Abstract
In a wind-tunnel study, the upwind flight and source location of female Aedes aegypti to plumes of carbon dioxide (CO2) gas and odour from human feet is tested. Both odour sources are presented singly and in combination. Flight upwind along the plumes is evident for both CO2 and odour from human feet when the odours are presented alone. Likewise, both odour sources are located by more than 70% of mosquitoes in less than 3 min. When both CO2 and odour from human feet are presented simultaneously in two different choice tests (with plumes superimposed or with plumes separated), there is no evidence that females orientate along the plume of CO2 and only a few mosquitoes locate its source. Rather, the foot odour plume is navigated and the source of foot odour is located by over 80% of female Ae. aegypti. When a female is presented a plume of CO2 within a broad plume of human foot odour of relatively low concentration, the source of CO2 is not located; instead, flight is upwind in the diffuse plume of foot odour. Although upwind flight by Ae. aegypti at long range is presumably induced by CO2 and the threshold of response to skin odours is lowered, our findings suggest that once females have arrived near a prospective human host, upwind orientation and landing are largely governed by the suite of odours from a human foot, while orientation is no longer influenced by CO2.
Keywords: Anemotaxis, orientation wind tunnel, yellow fever mosquito
Introduction
A female mosquito must locate a suitable host to blood feed. Studies attempting to identify the suite of cues used in host finding have focused principally on odours emitted by hosts, although thermal and visual cues can be important to host-location once a mosquito has arrived in the vicinity of a host. Carbon dioxide (CO2) has long been viewed as a principal cue (Rudolfs , 1922), inducing upwind flight at some distance (Gillies, 1980; Cardé & Gibson, 2010). Great effort (e.g., Bernier et al., 2000, 2002; Okumu et al. 2010; Mukabana et al. 2012) has been expended to establish the identity of other host odours that enable host finding, particularly among the highly anthropophilic species that transmit human diseases.
Establishing which individual compounds or combinations thereof mediate upwind orientation (anemotaxis), landing on a host or, in some highly anthropophilic species, house-entering behaviour (as a prelude to host-location during the normal time for biting) requires diagnostic bioassays. Most behavioural bioassays, however, do not present odours in a natural context but rather use surrogate measures such as field capture in suction traps co-emitting CO2 or, in laboratory trials, entrance into ports over assay intervals of many minutes. Suction traps do not assay landing and, at least with some Culex species, these capture only a small proportion of mosquitoes lured to a trap's vicinity (Cooperband & Cardé, 2006). Port-entrance bioassays also may fail to document flight near the port that does not lead to capture and long assay times (many minutes) may not reveal differences among odours that induce rapid vs. delayed responses (Dekker et al., 2001). Furthermore, most assay systems do not present odour plumes in spatial structures that mimic the natural pattern of odour dispersion in nature.
Wind-tunnel studies using video recordings of flight and landing responses attempt to mimic many of the natural conditions that a mosquito would encounter in host searching in the field (Takken et al., 1997; Dekker et al., 2005; Dekker & Cardé, 2011; Lacey & Cardé, 2011, 2012; Spitzen et al., 2013) and offer a new paradigm for understanding which of the hundreds of compounds released from the surface of human skin and possibly human breath (in addition to CO2) and human sweat mediate host finding.
The stimuli inducing host finding is perhaps best understood in the yellow fever mosquito, Aedes aegypti (L.). CO2 is a potent activator of female Ae. aegypti and, even at low concentrations (> 0.04 % above ambient), a turbulent plume evokes upwind flight along the plume and rapid source finding (Dekker et al., 2005). Furthermore, a single contact with a filament of CO2 gas reduces the threshold for response to human skin odour (Dekker et al., 2005). This change in salience likely is facilitated by the CO2 receptor also being sensitive to human skin odours (Tauxe et al., 2013). Once a female has arrived in close proximity to a human host, however, it may no longer be within the plume of CO2 and how at this stage of orientation this cue interacts with skin odour is unclear. This interaction may be temporally complex, given that the mosquito may be moving in and out of the CO2 plume.
Orientation to human skin odour is optimal when the odour plume is relatively homogeneous in structure or when the CO2 plume is turbulent (Geier et al., 1999a; Dekker et al., 2011). Several skin odorants attract Ae. aegypti, including l-lactic acid (Smith, 1970; Dekker et al. 2002), ammonia (Geier et al., 1999b), and short-chain carboxylic acids (Bosch et al., 2000). Logan et al. (2008) suggest that the ratio of skin odorants is crucial to inducing orientation in Ae. aegypti because increased levels of some specific components decrease close-range attraction to a human hand. Therefore, variability in the ratio of natural odorants would account in part for the variation in attractiveness among individual humans. Creation of a blend of compounds mimicking at physiological concentrations the attractiveness of human skin has remained an elusive goal (Williams et al., 2006; Okumu et al., 2010; Mukabana et al., 2012).
In this study, human foot odour is employed as a representative human skin odour (hereafter “foot odour” in reference to presented research and “skin odour” in reference to odours from the surface of the human body as a-whole) and CO2 in wind-tunnel manipulations to examine the interactions of the odours on the plume following and landing behaviours of female Ae. aegypti. The hypothesis is tested that when a mosquito is in close proximity to sources of these two odours, human foot odour supersedes CO2 in the induction of orientation.
Materials and methods
Mosquitoes
A colony of Aedes aegypti L., Rockefeller strain, was maintained under LD 14:10 h photocycle, at ~27°C and 70% relative humidity. The colony was propagated by providing adult female mice, Mus musculus, as a blood meal (Animal Use Protocol #A-0303007-1). Larvae were reared in plastic containers filled with deionized (d.i.) water and fed rabbit pellets (Purina Mills, LLC, St. Louis, Missouri). Pupae were transferred to screen cages (30 × 30 × 30 cm) prior to eclosion and adults of both sexes were enclosed together and provided a 10% solution of sucrose in d.i. water ad libitum. Females used in experiments were 6-12 d post-eclosion, assumed to have mated, and not blood-fed. A single mosquito was aspirated into each release cage 1 h before bioassays were initiated in hours 1-4 of photophase. Release cages consisted of cylinders of clear acrylic (~7 cm long and 8 cm i.d.) covered on one end with insect screen and open on the other. A slit was cut through the side of the cylinder near the open end and a card-stock partition was inserted to confine mosquitoes. Release cages were housed in a vented box in the room with the wind tunnel until used in bioassays.
Wind tunnel
Bioassays were conducted in a wind tunnel constructed from transparent Plexiglas, 1.5 m long × 0.5 m height × 0.5 m width (see Dekker et al., 2005; Lacey & Cardé, 2011). Air was pushed into the tunnel from outside the building by an in-line duct fan (Kanalflakt, Inc. Sarasota, Florida), steam humidified (IMBA9, Sussman® electric boilers, Long Island City, New York), and passed through an activated charcoal filter, an aluminium honeycomb laminizer (cells 1.5 cm dia. × 15 cm long) and two fine-mesh screens before entering the arena. The airflow (0.2 m s−1, 27-29°C, ~70% R. H.) was even throughout the tunnel (measured with an Omega HHF 52 anemometer; Omega Engineering, Stamford, Connecticut). Air was exhausted into the room. A wall-mounted exhaust fan was operated between assays to remove contaminated air from the room. The inside of the wind tunnel and all accessories (e.g. release cage, pedestals, CO2 source; see below) were cleaned with ethanol after bioassays with human foot odour (see below) and latex gloves were worn while working inside the wind tunnel.
The floor and walls of the wind tunnel were covered with non-reflective black polyvinylchloride sheet (1 mm thickness; S & W plastic, Ontario, California) to provide a matte background for viewing and recording flight of mosquitoes in flight (see below). Light for viewing, video recording, and subsequent digital analysis of flight (see below) was provided by four infrared light arrays (60 LEDs / light, 940 nm; Rainbow, Irvine, California) directed upwind from the downwind end of the tunnel. Four fluorescent bulbs (cool white, 34 W) on the ceiling of the room provided moderate to low visible light (~0.04W m−2, Gossen Ultra-Pro light meter; Gossen Foto und Lichtmesstechnik, Nurnberg, Germany). Videos of mosquito flight were recorded by a CCTV video camera (ICD 48; Ikegami Electronics, Maywood, New Jersey) with 6 mm lenses mounted above the wind tunnel.
A single mosquito was released on the midline of the tunnel 120 cm from the upwind screen and 25 cm above the floor (midline of release cage). An open-ended acrylic cylinder (15 cm long, 9.5 cm i.d.) fitted with a swinging screen door that could be opened remotely by pulling a string accommodated the release cages and allowed release without evident disturbance of the mosquitoes. Mosquitoes were allowed to acclimate in the wind tunnel for 1 min prior to release. When beads with foot odour were a treatment, the mosquitoes were exposed to foot odour during the acclimatization period to avoid entering the wind tunnel and disturbing mosquitoes before release. The flow of CO2 laden air was initiated 10 s before opening the cage.
Odour sources
Glass beads (black, 2mm; Darice Inc., Strongsville, Ohio) were used to collect human foot odour and as a source of odour in the wind tunnel (see, Bernier et al., 2000; Qui et al., 2006; Lacey & Cardé, 2011). Though odour from human skin varies widely between regions of the human body (Dormont et al., 2013) and odour from human feet may or may not have specific characteristics that elicit specific behaviours from some species of mosquito (de Jong & Knols, 1995; Knols et al., 1996; Dekker et al., 1998), foot odour was employed because of the relative simplicity of collection and vigorous response of female Ae. aegypti in preliminary trials. Beads were cleaned by agitating in a sonication bath in d.i. water and detergent (Sparkleen; Fisher Scientific, Pittsburgh, Pennsylvania), rinsed thoroughly in d.i. water, rinsed twice in acetone, and heated to 250 °C for > 12 hr. Beads (20 g) were held in contact with the toes and sole of a human foot (author E.S.L.) by ‘wearing’ them inside a clean cotton sock (Hanesbrands Inc., Winston-Salem, North Carolina) and athletic shoe (Converse Inc., North Andover, Massachusetts). Feet were cleaned with bar soap (Lever 2000; Unilever, Rotterdam, The Netherlands) ~1 h prior to insertion and beads were ‘worn’ for four hours prior to bioassays. Beads were presented in 7 cm-diameter Petri dishes elevated to 25 cm above the floor on pedestals constructed from steel. Clean beads were used as controls when CO2 was presented alone.
A plume of either CO2-enriched air or clean air without additional CO2 was presented with the wind flow and along the midline of the wind tunnel at a rate of 1 L min−1 from a glass tube (2 mm i.d.; 25 cm above the floor) bent at 90° with the opening directed downwind parallel to the wind flow, creating a turbulent plume. The CO2-laden air was produced by mixing air (medical grade; 0.032% CO2; Air Gas, Inc., Lakewood, California) and CO2 (100%; Air Gas, Inc., Lakewood, California) in a 1 L side-arm flask containing 100 ml of d.i. water heated to 100°C. Flow rates were regulated with flow meters (Cole-Parmer Instrument Company, Vernon Hills, Illinois) to produce air enriched to 4% CO2. The resulting CO2-laden airflow (at ~32°C, 85% r. h.) moved to the glass tube via vinyl tubing (6.4 mm i.d., Fisher Scientific, Pittsburgh, Pennsylvania). The resultant plume had a time-averaged concentration of CO2 along the centre of ~0.3% 10 cm downwind of the source and ~0.06% 20 cm upwind of the release cage (measured with GasHound LI-800, LI-COR Biotechnology, Lincoln, Nebraska). To avoid contamination of ‘clean’ air when treatments contained no additional CO2, separate tubing, flow meters, and side–arm flasks were used to deliver air to the wind tunnel. Visualization of plumes with TiCl4 ‘smoke’ indicated that the plumes from both the beads and the CO2 source maintained structure and dimensions along the length of the wind tunnel. The plumes from either the CO2 source or its clean air control fully engulfed the mosquito release cage. Bioassays testing response to foot odour were grouped together as were bioassays employing only clean beads (e.g., 4 trials with clean beads were conducted sequentially followed by 4 bioassays with foot odour present) to minimize possible behavioural effects from contamination on surfaces within the wind tunnel from odours volatilizing from beads. All treatments in an experiment were tested every day and treatments were alternated from early in the testing period to late in the testing period.
Experiment 1
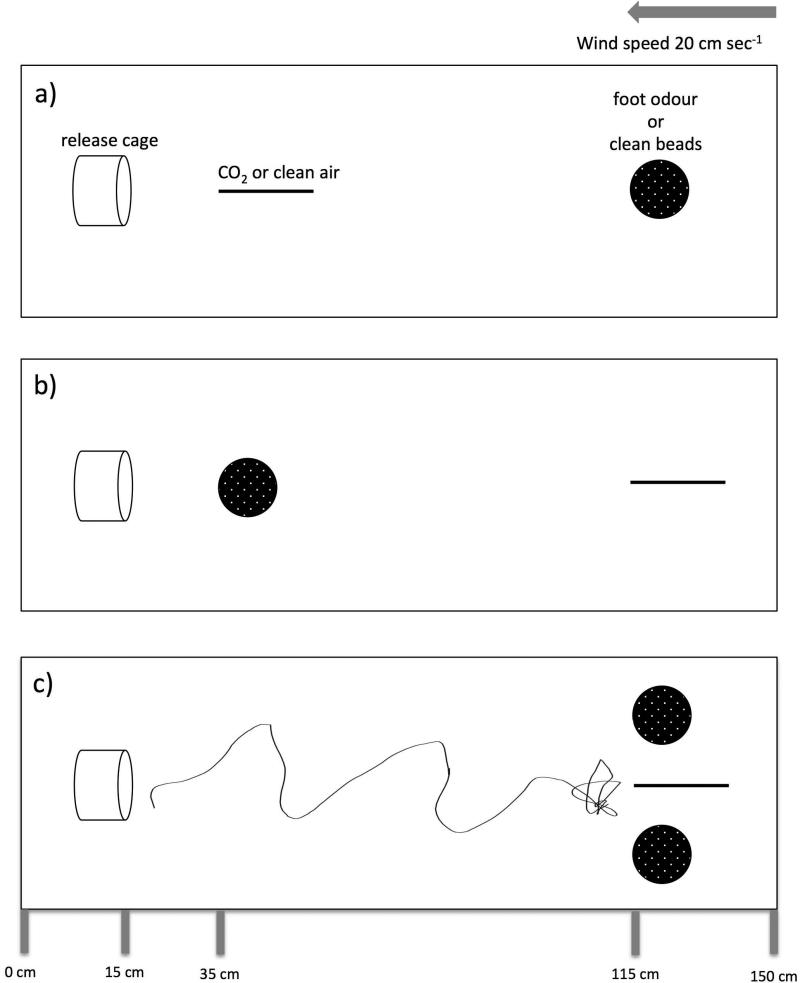
Response to CO2 and foot odour was tested when odour plumes were superimposed but sources were spatially separated along the long axis of the wind-tunnel (one odour source relatively close to the mosquito and the other further upwind). There were 8 treatments with 30 trials for each treatment (Figs. 1a, b):
Figure 1.
A top-view schematic of the wind tunnel for Experiment 1 (a & b) and Experiment 2 (c). Solid dark circles represent Petri dishes with beads (with foot odour or clean beads), dark bars represent the point source of carbon dioxide (CO2) or a control stream of clean air, and the cylinder represents the release cage. The line in (c) represents a 2-D track from a “typical” upwind flight and location of the source of CO2by a female Aedes aegypti.
Treatments experiment 1a:
4 % CO2 20 cm upwind and clean beads 100 cm upwind (CO2 alone)
clean air 20 cm upwind and beads with foot odour 100 cm upwind (foot odour alone)
4 % CO2 20 cm upwind and beads with foot odour 100 cm upwind (both odour treatments)
control (no CO2 or foot odour)
Treatments experiment 1b:
-
5
clean beads 20 cm upwind and 4 % CO2 100 cm upwind (CO2 alone)
-
6
beads with foot odour 20 cm upwind and clean air 100 cm upwind (foot odour alone)
-
7
beads with foot odour 20 cm upwind and 4 % CO2 100 cm upwind (both odour treatments)
-
8
control (no CO2 or foot odour)
The mosquito was allowed 3 min to respond to an odour source. A response to foot odour was scored when the beads were landed upon by a mosquito. A response to CO2 was scored after the mosquito navigated to the source of the CO2 plume and flew in ~5 cm ‘zigzags’ immediately downwind (< 10 cm) of the source (see Fig. 1c for visualization of source location). Differences in numbers of mosquitoes responding to one-or-the-other odours within treatments and numbers responding to the same odour between treatments were compared with Fisher's Exact Test (Sokal & Rohlf, 1995).
Experiment 2
Responses to upwind sources of CO2 and foot odour were tested when odours were the same distance from the release cage and odour plumes did not overlap. The CO2 source and two dishes of beads were presented 100 cm upwind from the release cage. The beads were 10 cm from the respective walls of the wind tunnel (30 cm apart) and the CO2 was along the midline of the wind tunnel between the dishes of beads (Fig. 1c). The plumes from the bead dishes (foot odour or control) passed to either side of the release cage, such that to enter these plumes a mosquito would need to move laterally from the release cage. Treatments were: 1) CO2 alone, 2) foot odour alone, and 3) both odour treatments. As in Experiment 1, landing on beads and/or location of the CO2 source was recorded and compared with Fisher's Exact Test (Sokal & Rohlf, 1995).
Flights resulting in location of an odour source were analysed by tracking mosquito flight digitally with the software Ethovision XT (v. 7.1, Noldus Information Technology, Wageningen, The Netherlands). Flights were tracked to provide frame-by-frame coordinates of the mosquito that were reconstructed (R, v 2.13.0) to provide a visual representation of orientation (Fig. 2). As well, dimensions of odour plumes (in 2D) were configured in the tracking software (CO2 ~ 8 cm wide plume, foot odour ~ 10 cm wide plume; see Lacey & Cardé, 2011) and used to calculate time spent in odour plumes during flight and to determine orientation strategies when both a plume of foot odour and CO2 where both present at the same time. Flights were tracked from activation to location of an odour source. The mean time spent in odour plumes from CO2, foot odour, and control (and time spent in respective area of plume when either CO2 or foot odour was absent) was compared with nonparametric Kruskal-Wallis test (Statistix, Analytical Software, Tallahassee, Florida).
Figure 2.
Overlaid top views of 2-D tracks of multiple flights by female Aedes aegypti in response to odour plumes in the wind tunnel in Experiment 2. Tracks illustrate only segments of flights that resulted in location of an odour source and do not illustrate total flight in each trial. Odours were: a) carbon dioxide (CO2) (n = 20 tracks), b) CO2 and foot odour on beads (n = 21 tracks), and c) foot odour alone (n = 13 tracks). Circles represent Petri dishes containing beads with foot odour. Dark lines represent source of CO2. The grey shaded areas represent the approximate location and dimensions of the odour plumes. The x-axis is the long axis of the wind tunnel with the scale (in mm) representing the space between the release cage and the odour treatments (the ‘arena’ in which Ethovision could track flights). Wind flow of 20 cm • sec−1 is from right to left. Along the y-axis is the width of the wind tunnel (in mm). The CO2 plume was along the midline (0 mm) of the wind tunnel and foot odour was 150 mm to the sides. Location of the foot odour was switched between trials. Location of dishes with foot odour on beads was switched between trials, for purpose of superimposing tracks for illustration, locations of the mosquito on the y-axis in some flights to foot odour were multiplied by −1.
Experiment 3
Response of female Ae. aegypti to CO2 was tested when the source of foot odour was less concentrated and the plume larger and more diffuse than in the previous experiments. To create a broad plume of foot odour, the fine-mesh screen from the upwind end of the wind tunnel was ‘coated’ with beads. The screen was removed from the frame of the wind tunnel and held horizontally (while wearing latex gloves). The same quantity of beads with foot odour used previously (20 g) was poured onto the screen and the beads gently rolled on the screen for 10 min. while taking care to cover all areas of the screen evenly while avoiding contact with the aluminium frame. As a control, this procedure was repeated with clean beads on an identical second screen. A screen (treatment or control) was replaced in the wind tunnel and mosquitoes were tested for location of the CO2 source. Release of mosquitoes and quality and position of the CO2 source was the same as in Experiment 2. Screens were washed in detergent (Sparkleen; Fisher Scientific, Pittsburgh, Pennsylvania) and water, rinsed in d.i. water, wiped with ethanol, and air-dried. Screens were replaced and washed after 10 min. (~ 3 trials) because preliminary trials demonstrated the effect of foot odour on orientation to CO2 (see results) waned after a short period of time (unpublished data; presumably because of loss of the low quantity of foot odour applied to screens). Only location of the source of CO2 was scored, as response to foot odour was ambiguous (e.g. no point source of foot odour). Differences in numbers of mosquitoes responding to CO2 between treatments were compared with Fisher's Exact Test (Sokal & Rohlf, 1995).
Results
Experiment 1
When CO2 was presented with clean beads, the source of CO2 was located by >80% of the female Ae. aegypti. When beads with foot odour were presented alone, the beads were landed on by >70% of the mosquitoes after upwind navigation. When foot odour beads and CO2 were presented together, the beads were landed on by >80% of mosquitoes that flew upwind and the source of CO2 was located by <5% of mosquitoes (Table 1).
Table 1.
Numbers of female Aedes aegypti locating the source of carbon dioxide (CO2) or odour from human foot within 3 min. in the wind tunnel in experiments 1a (odours plumes superimposed, CO2 downwind of foot odour), 1b (odours plumes superimposed, foot odour downwind of CO2), and 2 (odour sources equidistant and plumes distinct).
| Experiment | Treatment | n | CO2 | Foot odour | P < 0.05 |
|---|---|---|---|---|---|
| 1a | CO2 alone | 28 | 24 | - | a |
| Foot odour alone | 23 | - | 17 | b | |
| CO2 + foot odour | 27 | 3* | 21 | b | |
| 1b | CO2 alone | 27 | 21 | - | a |
| Foot odour alone | 26 | - | 19 | b | |
| CO2 + foot odour | 27 | 0 | 22 | b | |
| 2 | CO2 alone | 27 | 21 | - | a |
| Foot odour alone | 21 | - | 16 | b | |
| CO2 + foot odour | 27 | 0 | 22 | b |
Different letters in the column to the right identify significant differences in numbers locating the CO2 source (CO2 location between all treatments in experiments 1 & 2 were compared pair-wise, Fisher's exact test P < 0.05).
The 3 females responding to CO2 located the source and then immediately navigated to and landed on beads with foot odour. n = number of females (out of 30) released that flew upwind.
Experiment 2
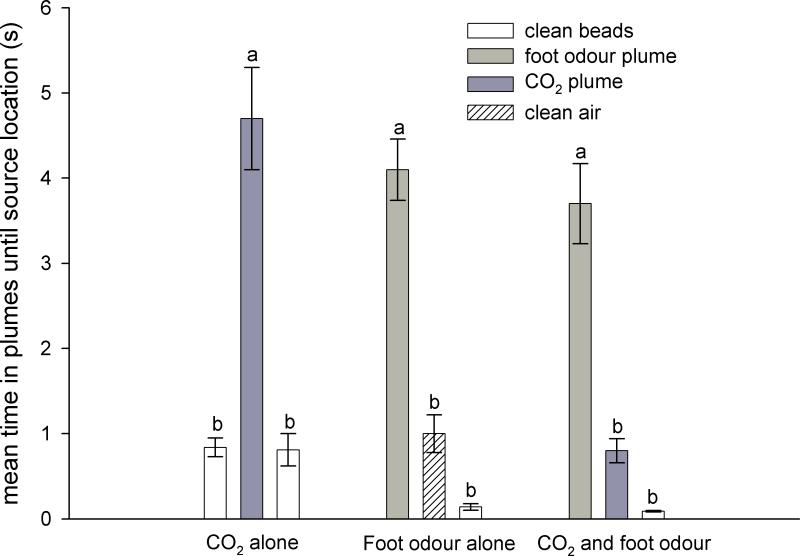
Similar to Experiment 1, when CO2 was presented in the wind tunnel with no foot odour >80%of female Ae. aegypti oriented upwind and located the source of CO2 (Table 1). When foot odour beads were presented along with CO2, > 80% oriented upwind and landed on the beads and none went to CO2. Analysis of flight tracks revealed female Ae. aegypti navigated along plumes of CO2 only when foot odour was absent and navigated along plumes of foot odour regardless of the presence or absence of CO2 (Figs. 2 & 3). Mean time spent in odour plumes was significantly different among treatments (Kruskall-Wallis χ28,61= 53.67, P < 0.0001; Fig. 3). When only the CO2 plume was present, mean time spent flying in it was significantly longer than in the space that was occupied by the ‘plume’ from clean beads flanking the source of CO2 (Kruskal-Wallis χ22,22 = 41.80, P < 0.0001; Fig. 3). When only foot odour was present, mean time spent flying in the plume from foot odourwas significantly longer than in adjacent ‘plumes’ from clean air or clean beads (Kruskal-Wallis χ22,16 = 29.37, P < 0.001; Fig. 3). When both CO2 and foot odour were present, mean time spent flying in the plume from foot odour was significantly longer than for CO2 or clean beads (Kruskal-Wallis χ22,23 = 47.01, P < 0.0001; Fig. 3), whereas the mean time for flights in the plumes from CO2 from clean beads did not differ.
Figure 3.
Mean times (± SEs) spent in odour plumes by female Aedes aegypti within the trackable area of the wind tunnel in Experiment 2. Time is from activation to odour location. Within each comparison, bars with different letters above them are significantly different (Kruskal-Wallis test, P < 0.05).
Experiment 3
With a clean (odour-free) screen at the upwind end of the wind tunnel, female Ae. aegypti navigated along the plume of CO2 and located its source >80 % of the time (Table 2). With a screen impregnated with foot odour at the upwind end of the wind tunnel, there was no discernible navigation along the plume of CO2 and zero mosquitoes located its source. Rather mosquitoes flew (somewhat) directly towards the upwind screen and beyond the field of view.
Table 2.
Numbers of female Aedes aegypti in Experiment 3 locating the source of carbon dioxide (CO2) when either a clean screen was in place in the upwind end of the wind tunnel or a screen coated with foot odour.
| Treatment | n | CO2 | P < 0.05 |
|---|---|---|---|
| CO2 alone | 18 | 16 | a |
| CO2 + foot odour | 16 | 0 | b |
Females that did not locate the source of CO2 flew to the screen at the upwind end of the wind tunnel. Different letters in the column to the right identify significant differences in numbers locating the odour source (CO2 location between treatments was compared with Fisher's exact test P < 0.05). n = number of females (out of 20) released that flew upwind.
Discussion
Odours from human skin and human breath offer two spatially and chemically distinct sources of odorants. Breath is expelled from the mouth and nose and, although additional potential kairomones are present in breath (Takken et al., 1997), CO2 is the primary cue evoking attraction from a distance. Likewise, very small amounts of CO2 emanate from the skin (Clements, 1999), but the primary kairomones from skin are presumably some combination of a few of the hundreds of different odorants that volatize from its surface. In a wind tunnel, both CO2 and odours from skin (in relatively high concentration) are attractive to female Ae. aegypti and lead to rapid location of the respective odour sources (Dekker et al., 2005; Dekker & Cardé, 2011; and results herein). A brief exposure to a filament of CO2 instantly lowers the threshold for response to skin odour (Dekker et al., 2005) perhaps because receptors for CO2 also detect human skin odours (Tauxe et al., 2013). The findings presented here demonstrate that in an assay that mimics the close encounter of a mosquito with sources of foot odour and CO2, odours from the foot supersede CO2 in the induction of orientation.
The physiological mechanism governing behavioural responses to a combination of CO2 and foot odours is not fully understood. Nor is it clear in Ae. aegypti if sensitization to skin odours after detection of CO2 is distinct from the reduced behavioural response to CO2 in the presence of foot odour or if they are reciprocal behavioural responses to a single molecular interaction (e.g. behavioural response to CO2 is reduced due to increased response to foot odours). Turner et al. (2011) show that odorants can interact with the CO2 receptor neurons and perhaps this effect could influence behaviour. For example, in Drosophila melanogaster molecules produced from ripening fruit can inhibit the CO2 receptor and mask the repellent effects of CO2 emanating from the fruit (Turner & Ray, 2009).
Carbon dioxide is most attractive to female Ae. aegypti in a turbulent plume (Dekker & Cardé, 2011) and it induces orientation when encountered in filaments at concentrations of only 10 parts-per-million above ambient (Dekker et al., 2005). These wisps of CO2 persist and are measureable at concentrations above ambient over relatively long distances (tens of meters) as the plume is transported downwind (Zöllner et al., 2004). These factors suggest that CO2 can function at a distance from a host. Conversely, odours from human skin collectively evoke stronger responses (i.e. activation, orientation, and location) by female Ae. aegypti in a homogenous plume (Dekker & Cardé, 2011) and a homogenous plume of skin odour is most likely to be found close to the host. This study supports the hypothesis that CO2 functions as a long-distance attractant because, close to the host, volatiles from the foot usurp the behavioural response to CO2.
The role of individual components of skin odour on orientation to a host is largely unknown. The vast collection of different molecules varies greatly in physical characteristics (i.e., volatility, solubility, ionic state) and some of these may act only close to the host (e.g., inducing landing [Healy & Copland 2000; Healy et al., 2002]). As well, little is known about the distances over which mosquitoes navigate to a host and so ‘long-range’ and ‘short-range’ are relativistic terms. Regardless, at some point during navigation, the strategy must shift from location of the host as a whole and to location of a site suitable for landing and biting. CO2 is a potent activator of female Ae. aegypti and even low concentrations (<0.04 % above ambient; expired breath ≅3.5-4%) lead to rapid upwind orientation and source finding, whereas skin odour reduced by 80% in concentration did not evoke a strong response without the presence of CO2 (Dekker et al., 2005). Subsequently, increasing concentrations of skin odour would divert the mosquito from orientation along the plume of CO2 to the skin surface, perhaps also guided by heat cues, elevated moisture levels, and visual cues. Not only would this strategy provide a transition from location of a host as-a-whole to location of a suitable site to land and feed, it would circumvent the need for a mosquito to make a choice between two distinct odorants that separate spatially as a mosquito nears a host.
Profiles of odorants from human skin vary among individual humans (Bernier et al., 2002) and some of these compounds are ubiquitous in the natural environment and are not human-specific (Bernier et al., 2007). A turbulent plume of CO2 with filaments above ambient concentrations is indicative of a nearby, potential host (Dekker & Cardé, 2011). Human hosts of diurnal Ae. aegypti are typically awake, mobile, and transient (unlike anthropophilic nocturnal mosquitoes such as several Anopheles species that typically bite at-rest hosts [Dekker et al., 2005]). Therefore, Ae. aegypti should favour a strategy of rapid detection of, navigation to, and landing on a human.
Although identification of potential kairomones and the responses they evoke in mosquito's sensory system are being characterized at an increasing rate (Carey et al. 2010; Wang et al. 2010; Carey & Carlson, 2011; Tauxe et al., 2013), which of these compounds in combination govern attraction, landing and biting remains uncertain. Even for Ae. aegypti, a species generally amenable to study in a wind-tunnel setting, the behavioural output in response to natural complex of odours and other host-associated cues is not fully understood. For ‘next-generation’ strategies of vector control that rely either on manipulation of host finding or trap-out to succeed, it is likely that an improved understanding is needed of the cues governing the orientation behaviours of Ae. aegypti, and species such as Anopheles gambiae and Culex quinquefasciatus, which so far have proven difficult to assay in wind tunnels.
Acknowledgements
We thank C. Ingell-Stouthamer for assistance with illustrations of flight tracks and K. A. Klingler for providing mosquitoes. This work was supported by grants from the NIAID (NIH) to A.R and R.T.C, award numbers RO1AI087785 and R56AI099778 , and by the Mosquito Research Foundation (to R.T.C.).
References
- Bernier UL, Kline DL, Barnard DR, et al. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Analytical Chemistry. 2000;72:747–756. doi: 10.1021/ac990963k. [DOI] [PubMed] [Google Scholar]
- Bernier UL, Kline DL, Barnard DR, et al. Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae). Journal of the American Mosquito Control Association. 2002;18:186–195. [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Posey KH. Human emanations and related natural compounds that inhibit mosquito host-finding ability. In: Debboun M, M., Frances SP, Strickman D, editors. Insect Repellents. Principles, Methods, and Use. CRC Press; Boca Raton, Florida: 2007. pp. 77–100. [Google Scholar]
- Bosch OJ, Geier M, Boeckh J. Contribution of fatty acids to olfactory host finding of female Aedes aegypti. Chemical Senses. 2000;25:323–330. doi: 10.1093/oxfordjournals.chemse.a014042. [DOI] [PubMed] [Google Scholar]
- Cardé RT, Gibson G. Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues. In: Takken W, Knols BGJ, editors. Ecology of Vector-Borne Diseases, V. 2, Olfaction in Vector-Host Interactions. Wageningen Academic Publishers; Wageningen, The Netherlands: 2010. pp. 115–141. [Google Scholar]
- Carey AF, Wang G, Su C-Y, et al. Odorant reception in the malaria mosquito Anophelesgambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AF, Carlson JR. Insect olfaction from model systems to disease control. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12987–12995. doi: 10.1073/pnas.1103472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes, vol. 2, Sensory Reception and Behaviour. CAB International; Wallingford, Oxford, U.K.: 1999. [Google Scholar]
- Cooperband MF, Carde RT. Orientation of Culex mosquitoes to carbon dioxide-baited traps: flight manoeuvres and trapping efficiency. Medical and terinary Entomology. 2006;26:11–26. doi: 10.1111/j.1365-2915.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- de Jong R, Knols BGJ. Selection of biting sites by mosquitoes. Olfaction in Mosquito-Host Interactions. Ciba Foundation Symposium. 1996;200:89–108. doi: 10.1002/9780470514948.ch8. [DOI] [PubMed] [Google Scholar]
- Dekker T, Takken W, Knols BGJ, Bouman E, van de Laak S, de Bever A, Huisman PWT. Selection of biting sites on a human host by Anophelesgambiae s. s., An. arabiensis, and An. quadriannulatus. Entomologia Experimentalis et Applicata. 1998;87:295–300. [Google Scholar]
- Dekker T, Takken W, Carde RT. Structure of host-odour plumes influences catch of Anopheles gambiae s.s. and Aedes aegypti in a dual-choice olfactometer. Physiological Entomology. 2001;26:124–134. [Google Scholar]
- Dekker T, Steib B, Carde RT, Geier M. L-lactic acid: a human-signifying cue for the anthrophilic mosquitoes. Medical and Veterinary Entomology. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- Dekker T, Geier M, Cardé RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. Journal of Experimental Biology. 2005;208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- Dekker T, Cardé RT. Moment-to-moment flight manoeuvres of the female yellow fever mosquito (Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. Journal of Experimental Biology. 2011;214:34743479. doi: 10.1242/jeb.055186. [DOI] [PubMed] [Google Scholar]
- Dormont L, Bessiere JM, Cohuet A. Human skin volatiles: a review. Journal of Chemical Ecology. 2013;39:569–578. doi: 10.1007/s10886-013-0286-z. [DOI] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. Journal of Experimental Biology. 1999a;202:1639–1648. doi: 10.1242/jeb.202.12.1639. [DOI] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chemical Senses. 1999b;24:1–7. doi: 10.1093/chemse/24.6.647. [DOI] [PubMed] [Google Scholar]
- Gillies MT. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bulletin ofEntomological Research. 1980;80:525532. [Google Scholar]
- Healy TP, Copland MJW. Human sweat and 2-oxopentanoic acid elicit a landing response from Anophelesgambiae. Medical and Veterinary Entomology. 2000;14:195–200. doi: 10.1046/j.1365-2915.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- Healy TP, Copland MJW, Cork A, et al. Landing responses of Anopheles gambiae elicited by oxocarboxylic acids. Medical and Veterinary Entomology. 2002;16:126–132. doi: 10.1046/j.1365-2915.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- Knols B, van Loon J, Cork A, et al. Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bulletin of Entomological Research. 1997;87:151–159. [Google Scholar]
- Lacey ES, Cardé RT. Activation, orientation and landing of female Culex quinquefasciatus in response to carbon dioxide and odour from human feet: 3-D flight analysis in a wind tunnel. Medical and Veterinary Entomology. 2011;25:94–103. doi: 10.1111/j.1365-2915.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Lacey ES, Cardé RT. Location of and landing on a source of human body odour by female Culex quinquefasciatus in still and moving air. Physiological Entomology. 2012;37:153–159. doi: 10.1111/j.1365-3032.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JG, Birkett MA, Clark SJ, et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. Journal of Chemical Ecology. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- Mukabana WR, Mweresa CK, Otieno B, et al. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. Journal of Chemical Ecology. 2012;38:235–244. doi: 10.1007/s10886-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu FO, