Human Tendon Cells Form Functional Gap Junctions in a 3-D Collagen Gel
Finally, to check the gap junction function in an environment mimicking in vivo tendon, a 3-D culture model was setup. Similar to the human tenocytes grown in monolayer, two conditions were compared. Cells were selected for photobleaching based on the presence of cell-to-cell contacts. Consequently, for the isolated condition, only cells that formed sporadic cell-to-cell contacts were selected and for the connected condition, cells that formed numerous cell-to-cell contacts were chosen. As demonstrated in Fig. 4, isolated cells subjected to photobleaching showed a decrease in intercellular communication in comparison to tenocytes forming many cell-to-cell contacts. The mobile fraction percentage values in isolated tenocytes and well-connected cells in 3-D were similar to the 2-D condition showing values reaching about 50% in connected cells and 8% isolated cells (Fig. 4). The slightly lower numbers reflect the general lower density of cells found in 3-D gels. The FRAP curves for both 3-D and 2-D conditions showed the same patterns in recovery [Fig. 5(b)]. Together these results show that human tenocytes maintain functional gap junctions in 2-D and 3-D culture conditions.
Fig. 4.
Gap junctional communication in isolated versus connected human tenocytes cultured in 3-D collagen gels. Isolated cells grown in 3-D and subjected to photobleaching recover slower than tenocytes forming many cell-to-cell contacts. The mobile fraction percentage values in isolated tenocytes and well-connected cells. Results shown are of three patients. Statistical differences are indicated as ***.
4. Discussion
In the present study, we investigated gap junctional communication in healthy human tenocytes using a FRAP technique. This assay has been introduced by Wade and collaborators as an alternative approach to evaluate gap junction function in human fibroblasts and teratocarcinoma cells without disrupting the cell integrity. It measures the redistribution of a gap junction-permeable fluorescent dye following photobleaching.16 Previously, FRAP was used to study the differences in the intercellular communication between tendon-derived cells from foals and adult horses.20 A similar technique, namely fluorescent loss induced by photobleaching, was used to investigate the effects of tensile load on rat tendon fascicles.8 Using FRAP, we were able to demonstrate that human tendon cells cultured in vitro in both 2-D and 3-D form functional gap junctions. Human tenocytes grown in a confluent monolayer expressed Cx43 at cell-to-cell borders and rapidly recovered after photobleaching. At the same time, cells with no or only a few apparent cell-to-cell contacts demonstrated no or a significantly impaired recovery. The same was also true for 3-D cultures, where significant differences were observed in recovers depending on the degree of cell-to-cell contact formed. Similar observations were made previously by Hunter et al., who showed that adult notochordal nucleus pulposus cast into thin agarose gels and subjected to FRAP recovers only in the presence of adjacent cells.25
The use of different gap junction inhibitors, such as GA, octanol, mimetic peptides, and antisense oligonucleotides, has been described to study gap junction function in tenocytes.8,9 To corroborate our results, we used two gap junction blockers, 18β-GA and its synthetic derivative CBX, to modulate gap junction communication in human tenocytes. Both inhibitors were described to induce conformational changes in connexin structure, leading to changes in gap junction permeability.26 Although CBX has not been used previously in gap junction blocking experiments in tendon, it was used to inhibit intercellular communication in other cell types.18,19 We have shown that GA and CBX significantly decrease gap junction-mediated communication in treated tenocytes. Interestingly, in some experiments, CBX action was only transient and after a period of complete inhibition, cells were able to recover despite the presence of an inhibitor (data not shown). Collectively, these results confirm the presence of functional gap junctions in human tenocytes.
Although calcein is commonly utilized as a viability indicator, it can also be used to study intercellular communication in FRAP experiments.8,17,20,23 It is a membrane-impermeable hydrolysis product of calcein AM. The dye is converted by cellular esterases when it enters the cell and can be transported across functional gap junctions. To control the possibility that uncatalyzed calcein AM was still present in the medium or leaking out of stained cells and was being taken back into the bleached cells, we tested HeLa cells which do not form functional gap junctions. As demonstrated, HeLa cells did not recover after FRAP. This is further supported by the action of both GA and CBX in preventing recovery from FRAP as well as significant differences in recovery between isolated and connected cells. To test that the inhibitors were not interfering with cellular esterase activity and therefore preventing recovery after photobleaching, cells were first treated with CBX and then stained with calcein AM. All cells were stained normally (data not shown).
