Abstract
Background
To determine the validity of body mass index (BMI) to identify excess fatness in youth with Down syndrome (DS).
Methods
Using the CDC growth reference, we defined overweight (≥85th percentile) and obesity (≥95th percentile) based on participants’ age- and sex-specific BMI z-scores, calculated from measured height and weight. Percentage body fat (%BF) was measured by dual-energy X-ray absorptiometry. We determined sensitivity, specificity, positive predictive value, negative predictive value, and efficiency of BMI percentiles to identify excess adiposity relative to elevated %BF cut-offs developed from the Pediatric Rosetta Body Composition project (Freedman et al., 2009b) in 32 youth (20M/12F), ages 13–21 years with Down syndrome.
Results
For adolescents with Down syndrome using the cut-off points of 95th percentile for BMI (obesity), sensitivity and specificity were 71% and 96%, respectively. Positive predictive value was 83% and negative predictive value was 92%. Overall efficiency was 91%. Sensitivity and specificity for BMI cut-offs above the 85th percentile (overweight) were 100% and 60%, respectively. The positive predictive value was 41% and negative predictive value was 100%. Overall efficiency was 69%.
Conclusion
On the whole, the obesity (≥95th percentile) cut-off performs better than the overweight cut-off (85th–94th percentile) in identifying elevated fatness in youth with DS.
Introduction
Obesity in the general population of children in the US has become a significant health concern, with a tripling in prevalence of childhood obesity over the last 20 years (Ogden et al. 2010). With its tendency to track into adulthood (Guo et al. 2002) and association with cardiovascular disease, diabetes, and certain cancers (Must and Strauss 1999), childhood obesity threatens the gains in health and longevity that have occurred over the last century. Despite the high prevalence of obesity in the general population, there has been limited information on the prevalence of obesity in children with Down syndrome (DS).
Down syndrome, a chromosomal disorder associated with trisomy 21, has an estimated incidence in the US of 1 in 691 births per year (Centers for Disease Control and Prevention 2011b). Recent data suggest that the prevalence of DS is higher among males than females (Shin et al. 2009). Children and adolescents born with DS may have congenital heart abnormalities (Freeman et al. 1998) or other medical problems and are often overweight (Chumlea and Cronk 1981, Cronk et al. 1985). Growth patterns of children with DS are different from patterns in typically developing children; the pubertal growth spurt has been reported to occur earlier (Arnell et al. 1996) and is decreased in children with DS (Myrelid et al. 2002). Children with DS are reported to be significantly shorter than children of similar ages without DS (Cronk et al. 1988) and to have shorter limb length (Jaswal and Jaswal 1981). Data from the UK suggest that children with DS tend to grow along the 2nd percentile on growth charts for the general population (Styles et al. 2002). Growth charts for children and adolescents with DS have been developed in the UK and Republic of Ireland (Styles et al. 2002), US (Cronk et al. 1988), and Sweden (Myrelid et al. 2002). Despite these well-documented alternations in growth of children and adolescents with DS, data on their body composition are scant. Fat-free mass (FFM) is known to increase with stature (Kelly et al. 2009); thus the decreased stature in adolescents with DS would be expected to be associated with lower FFM. One study reported decreased levels of FFM as measured by dual-energy X-ray absorptiometry (DXA) in children and adolescents with DS compared to typically developing (TD) children (Gonzalez-Aguero et al. 2011), while a second small study (n=12 DS and n=10 TD) found no differences in FFM between children with DS and TD children aged 5–11years (Luke et al. 1994).
Rimmer et al reported an obesity prevalence of 31% based on CDC criteria in a convenience sample of 81 youth with DS surveyed via the internet (Rimmer et al. 2010), providing some evidence that obesity is significantly higher in this population. Self-reported secondary conditions such as elevated blood cholesterol and diabetes were more prevalent in adolescents with cognitive disabilities who were overweight than those who were at a healthy weight (Rimmer et al. 2010), highlighting the importance of identifying adolescents with cognitive impairments who are overweight in order to prevent and treat potential co-morbidities.
The purpose of this study was to evaluate the accuracy of the CDC growth reference to identify excess adiposity in a cohort of adolescents with DS. Given their short stature, distinct growth patterns, and decreased FFM, it is unclear whether the CDC BMI growth reference used to identify obesity in TD children are appropriate for use in children with DS. Accordingly, we hypothesized that adolescents with DS and a BMI in the range of 85–94th percentile would have excess body fatness but would not be categorized as obese using the recommended BMI cut-off for obesity.
