Abstract
There are few studies examining changes in waking function in a laboratory environment with no sleep deprivation and mood has been largely overlooked in this context. The present study examined changes in mood, performance, sleep and sleepiness in the laboratory study with no sleep deprivation. Nineteen participants (10M, 9F; 22y ± 4.2y) were given nine 9h sleep opportunities (2300-0800). Every two hours during wake, participants completed the Mood Scale II, a 10-minute Psychomotor Vigilance Task and measures of sleepiness and fatigue. Sleep was monitored using an electroencephalographic montage. Findings revealed significant negative mood change, performance impairment, reduced total sleep time and sleep efficiency (all p < .05). These findings suggest that the laboratory environment or procedural factors may impair mood, performance and sleep. These findings may have implications for interpreting impairments in mood, performance and sleep when observed in laboratory environments.
Keywords: laboratory environment, sleep, sleep loss, mood, performance, control condition, laboratory
1. Introduction
A large body of laboratory-based research demonstrates the negative consequences of sleep loss for waking function.1–3 Fewer studies have investigated waking function in a laboratory study with no sleep loss. The laboratory environment is associated with a high level of environmental, behavioural and social control, thus it is intuitive that such confinement may affect multiple aspects of functioning. The limited existing studies in this area have focussed on neurobehavioural performance whereas mood has been overlooked. Given its sensitive and changeable nature, mood may be one variable negatively impacted by confinement. Indeed, in a recent review concerned with the ambulatory and laboratory measurement of emotion, a construct closely related to mood, Wilhelm and Grossman4 assert that emotional reactions to the laboratory environment and experimental procedures may “interfere with the natural unfolding of emotion in the laboratory”.4 These authors propose that laboratory research methods themselves may influence emotion. This may be of particular importance in sleep research given evidence demonstrating a relationship between negative mood states and impaired performance.5 The present study examined changes in mood, as well as performance, sleep and sleepiness, during extended time in the laboratory with no sleep loss.
There is a small body of research concerned with the effects of confinement on mood. Shimamiya and colleagues6, 7 isolated participants for 10 days in a dive simulation chamber approximately 34m2 in size and containing bunk beds and a desk. Negative mood change was noted in some, but not all, participants. Participants had no direct contact with researchers or with friends and family, but it is unclear whether participants were isolated from each other or whether they shared living quarters. Similarly, Palinkas and colleagues8 found that individuals isolated in work stations in Antarctica and the South Pole for one year reported increases in depressed mood, tension, anxiety and anger. Based on these findings, it may be reasonable to assume that the confinement experienced by participants in laboratory sleep studies has similar negative consequences for mood.
Only two studies have examined mood changes during confinement in the laboratory with no sleep loss. In both studies, this was achieved by the inclusion of a control condition. Lipsey9 defines a control condition as a sample of individuals that experience an experimental protocol without the manipulation of the independent variable, in this case sleep loss. In one study, a 4h time in bed (TIB) sleep restriction condition was associated with significant decreases in mood relative to an 8h TIB condition over a 12 day laboratory protocol.10 In the other study, Franzen et al.11, 12 used a between-subjects design to look at changes in emotional reactivity, mood, performance and sleepiness following a night of either sleep deprivation or normal sleep opportunity. Sleep deprived participants were significantly impaired on all mood and emotional reactivity measures, excluding one measure of negative affect on which neither group changed, compared to control participants. In both Haack and Mullington10 and Franzen and colleagues11, 12 studies it is unclear whether control participants’ mood changed significantly compared to baseline.
There are a small number of studies addressing performance changes in a laboratory study with no sleep loss. For example, Franzen et al.11 included performance measures in their study and found performance on the Psychomotor Vigilance Task (PVT) was significantly impaired in sleep-deprived participants compared to control participants. Van Dongen et al.13 examined changes in sleep, sleepiness and performance during three days of acute sleep loss or 10 days of sleep restricted to 4h or 6h TIB. An 8h TIB condition was included. No significant change was noted in the 8h condition; however, the authors did note non-significant increases in behavioural lapses and subjective sleepiness.13 Similarly, Mollicone et al.14 noted non-significant increases in PVT lapses and subjective sleepiness in a control condition given 8.2h TIB per night.