The FRAP technique has been previously used by others to study gap junction function in 3-D in cartilage and ligament shaving.23,27 Nevertheless, difficulties with FRAP in tendon tissue explants due to postmortem cell death and problems with visualization of widely spaced cells in the native tissue have been described.20 The 3-D system described in this report allows studying gap junction communication in a condition mimicking more the in vivo organization of the cells, but at the same time takes advantage of the in vitro culture system. The 3-D cell culture approach has been explored previously and proven to be suitable for tenocytes culture. The cells cultured in collagen I gels behave similarly to the cells found in a whole tendon forming numerous cell-to-cell contacts and retaining their phenotype.28 Here, we were able to show functional gap junctions in 3-D culture conditions using collagen I gels.
The role of gap junctions in human tendons has not been sufficiently studied, as the majority of work has been performed in rat and chicken tissues. This is the first study showing functional gap junctions in human tendon cells and, to our knowledge, the first demonstration of FRAP utility in 3-D tenocyte culture. We also provide evidence that this gap junctional communication is druggable and, therefore, offers novel therapeutic opportunities. The FRAP assay provides a useful tool to quantify tenocyte function as an interconnected network that carries out mechanosensing and injury responses. A better understanding of tenocyte biology will help to develop improved treatment strategies for tendon injuries.
Acknowledgments
This project was funded by Orthopaedic Research UK Contract Grant No. ORUK478. We acknowledge support from the Oxford Musculoskeletal Biobank and the Oxford NIHR BRU. For donating patients and surgeons, Mr. Andrew Price, Mr. Chethan Jayadev, and Mr. Ben Davies are gratefully thanked.
Biographies
Maria Kuzma-Kuzniarska received her PhD degree from the University of Liverpool. Currently, she is working as a postdoctoral research assistant at Botnar Research Center, University of Oxford, and as a freelance scientific illustrator.
Clarence Yapp received his MSc and DPhil degrees from Oxford University. He is currently a researcher at the Structural Genomics Consortium and also heads the imaging facility at the Botnar Research Centre. His research interests include nonlinear optics, high-content imaging, and automated solutions for biomedical research.
Thomas W. Pearson-Jones is a medical student at the University of Oxford.
Andrew K. Jones received his PhD in molecular biology from University of Leeds. He is a senior lecturer in molecular biology and genomics at Oxford Brookes University.
Philippa A. Hulley is a university lecturer and biomedical scientist studying the adaptive mechanisms deployed by cells of the bones and joints during normal activity, aging, and disease.
References
- 1.Voleti P. B., Buckley M. R., Soslowsky LJ L. J., “Tendon healing: repair and regeneration,” Annu. Rev. Biomed. Eng. 14, 47–71 (2012). 10.1146/annurev-bioeng-071811-150122 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin M., Kaiser E., Milz S., “Structure-function relationships in tendons: a review,” J. Anat. 212(3), 211–228 (2008). 10.1111/joa.2008.212.issue-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins R., Bisson L. J., “Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials,” Am. J. Sports Med. 40(9), 2154–2160 (2012). 10.1177/0363546512453293 [DOI] [PubMed] [Google Scholar]
- 4.Silbernagel K. G., et al. , “Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy,” Br. J. Sports Med. 41(4), 276–280; discussion 280 (2007). 10.1136/bjsm.2006.033464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P., Maffulli N., “Tendinopathy and tendon injury: the future,” Disabil. Rehabil. 30(20–22), 1733–1745 (2008). 10.1080/09638280701788274 [DOI] [PubMed] [Google Scholar]
- 6.McNeilly C. M., et al. , “Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions,” J. Anat. 189(Pt 3), 593–600 (1996). [PMC free article] [PubMed] [Google Scholar]
- 7.Sohl G., Willecke K., “Gap junctions and the connexin protein family,” Cardiovasc. Res. 62(2), 228–232 (2004). 10.1016/j.cardiores.2003.11.013 [DOI] [PubMed] [Google Scholar]
- 8.Maeda E., et al. , “Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading,” Biomech. Model. Mechanobiol. 11(3–4), 439–447 (2012). 10.1007/s10237-011-0323-1 [DOI] [PubMed] [Google Scholar]