Methods
Participants
Adolescents and young adults with DS between the ages of 13–21 years participated in this study. Participants were recruited from several sources, including an ongoing family-based weight reduction study for adolescents with DS, the Massachusetts Down Syndrome Congress, Special Olympics Massachusetts, and Craigslist™. The study was conducted at the Clinical Research Center (CRC) at the Massachusetts Institute of Technology. Parents gave informed consent for their offspring; participants themselves completed a brief assent form. All females took a pregnancy test to ensure that they were not pregnant (a criterion for participation). No females were excluded due to pregnancy.
Protocol
Participants were asked to abstain from eating two hours prior to their scheduled appointment time. Weight was measured in light clothing or a hospital gown and pants on a Seca scale. Height was measured on a wall-mounted stadiometer without shoes (Holtain, Crosswell, UK). Body composition was measured by DXA. Participants were asked to remove all metal items from their person, including items with clothing fasteners (i.e. zippers, snaps), jewelry, and accessories. They were asked to lie still for approximately 2–5 minutes per scan. After the measurement of body composition, participants were offered a light snack. The study was approved by the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects and the University of Massachusetts Medical School Institutional Review Board.
Analyses
We used the CDC growth reference charts (Centers for Disease Control and Prevention 2011a) to identify overweight and obesity in a group of adolescents with DS because the growth charts developed for children with DS in the UK and US do not include BMI percentiles. Overweight was defined as a BMI ≥85th percentile and obesity was defined as a BMI ≥95th percentile, based on participants’ age- and sex-specific BMI z-score calculated from measured height and weight. Five of the male participants and one of the female participants were between 20 and 21 years of age. We used the 20-year old cut-off point for these six individuals to determine BMI percentile.
Body Composition by DXA
Body composition was determined by whole body DXA measures using a Hologic QDR 4500A (S/N 45669 version 11.2, fan beam densitometer, Waltham, MA). Lean soft tissue mass (LSTM) and bone mineral content (BMC) were summed to determine FFM. Schoeller et al. (Schoeller et al. 2005) have determined that the Hologic QDR 4500 overestimates FFM and underestimates fat mass in comparison to criterion body composition measures and recommend that LSTM determined from the QDR 4500 be reduced by 5%. We applied the following equations recommended by Schoeller et al. to calculate FFM and fat mass:
This correction factor is used by NHANES (Ogden et al. 2011) in both children and adults.
Percentage body fat cut-offs
We classified obesity relative to elevated percentage body fat (%BF) cut-offs, developed from the Pediatric Rosetta Body Composition Project which enrolled 1196 participants, ranging from 5 to18 years of age (Freedman et al. 2009b). These values correspond to a percentage body fat greater than 32% for males 12 to14.9 years of age and 29% for males > 15 years. For females the cut-off points are 39% for 12–14.9 year olds, and 42% for females > 15years.
We categorized participants as overweight or obese based on CDC BMI percentile cut-off points, and as having elevated body fatness measured by DXA based on cut-off points from the Pediatric Rosetta Study (Freedman et al. 2009b). We considered the latter to be the gold standard. We also calculated the fat-free mass index as FFM/height2.
Statistical analysis
Basic descriptive statistics (means, and standard deviations (SD), ranges, percentages) were used to characterize the sample. The distributions of the key outcome variables, percentage body fat and BMI z-score were examined for normality. We used Spearman’s correlation coefficient as the measure of association between percentage body fat measured by DXA and BMI z-score because BMI z-scores were not normally distributed among males.
We calculated standard screening validity measures for BMI based definitions of obesity (95th percentile BMI z-score) and overweight (85th percentile BMI z-score) to assess the validity and accuracy of BMI with percentage body fat by DXA as the gold standard using a two-by-two table (Himes and Bouchard 1989). We then determined the sensitivity, specificity, positive predictive ability, negative predicative ability, and efficiency of BMI to accurately identify elevated body fatness using standard definitions. Sensitivity is defined as the proportion of truly obese subjects so classified (true positives); specificity is the proportion of truly non-obese subject so classified (true negatives). The positive predictive value of an obesity screening measure is the proportion of those who are identified as obese by the indicator who are truly positive (obese). The negative predictive value of an obesity screener is the proportion of those who are truly negative (non-obese). Efficiency is defined as the proportion of all subjects who are correctly classified by the screening measure and is equal to the sum of the true positives and true negatives divided by the total sample size. These measures were calculated for the whole sample and for males and females separately.
Results
Thirty-four subjects, 22 males and 12 females, between the ages of 13 and 21 years participated in the study; two males were excluded because their scans contained artifacts due to prior surgeries— and it was deemed that these could affect the estimates of lean soft tissue mass. Mean height-for-age was at the 3rd percentile (z-score± SD; −2.546± 0.903) weight-for-age was at the 43rd percentile (z-score was −0.346 ± 1.166) using the CDC percentiles for TD children. Characteristics of the participants are presented in Table 1.
Table 1.
Participant characteristics
| Males (n = 20) | Females (n = 12) | |
|---|---|---|
| Age (yr)* | 17.4 (2.6) | 17.1 (2.5) |
| Race† | ||
| White | 17 (85.0%) | 11 (91.7%) |
| Black | 0 (0.0%) | 1 (8.3%) |
| Multiple Race | 2 (10.0%) | 0 (0.0%) |
| Missing | 1 (5.0%) | 0 (0.0%) |
| Height (cm) * | 155.9 (5.6) | 142.6 (3.9) |
| Weight (kg) * | 63.5 (11.6) | 50.2 (9.4) |
| BMI* | 26.1 (4.1) | 24.7 (4.4) |
| BMI z-score* | 1.1 (0.9) | 0.8 (0.7) |
| BMI Category† | ||
| Normal | 8 (40.0%) | 7 (58.3%) |
| Overweight (85–94th percentile BMI) | 7 (35.0%) | 4 (33.3%) |
| Obese (≥95th percentile BMI) | 5 (25.0%) | 1 (8.3%) |
| FFM (kg) *,‡ | 46.0 (63.7) | 32.5 (37.6) |
| % Body Fat*,‡ | 27.3 (5.0) | 34.8 (5.1) |
BMI z-score and percentage body fat
Mean BMI corresponded to the 78th percentile (z-score was 0.971 ± 0.81) in the sample. Using overweight and obesity definitions based on BMI, 18.8% of the adolescents were above the 95th percentile and thus classified as obese, and 34.4 % were between the 85th and 95th percentile and classified as overweight. For males, 25% were obese and 35% were overweight. For females, 8.3% were obese and 33.3% were overweight. None of the participants had BMI percentiles above the 99th percentile. Using the cut-off values for percentage body fat from the Pediatric Rosetta Body Composition study, 21.9% of participants had elevated body fat (25% of males and 16.6% of females). The fat-free mass index, FFM/height2 in 21 of the 32 participants was above the mean for TD children.
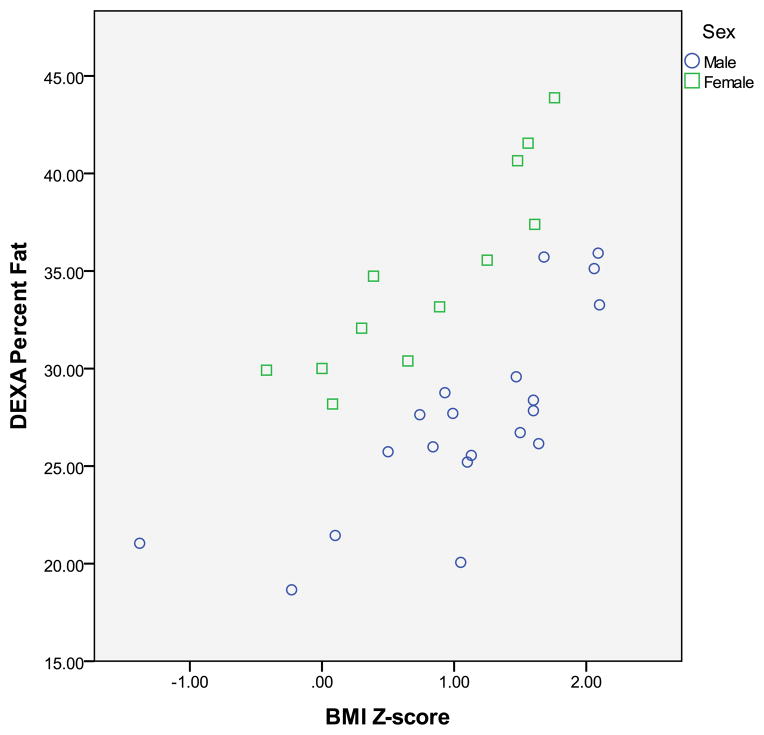
A scatterplot of BMI z-score and percentage body fat by DXA is displayed in the Figure. BMI z-score was moderately correlated with percentage body fat by DXA in both males and females combined (r=0.50 p<0.01). In females, BMI z-score was highly correlated with %BF by DXA (r=0.92, p<0.001). In males, BMI z-score was highly correlated with %BF (r=0.76, p<0.001).