Despite sleep opportunities of 8h or more, habitual sleep was not preserved. Participants in Van Dongen et al’s13 8h condition obtained between approximately 6.5h and 7.5h total sleep time (TST) throughout the protocol, slightly less than their pre-study habitual sleep time of approximately 7.64h. Similarly, participants in Haack and Mullington’s10 study slept for an average of 6.52h during an 8h opportunity, despite habitually obtaining between seven and nine hours. Sleep time data are not reported in Franzen et al’s11 paper but it is stated that control participants were provided with ‘habitual’ sleep opportunity. Participants in Mollicone et al.’s study15 habitually spent approximately 8h TIB per night and obtained approximately 7h sleep per night during the study when given opportunities of 8.2h TIB. The authors reported no significant changes in TST or sleep efficiency (SE) over the 13-day study. Taken together, these findings may indicate a negative impact of the laboratory or procedural factors on TST and/or that a longer than habitual sleep opportunity is necessary for control participants to obtain ‘habitual’ sleep amount in the laboratory.
Only one published study has included a condition consisting of a sleep opportunity substantially longer than 8h TIB. Belenky et al.16 investigated performance and subjective sleepiness under conditions of sleep restriction (3, 5, 7h TIB), including a 9h TIB condition. No significant changes in performance or sleepiness were noted in the 9h condition.16 This sleep opportunity resulted in a significant increase in TST for participants in the 9h condition compared to baseline, but an average overall TST of 7.93h. Habitual sleep times were not reported. Importantly, mood was not measured in this study.
In summary, it is currently unclear how mood changes in a highly controlled laboratory environment with no sleep loss. Research addressing performance measures in a laboratory study with no sleep loss indicated sleep opportunities less than 9h resulted in sleep reduced below habitual times. The only study to provide 9h sleep opportunities did not include measures of mood. It is important to address this deficit so that we can more clearly understand the impact of both the laboratory environment and sleep loss on mood and other aspects of waking function.
2. Materials and Methods
Ethical clearance was granted for this project by the University of South Australia’s Human Research Ethics Committee.
2.1 Participants
Nineteen participants took part in the study (10M, 9F; 22y ± 4.2y). Participants were of sound mental and physical health, with no personal or family history of sleep or mental disorders and no recent history of shift work or transmeridian travel. Participants were non-smokers, non-nappers and did not habitually consume more than two caffeinated beverages per day.
2.2 Procedures
Potential participants were screened using a General Health Questionnaire (GHQ) and required to attend an interview in the laboratory, written informed consent was obtained at this time. Sleep diaries were completed by potential participants during the week prior to commencement of the study to ensure the maintenance of normal sleep wake patterns (bed time between 2200 and 2400, wake time between 0700 and 0900). Two days, including an adaptation and baseline night, were spent training participants on study procedures and testing materials. On the baseline night subjects retired at 2300 and were woken at 0800. This was followed by seven nights of nine-hour sleep opportunities (2300-0800). Nine-hour sleep opportunities were chosen in order to avoid any unintentional reduction in sleep time, as previous research indicates that a longer than habitual sleep opportunity may be necessary in the laboratory environment for participants to obtain habitual sleep durations.10, 13, 15 In addition, the bed times and wake times imposed in the study were similar to habitual sleep times self-reported by participants and confirmed by actigraphy. Testing occurred every two waking hours, commencing at 0900. Meals were served at 0800, 1200 and 1800 each day, and snacking was permitted during the first 30 minutes of every even hour (e.g. 1400–1430). Participants each had their own bedroom (each approximately 4.2m × 2.6m). Testing sessions were completed by participants in their individual bedrooms. All non-testing time was spent in the open-plan living and kitchen area (approximately 8.5m × 5m) of the laboratory to ensure compliance with study procedures. During this time, participants were permitted to read, watch television or socialize with each other. Participants were not allowed to engage in physical activity. During the entire experimental protocol participants inhabited a light (300 lux), sound and temperature (21°c) controlled sleep laboratory facility (see Fig. 1).
Fig. 1.
Floor plan of the laboratory environment (not to scale).
2.3 Measurement of mood
The Mood Scale II (MSII) was used to assess mood change. This scale presents individuals with 36 mood-related adjectives (i.e. frustrated, cheerful, uneasy), which participants respond to on a three-point Likert scale indicating their current experience of that adjective (1=not at all, 2=somewhat/sometimes, 3=mostly/generally). These 36 adjectives then load on to one of six mood dimensions: activation, happiness, depression, anger, fear and fatigue. The scale takes approximately five minutes to complete and is completed as the second measure in the Walter Reed Performance Assessment Battery (WR-PAB).17
2.4 Measurement of performance
Participants completed a performance VAS asking ‘How well do you think you will perform?’ and anchored by ‘Extremely poorly’ and ‘Extremely well’. The performance VAS was administered at the beginning of every test session and refers to performance on all neurobehavioural tests administered in the study; however, only data from a ten-minute psychomotor vigilance task18 are reported here. The PVT is a small hand-held device that involves a button-press response to a visual stimulus. The outputs used in the present study were response time (RT; the time elapsed between the presentation of the stimulus and the delivery of the response), lapses (the number of RTs greater than 500 milliseconds) and reciprocal response time (RRT; 1/RT). The PVT has been demonstrated as sensitive to sleep loss,19, 20 as a reliable measure of neurobehavioural performance21 and as robust to repeated testing within individuals.22
2.5 Measurement of sleep
Data from all sleep periods were recorded using polysomnography (PSG). In all instances, a five-channel electroencephalographic (EEG) montage was used. Sleep data were analysed and scored in 30-second epochs and in accordance with the criteria of Rechtschaffen and Kales.23 Total Sleep Time (TST) and Sleep Efficiency (SE) are reported. Total Sleep Time (TST) was calculated as the total time spent in any stage of sleep (Stages 1–4, REM), not including nocturnal awakenings exceeding 16 seconds and sleep onset (defined as the first 1.5 minutes of sleep after lights out). Sleep efficiency (SE) was calculated in the following way to allow expression as a percentage:
2.6 Measurement of sleepiness
At the commencement of each test battery, participants completed the Samn-Perelli Fatigue Scale.24 This scale requires responses on a seven-point Likert scale (one=fully alert, wide awake; 7=completely exhausted, unable to function effectively).24 Following this, participants completed a visual analogue scale (VAS) assessing alertness, asking ‘How alert do you feel?’ and anchored by ‘Struggling to remain awake’ and ‘Extremely alert and wide awake’. Finally, participants completed the Stanford Sleepiness Scale (SSS).25 The SSS requires responses on a seven-point Likert scale (1=feeling active, vital, alert or wide awake; 7=no longer fighting sleep, sleep onset soon, having dream-like thoughts).
2.7 Statistical analysis
Mixed effects Analysis of Variance (ANOVA) were used to test for main effects of day (all variables) and time (all variables excluding TST and SE). In the case of day, data for each study day were compared to baseline. In the case of time, data for each testing session time point (1100, 1300, 1500, 1700, 1900 and 2100) were compared to the first testing session time point upon waking (0900). Upon identification of significant main effects, post-hoc analyses were undertaken to determine which specific day and time were associated with significant change (from baseline or 0900) for that particular variable.
3. Results
Mixed effects ANOVAs revealed a significant main effect of day for activation, anger, depression, fatigue, fear, happiness, PVT lapses, PVT RRT, PVT RT, Samn-Perelli fatigue scores, VAS performance, TST and SE (all p < .05). There was also a significant main effect of time for activation, fatigue, Samn-Perelli fatigue scores, SSS scores, VAS alertness and VAS performance (all P < .05). F, df and p values are shown in Table 1.
Table 1.
f, df, and p values for mixed effects ANOVA
| day f |
df | p | time f |
df | p | day × time f |
df | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mood | Activation | 4.31 | 7,981 | <.001 | 12.42 | 6,981 | <.001 | 1.12 | 42,981 | 0.269 |
| Anger | 5.90 | 7,981 | <.001 | 0.84 | 6,981 | 0.538 | 0.87 | 42,981 | 0.692 | |
| Depression | 8.75 | 7,981 | <.001 | 0.53 | 6,981 | 0.784 | 0.84 | 42,981 | 0.743 | |
| Fatigue | 2.18 | 7,981 | 0.033 | 7.12 | 6,981 | <.001 | 0.93 | 42,981 | 0.583 | |
| Fear | 3.00 | 7,981 | 0.004 | 1.37 | 6,981 | 0.223 | 0.71 | 42,981 | 0.912 | |
| Happiness | 18.00 | 7,981 | <.001 | 1.63 | 6,981 | 0.135 | 0.90 | 42,981 | 0.652 | |
| Performance | VAS Performance | 2.32 | 7,973 | 0.024 | 12.43 | 6,973 | <.001 | 1.21 | 42,973 | 0.170 |
| Lapses | 18.31 | 7,920 | <.001 | 2.04 | 6,920 | 0.057 | 0.51 | 42,920 | 0.996 | |
| RT | 19.18 | 7,920 | <.001 | 1.31 | 6,920 | 0.249 | 0.48 | 42,920 | 0.998 | |
| RRT | 20.25 | 7,920 | <.001 | 1.82 | 6,920 | 0.092 | 0.42 | 42,920 | 0.999 | |
| Sleep | TST | 5.85 | 7,126 | <.001 | – | – | – | – | – | – |
| SE | 5.74 | 7,126 | <.001 | – | – | – | – | – | – | |
| Sleepiness | Samn-Perelli | 2.45 | 7,974 | 0.017 | 37.89 | 6,974 | <.001 | 1.09 | 42,974 | 0.316 |
| VAS alertness | 1.43 | 7,974 | 0.188 | 18.63 | 6,974 | <.001 | 1.23 | 42,974 | 0.149 | |
| SSS | 1.66 | 7980 | 0.115 | 30.97 | 6,980 | <.001 | 1.03 | 42,980 | 0.411 |
Post hoc analyses were conducted to investigate the significant main effects noted above. Findings of these analyses for the main effect of day are presented in Fig. 2 (mood), Fig. 3 (performance), Fig. 4 (sleep) and Fig. 5 (sleepiness), below. In terms of the main effect of time, post hoc analyses revealed significant increases in subjective fatigue on the Samn-Perelli scale and the MSII, increased SSS scores, decreased VAS alertness and decreased VAS performance between 1100 and 1900, compared to 0900 (P < .05). Fatigue on the Samn-Perelli scale was also significantly elevated at 2100, relative to 0900 (P < .05). Activation scores on the MSII were significantly reduced relative to 0900 between 1100 and 1700 (P < .05).
Fig. 2.
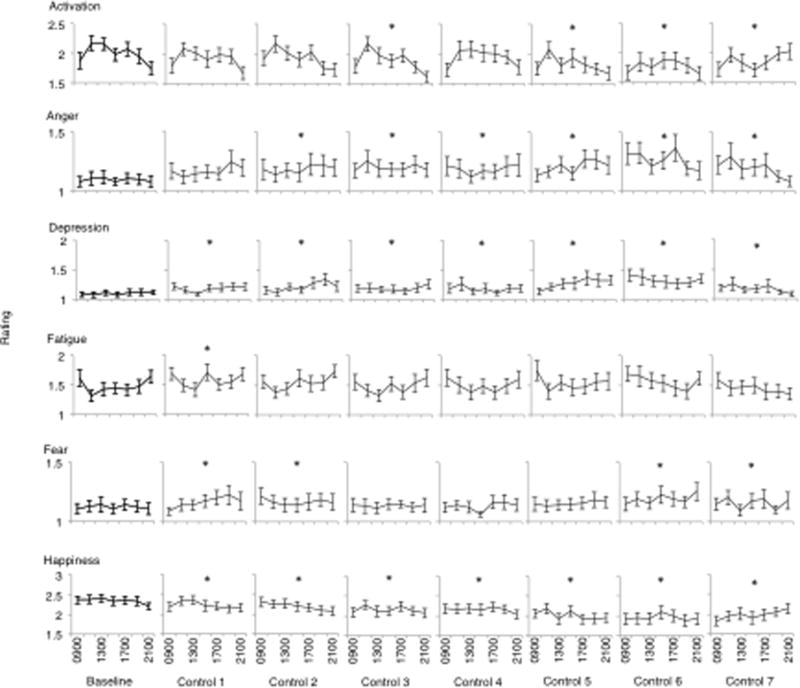
Responses on Mood Scale II dimensions (activation, anger, depression, fatigue, fear and happiness) from Baseline to Control Day 7. Data are plotted from 0900 until 2100 within days. Stars indicate significant difference on that study day compared to Baseline (p <.05).
Fig. 3.
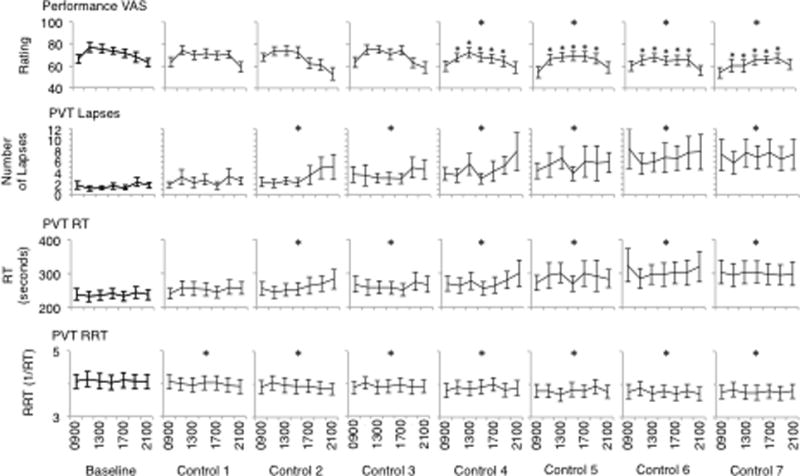
Performance measures (performance VAS, PVT lapses, PVT response time and PVT reciprocal response time) from Baseline to Control Day 7. Data are plotted from 0900 until 2100 within days. Stars indicate significant difference on that study day compared to Baseline (p <.05).
Fig. 4.
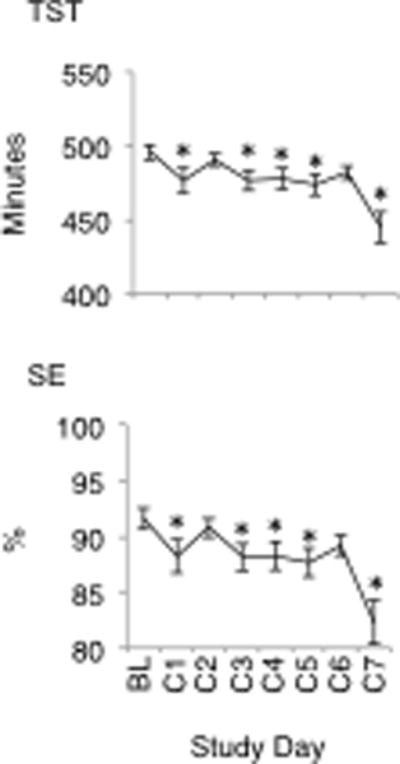
Total Sleep Time (TST) and Sleep Efficiency (SE) from Baseline to Control Day 7. Stars indicate significant difference compared to Baseline (p <.05).
Fig. 5.
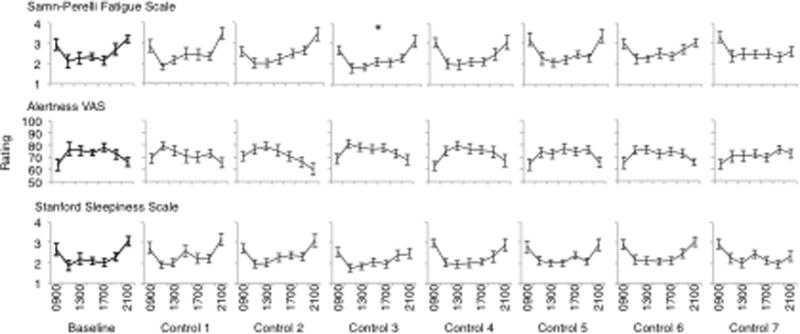
Responses on sleepiness measures (Samn-Perelli fatigue scale, alertness VAS and Stanford sleepiness scale) from Baseline to Control Day 7. Data are plotted from 0900 until 2100 within days. Stars indicate significant difference on that study day compared to Baseline (p <.05).
4. Discussion
This study is one of the first to focus on changes in mood, as well as performance, sleep and sleepiness, in a laboratory environment with no sleep loss. Findings revealed significant negative mood change, performance impairment, reduced TST and SE. These findings suggest that the laboratory environment or procedural factors may impair mood, performance and sleep in research participants.
There was significant negative mood change during the protocol. The most dramatically affected mood dimensions were happiness and depression, which were impaired relative to baseline for the entirety of the study protocol. While this study is one of the first to demonstrate a potential relationship between the laboratory environment and negative mood, this finding is in line with previous research that has indicated negative mood in response to environmental factors such as temperature, odour and confinement.6–8, 26–28 While the laboratory was highly controlled in regards to temperature and cleanliness, confinement or other aspects of the laboratory environment, such as restricted access to sunlight and exercise, may have negatively influenced mood. Indeed, Wilhelm and Grossman4 suggest that the laboratory as an unfamiliar and potentially threatening environment may influence emotional responses in experimental research.
All measures of PVT performance were impaired relative to baseline after two days and nights in the laboratory environment. The degree of performance impairment noted in the present study is comparable to that recorded by Franzen et al.11 Control participants in Franzen et al.’s11 study recorded an average of 4.7 lapses and average RTs of 309.7ms per 10 minute PVT after one night of adaptation sleep and one night of habitual sleep opportunity. In the present study, an average of 4.7 lapses per testing session occurred after four days in the laboratory. Lapses continued to increase until the study’s conclusion. The highest average RTs were recorded on the final day of the present study and were 299ms; lower than those recorded after two days in Franzen et al.’s11 study. The performance impairment observed in the present study may be related to the negative mood also noted. Given that positive mood has been associated with increased energy and willingness to complete complex and demanding tasks,29 it is possible that the cognitive cost of negative mood may be decreased motivation and thus poorer performance. There is an obvious need for future research to explore the relationship between mood and performance in laboratory studies, particularly the extent to which changes in mood may influence motivation to perform.
Previous laboratory research has identified that some measures of sleep quality are sensitive to environmental influence.30 Consistent with this, sleep time in the present study was reduced relative to baseline for a majority of the study by between 19 and 51 minutes. In turn, SE was reduced to between 88% and 82%, compared to 91% at baseline. The overall average TST throughout was 7.9h and the lowest average TST was 7.4h on the last night of the study. Sleep durations of this length and reductions in TST of this magnitude are not typically associated with performance or mood impairment and participants did not report feeling significantly more sleepy. However, these sleep times represent a significant decline relative to participants’ habitual sleep durations. Thus, these findings are consistent with those of previous research demonstrating that despite adequate sleep opportunity, participants may not obtain habitual sleep amounts in the laboratory environment.7, 10 While reductions below habitual sleep time are associated with mood and performance impairment,31 it is difficult to determine whether the substantial impairments in waking function observed in the present study are a result of these minor changes in TST and SE. Indeed, participants in Van Dongen et al.’s13 study obtained approximately 6.5h to 7.5h sleep per night, less than habitual sleep times (7.64h) and less than participants in the present study, and recorded only non-significant performance impairments. Perhaps the influence of the laboratory environment is more pervasive in some populations, under certain protocols or dependent on the nature of the research team or the other participants with whom subjects interact. In the present study, participants were permitted to interact with each other. These interactions may have had positive or negative effects on mood. Future research should address the potential for group dynamics to affect mood, or other outcomes measures, in experimental sleep research.
The laboratory environment in the present study was highly controlled and there are no obvious differences in this environment and those described in other laboratory sleep studies. Indeed, control participants in Franzen et al.’s11 study received one night of habitual sleep in the laboratory yet recorded greater impairment on the PVT than observed in the present study. Experimental laboratory research is associated with a high level of environmental, behavioural and social control and it is intuitive to expect that confinement in this environment will affect waking function in some way. However, the source of this ‘laboratory effect’ may be difficult to determine, measure and control and may differ across research facilities. Compared to other laboratory study protocols, for example forced de-synchrony or constant routine, living conditions in this study were relatively naturalistic. It may be the case that in more stressful protocols, the consequences of the laboratory environment or procedural factors would be more substantial. This should be addressed in future research. The most effective way to monitor and compensate for this source of potential variance may be to include a control condition, as in the present study. Indeed, as Dinges et al.1 noted, without the inclusion of a control condition in laboratory research it is difficult to know what proportion of change may be attributed to experimental manipulation or procedural factors. It may be the case that exposure to an experimental manipulation moderates the negative impact of confinement. That is, when confinement in a laboratory is the greatest challenge presented to an individual, mood is negatively affected. On the other hand, when a challenge like sleep deprivation is experienced concurrently with confinement in a laboratory, the negative effects of the confinement may be overshadowed. Lack of experimental manipulation may alter an individual’s perception of what constitutes a mood altering negative experience. This principle may also explain changes in neurobehavioural performance observed in the present study, particularly in light of research indicating a relationship between mood and performance.5
As such, it is also worth considering the possibility that a control condition itself is associated with unique consequences for waking function. That is, while experimental manipulation affects participants in one way, lack of experimental manipulation may affect them in another way. As discussed above in relation to PVT performance, motivation is one domain that may be negatively affected by participation in a control condition and may affect waking function. Participants in the present study were aware that they were part of the control condition and that other conditions of the study involved sleep loss. This knowledge may have resulted in a sense of boredom in the control condition, particularly compared to the perceived novelty of participating in an experimental sleep loss condition. One way to overcome this in future studies may be to employ protocols similar to those used in pharmacological studies. In this way, two kinds of control conditions are proposed; a ‘true’ control condition and a ‘placebo’ control condition. Participants in the latter would experience the same conditions as those in the former, but would also experience the perception of experimental manipulation. For example, using a time isolation protocol, participants could be given ‘normal’ sleep opportunity, i.e. nine hours, but be told they are receiving reduced sleep opportunity, i.e. three hours. This would facilitate an understanding of how individuals are affected by the perception of experimental manipulation and also of potential psychosomatic decrements in functioning as a result of perceived sleep loss. Using multiple control conditions, significant changes could be compared to experimental participants to determine the relative impacts of a) the independent variable, b) procedural or environmental factors and c) perception of experimental manipulation.
In conclusion, this study is one of the first published accounts of a potential dose-response relationship between time spent in the laboratory and impaired mood, performance and sleep. The findings of the present study may have implications for our understanding of the negative consequences of sleep loss for waking function when assessed in laboratory environments. These findings are to be interpreted conservatively; however, they do serve to highlight the importance of including control conditions in experimental, laboratory research.
Acknowledgments
This research was funded by the National Institutes of Health (NIH).
Footnotes
Disclosure Statement
All authors declare that they have no conflicts of interest.
References
- 1.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 2.Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D. The dynamics of neurobehavioural recovery following sleep loss. Journal of Sleep Research. 2007;16:33–41. doi: 10.1111/j.1365-2869.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet MH. Effect of sleep disruption on sleep, performance and mood. Sleep. 1985;8:11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm FH, Grossman P. Emotions beyond the laboratory: theoretical fundaments, study design, and analytic strategies for advanced ambulatory assessment. Biological Psychology. 2010;84:552–69. doi: 10.1016/j.biopsycho.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Bolmont B, Thullier F, Abraini JH. Relationships between mood states and performances in reaction time, psychomotor ability, and mental efficiency during a 31-day gradual decompression in a hypobaric chamber from sea level to 8848 m equivalent altitude. Physiology and Behavior. 2000;71:469–76. doi: 10.1016/s0031-9384(00)00362-0. [DOI] [PubMed] [Google Scholar]
- 6.Shimamiya T, Terada N, Wakabayashi S, Mohri M. Mood change and immune status of human subjects in a 10-day confinement study. Aviation, Space and Environmental Medicine. 2005;76:481–5. [PubMed] [Google Scholar]
- 7.Shimamiya T, Terada N, Hiejima Y, Wakabayashi S, Kasai H, Mohri M. Effects of 10-day confinement on the immune system and psychological aspects in humans. Journal of Applied Physiology. 2004;97:920–4. doi: 10.1152/japplphysiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- 8.Palinkas LA, Johnson JC, Boster JS. Social support and depressed mood in isolated and confined environments. Acta Astronautica. 2004;54:639–47. doi: 10.1016/s0094-5765(03)00236-4. [DOI] [PubMed] [Google Scholar]
- 9.Lipsey MW. Design sensitivity: Statistical power for experimental research. California: SAGE Publications; 1990. [Google Scholar]
- 10.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology. 2009;80:300–5. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 14.Mollicone DJ, Van Dongen HPA, Rogers NL, Dinges DF. Response surface mapping of neurobehavioral performance: Testing the feasibility of split sleep schedules for space operations. Acta Astronautica. 2008;63:833–40. doi: 10.1016/j.actaastro.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollicone DJ, Van Dongen HPA, Dinges DF. Optimizing sleep/wake schedules in space: sleep during chronic nocturnal sleep restriction with and without diurnal naps. Acta Astronautica. 2007;60:354–61. [Google Scholar]
- 16.Belenky G, Wesensten NJ, Thorne D, Thomas M, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. Journal of Sleep Research. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 17.Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehavioral Toxicology and Teratology. 1985;7:415–8. [PubMed] [Google Scholar]
- 18.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavioral Research Methods. 1985;17:652–5. [Google Scholar]
- 19.Jewett ME, Dijk DJ, Kronauer RE, Dinges DF. Dose-response relationship between sleep duration and human psychomotor vigilance and subjective alertness. Sleep. 1999;22:171–9. doi: 10.1093/sleep/22.2.171. [DOI] [PubMed] [Google Scholar]
- 20.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Archives Italiennes de Biologie. 2001;139:253–67. [PubMed] [Google Scholar]
- 21.Dorrian J, Rogers N, Dinges D. Behavioural alertness as assessed by psychomotor vigilance performance. In: Kushida C, editor. Sleep Deprivation: Clinical Issues, Pharmacology, and Sleep Loss Effects. New York: Marcel Dekker; 2004. pp. 39–70. [Google Scholar]
- 22.Lamond N, Dawson D, Roach GD. Fatigue assessment in the field: validation of a hand-held electronic psychomotor vigilance task. Aviation, Space and Environmental Medicine. 2005;76:486–9. [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington D.C.: U.S. Public Health Service; 1968. [DOI] [PubMed] [Google Scholar]
- 24.Samn S, Perelli L. Estimating aircrew fatigue: A technique with implications to airlift operations. Brooks; Texas: 1982. [Google Scholar]
- 25.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 26.Asmus CL, Bell PA. Effects of environmental odor and coping style on negative affect, anger, arousal, and escape. Journal of Applied Social Psychology. 1999;29:245–60. [Google Scholar]
- 27.Griffitt W. Environmental effects on interpersonal affective behavior: ambient effective temperature and attraction. Journal of Personality and Social Psychology. 1970;15:240–4. doi: 10.1037/h0029432. [DOI] [PubMed] [Google Scholar]
- 28.Palinkas LA, Houseal M. Stages of change in mood and behavior during a winter in Antarctica. Environment and Behavior. 2000;32:128–41. doi: 10.1177/00139160021972469. [DOI] [PubMed] [Google Scholar]
- 29.Dienstbier RA. The impact of humor on energy, tension, task choices, and attributions: exploring hypotheses from toughness theory. Motivation and Emotion. 1995;19:255–67. [Google Scholar]
- 30.Moser D, Kloesch G, Fischmeister FP, Bauer H, Zeitlhofer J. Cyclic alternative pattern and sleep quality in healthy subjects – is there a first-night effect on different approaches of sleep quality? Biological Psychology. 2010;83:20–6. doi: 10.1016/j.biopsycho.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]