- 9.Waggett A. D., Benjamin M., Ralphs J. R., “Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load,” Eur. J. Cell Biol. 85(11), 1145–1154 (2006). 10.1016/j.ejcb.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Ralphs J. R., et al. , “Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation,” J. Anat. 193(2), 215–222 (1998). 10.1046/j.1469-7580.1998.19320215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banes A. J., et al. , “Gap junctions regulate responses of tendon cells ex vivo to mechanical loading,” Clin. Orthop. Relat. Res. (367 Suppl), S356–S370 (1999). 10.1097/00003086-199910001-00034 [DOI] [PubMed] [Google Scholar]
- 12.Qi J., et al. , “Gap junctions in IL-1beta-mediated cell survival response to strain,” J. Appl. Physiol. 110(5), 1425–1431 (2011). 10.1152/japplphysiol.00477.2010 [DOI] [PubMed] [Google Scholar]
- 13.Churko J. M., et al. , “Human dermal fibroblasts derived from oculodentodigital dysplasia patients suggest that patients may have wound-healing defects,” Hum. Mutat. 32(4), 456–466 (2011). 10.1002/humu.21472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori R., et al. , “Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration,” J. Cell Sci. 119(24), 5193–5203 (2006). 10.1242/jcs.03320 [DOI] [PubMed] [Google Scholar]
- 15.Qiu C., et al. , “Targeting connexin43 expression accelerates the rate of wound repair,” Curr. Biol. 13(19), 1697–1703 (2003). 10.1016/j.cub.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Wade M. H., Trosko J. E., Schindler M., “A fluorescence photobleaching assay of gap junction-mediated communication between human cells,” Science 232(4749), 525–528 (1986). 10.1126/science.3961495 [DOI] [PubMed] [Google Scholar]
- 17.Abbaci M., et al. , “Gap junctional intercellular communication capacity by gap-FRAP technique: a comparative study,” Biotechnol. J. 2(1), 50–61 (2007). 10.1002/(ISSN)1860-7314 [DOI] [PubMed] [Google Scholar]
- 18.Vaiyapuri S., et al. , “Gap junctions and connexin hemichannels underpin hemostasis and thrombosis,” Circulation 125(20), 2479–2491 (2012). 10.1161/CIRCULATIONAHA.112.101246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasquez C., et al. , “Enhanced fibroblast-myocyte interactions in response to cardiac injury,” Circ. Res. 107(8), 1011–1020 (2010). 10.1161/CIRCRESAHA.110.227421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young N. J., et al. , “Maturational alterations in gap junction expression and associated collagen synthesis in response to tendon function,” Matrix Biol. 28(6), 311–323 (2009). 10.1016/j.matbio.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Poulsen R. C., Carr A. J., Hulley P. A., “Protection against glucocorticoid-induced damage in human tenocytes by modulation of ERK, Akt, and forkhead signaling,” Endocrinology 152(2), 503–514 (2011). 10.1210/en.2010-1087 [DOI] [PubMed] [Google Scholar]
- 22.Yao L., et al. , “Phenotypic drift in human tenocyte culture,” Tissue Eng. 12(7), 1843–1849 (2006). 10.1089/ten.2006.12.1843 [DOI] [PubMed] [Google Scholar]
- 23.Chi S. S., et al. , “Gap junctions of the medial collateral ligament: structure, distribution, associations and function,” J. Anat. 207(2), 145–154 (2005). 10.1111/joa.2005.207.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert R., Dunina-Barkovskaya A., Hulser D. F., “Biophysical characterization of gap-junction channels in HeLa cells,” Pflugers Arch. 424(3–4), 335–342 (1993). 10.1007/BF00384361 [DOI] [PubMed] [Google Scholar]
- 25.Hunter C. J., Matyas J. R., Duncan N. A., “The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc,” Spine (Phila Pa 1976) 29(10), 1099–1104 (2004). 10.1097/00007632-200405150-00010 [DOI] [PubMed] [Google Scholar]
- 26.Goldberg G. S., et al. , “Evidence that disruption of connexin particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative,” Exp. Cell Res. 222(1), 48–53 (1996). 10.1006/excr.1996.0006 [DOI] [PubMed] [Google Scholar]
- 27.Chi S. S., Rattner J. B., Matyas J. R., “Communication between paired chondrocytes in the superficial zone of articular cartilage,” J. Anat. 205(5), 363–370 (2004). 10.1111/joa.2004.205.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garvin J., et al. , “Novel system for engineering bioartificial tendons and application of mechanical load,” Tissue Eng. 9(5), 967–979 (2003). 10.1089/107632703322495619 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.