Figure.
Relationship between BMI z score and %body fat measured by DXA in youth with Down syndrome (○ boys; □ girls) Spearman correlation (r=0.496, p<0.01).
The validity of screening at both the 95th and 85th percentile is based on measures of sensitivity, specificity, positive predictive value, negative predictive value and efficiency for the total cohort and males and females separately, as illustrated in Table 2. In the entire cohort, application of the 95th percentile BMI for the cut-off point for obesity yielded sensitivity and specificity of 71% and 96%, respectively. The positive and negative predictive value was 83% and 92%, respectively, and the efficiency was 91%. When these measures were stratified by gender, sensitivity was 80% in males and 50% in females. There was essentially no difference in specificity by gender. Positive predictive value improved in females and efficiency was essentially the same for males and females.
Table 2.
Validity of screening measures based on CDC BMI-for-age and body fatness by DXA*
| Total n = 32 |
Males n = 20 |
Females n = 12 |
|
|---|---|---|---|
| 95th percentile BMI | |||
| Sensitivity | 71% | 80% | 50% |
| Specificity | 96% | 93% | 100% |
| Positive Predictive Value | 83% | 80% | 100% |
| Negative Predictive Value | 92% | 93% | 91% |
| Efficiency | 91% | 90% | 92% |
| 85th percentile BMI | |||
| Sensitivity | 100% | 100% | 100% |
| Specificity | 60% | 53% | 70% |
| Positive Predictive Value | 41% | 42% | 40% |
| Negative Predictive Value | 100% | 100% | 100% |
| Efficiency | 69% | 65% | 75% |
gold standard measures based on the Rosetta Study (Freedman et al. 2009b). See Methods
Measures of sensitivity were higher with the 85th percentile as a cut-off point in comparison to the 95th percentile, both in the entire cohort and when the sample was stratified by gender. However, specificity and positive predictive value was much lower. The negative predictive value using the 85th percentile cutoff was 100%. Efficiency was much lower in the entire cohort, as well as in males and females separately. These values are tabulated in Table 2. Four participants had a BMI less than the 50th percentile and 11 had a BMI percentile between the 50th and 85th percentile. None of these 15 participants had elevated body fatness. The number of youth misclassified at both the 85th and 95th percentile is presented in Table 3.
Table 3.
Number of youth whose body fatness status is misclassified based on BMI percentile
| Elevated body fatness* | ||||||
|---|---|---|---|---|---|---|
| Total | Males | Females | ||||
|
| ||||||
| BMI for age | Yes | No | Yes | No | Yes | No |
| 95th CDC percentile | ||||||
| Yes | 5 | 1 | 4 | 1 | 1 | 0 |
| No | 2 | 24 | 1 | 14 | 1 | 10 |
| 85th CDC percentile | ||||||
| Yes | 7 | 10 | 5 | 7 | 2 | 3 |
| No | 0 | 15 | 0 | 8 | 0 | 7 |
Discussion
In this study we evaluated the accuracy of BMI percentiles developed for TD children to identify obesity in adolescents with DS. We sought to determine whether the BMI growth charts for TD children would accurately predict excess fatness in adolescents with BMI scores above the 95th percentile. We hypothesized that due to their altered body composition, some adolescents with a BMI in the range of 85–94th percentile would have elevated body fatness and the 85th percentile cutoff would be associated with high sensitivity and positive predictive value.
We found that the obesity cut-off point in BMI growth charts for TD children performed well in identifying youth with elevated fatness, whereas contrary to our hypothesis, the overweight definition misidentified a large proportion of the participants as overweight who did not have excess body fatness. These findings are similar to those found in TD children. Freedman et al (Freedman et al. 2009b) report variable levels of body fatness in the 85–94th percentile range for BMI. Approximately 30% of boys and girls who were in the 85–94th percentile had body fat within the normal range and the rest were in the moderate and elevated range of body fatness. The high positive predictive value we found using the 95th percentile BMI cut point in our study of adolescents with DS may be due to the high prevalence of obesity in this population overall, as BMI performs well in children with elevated body fatness (Krebs et al. 2007).
The poor performance of the overweight cut-off was surprising, given that persons with DS have lower FFM compared to persons without DS. For approximately 2/3 of participants the FFM index was above the mean for TD children (Kelly et al. 2009), similar to findings by Magge et al (Magge et al. 2008) in a study of younger children with DS suggesting that FFM relative to body height may not be reduced. However because of known differences in body proportions in individuals with DS (Jaswal and Jaswal 1981) further analyses with measures of limb length would be required to verify this assertion.
There were several limitations in our study. First, we elected to include youth aged 13 – 21, a broad age range, in anticipation with the challenges of recruiting participants with a low incidence condition. Our initial recruitment was from our “Health U” weight reduction study which included adolescents and young adults with DS up to age 21. Additionally, because youth with disabilities are eligible for services up to their 22nd birthday, 20 and 21 years olds are often grouped with adolescents. Second, the choice of cut-off points for obesity and body fat influences findings (Freedman and Sherry 2009a) and there is no consensus on cut-off points for excess body fatness in children. We used the elevated percentage body fat cut-off points developed from the Pediatric Rosetta Body Composition Study, a large study of TD children, but acknowledge it was based on a convenience sample, and the choice of cut-off points were based on the distribution, rather than on health outcomes. Third, the body composition measures published by Freedman et al that we based our cut-off points on were based on a Lunar DPX while our scans were done on a Hologic QD4500A. There may be differences due to the type of scanner. However, these cut-off points are consistent with body fatness at the 70–85th percentile in the NHANES 1999–2004 which also used a Hologic QD4500A (Ogden et al. 2011). A 2010 paper by Flegal and al suggests that the 75–85th percentile of body fatness (Flegal et al. 2010) is indicative of high adiposity. Fourth, given that our convenience sample was small and predominately white generalizability may be limited. Several studies in adults and children have suggested racial differences in body composition and that cut-off points for identifying overweight and obesity should be set at different levels of body fatness (Misra 2003, Freedman et al. 2008). Our small number of non-white subjects precludes our ability to consider racial/ethnic differences in our study. Our sample consisted of more males than females. This may reflect recent national data that suggest that the prevalence of DS is higher in males than females (Shin et al. 2009). However, all of our analyses were stratified by sex a priori. Only one of the females in our study was obese. Future studies on a larger sample are needed to verify our findings. Finally, although DXA is widely used in the measurement of body composition and has been shown to correlate well with criterion measures for body composition (Tothill et al. 2001, Ellis and Shypailo 1998), there are assumptions used in the calculation of soft tissue that may lead to random and systematic error (Tothill et al. 2001).
Overall, in our relatively small sample of adolescents and young adults with DS, we found that the CDC cutoff points for identifying obesity performed well for those who were above the 95th percentile and those below the 85th percentile. The cut points perform well in identifying those with high BMI, high body fatness, and normal BMI. However, as seen in other studies for TD children, there is substantial misclassification in the range of 85–94th BMI percentile for youth with DS. Gonzalez et al (Gonzalez-Aguero et al. 2011) have shown that fat distribution is different for youth with and without DS. Further research is needed on a larger population to confirm these findings and to determine if these cut-off points are associated with adverse health outcomes in youth with DS.
References
- Arnell H, Gustafsson J, Ivarsson SA, Anneren G. Growth and pubertal development in Down syndrome. Acta Paediatr. 1996;85:1102–6. doi: 10.1111/j.1651-2227.1996.tb14225.x. [DOI] [PubMed] [Google Scholar]
- Centers For Disease Control and Prevention. Growth Charts. 2011a [Online] Available at: http://www.cdc.gov/obesity/childhood/defining.html (updated 9 September 2010)
- Centers For Disease Control And Prevention. Facts about Down Syndrome. 2011b [Online] Available at: http://www.cdc.gov/ncbddd/birthdefects/DownSyndrome/html (updated 8 June 2011)
- Chumlea WC, Cronk CE. Overweight among children with trisomy. J Ment Defic Res. 1981;25(Pt 4):275–80. doi: 10.1111/j.1365-2788.1981.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, Reed RB. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81:102–10. [PubMed] [Google Scholar]
- Cronk CE, Chumlea WC, Roche AF. Assessment of overweight children with trisomy 21. Am J Ment Defic. 1985;89:433–6. [PubMed] [Google Scholar]
- Ellis KJ, Shypailo RJ. Bone mineral and body composition measurements: cross-calibration of pencil-beam and fan-beam dual-energy X-ray absorptiometers. J Bone Miner Res. 1998;13:1613–8. doi: 10.1359/jbmr.1998.13.10.1613. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, Borrud LG. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91:1020–6. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009a;124(Suppl 1):S23–34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Wang J, Thornton JC, Mei Z, Pierson RN, Jr, Dietz WH, Horlick M. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 2008;16:1105–11. doi: 10.1038/oby.2008.30. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Wang J, Thornton JC, Mei Z, Sopher AB, Pierson RN, Jr, Dietz WH, Horlick M. Classification of body fatness by body mass index-for-age categories among children. Arch Pediatr Adolesc Med. 2009b;163:805–11. doi: 10.1001/archpediatrics.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, Khoury MJ, Saker DM. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80:213–7. [PubMed] [Google Scholar]
- Gonzalez-Aguero A, Ara I, Moreno LA, Vicente-Rodriguez G, Casajus JA. Fat and lean masses in youths with Down syndrome: gender differences. Res Dev Disabil. 2011;32:1685–93. doi: 10.1016/j.ridd.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–8. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- Himes JH, Bouchard C. Validity of anthropometry in classifying youths as obese. Int J Obes. 1989;13:183–93. [PubMed] [Google Scholar]
- Jaswal S, Jaswal IJ. An anthropometric study of body size in Down syndrome. Indian J Pediatr. 1981;48:81–4. doi: 10.1007/BF02895195. [DOI] [PubMed] [Google Scholar]
- Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- Luke A, Roizen NJ, Sutton M, Schoeller DA. Energy expenditure in children with Down syndrome: correcting metabolic rate for movement. J Pediatr. 1994;125:829–38. doi: 10.1016/s0022-3476(94)70087-7. [DOI] [PubMed] [Google Scholar]
- Magge SN, O’neill KL, Shults J, Stallings VA, Stettler N. Leptin levels among prepubertal children with Down syndrome compared with their siblings. J Pediatr. 2008;152:321–6. doi: 10.1016/j.jpeds.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A. Revisions of cutoffs of body mass index to define overweight and obesity are needed for the Asian-ethnic groups. Int J Obes Relat Metab Disord. 2003;27:1294–6. doi: 10.1038/sj.ijo.0802412. [DOI] [PubMed] [Google Scholar]
- Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- Myrelid A, Gustafsson J, Ollars B, Anneren G. Growth charts for Down’s syndrome from birth to 18 years of age. Arch Dis Child. 2002;87:97–103. doi: 10.1136/adc.87.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Li Y, Freedman DS, Borrud LG, Flegal KM. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999–2004. Natl Health Stat Report. 2011:1–7. [PubMed] [Google Scholar]
- Rimmer JH, Yamaki K, Lowry BM, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. 2010;54:787–94. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, Harris TB, Heymsfield SB, Horlick M, Lohman TG, Lukaski HC, Shepherd J, Siervogel RM, Borrud LG. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–25. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- Shin M, Besser LM, Kucik JE, Lu C, Siffel C, Correa A. Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009;124:1565–71. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- Styles ME, Cole TJ, Dennis J, Preece MA. New cross sectional stature, weight, and head circumference references for Down’s syndrome in the UK and Republic of Ireland. Arch Dis Child. 2002;87:104–8. doi: 10.1136/adc.87.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothill P, Hannan WJ, Wilkinson S. Comparisons between a pencil beam and two fan beam dual energy X-ray absorptiometers used for measuring total body bone and soft tissue. Br J Radiol. 2001;74:166–76. doi: 10.1259/bjr.74.878.740166. [DOI] [PubMed] [Google Scholar]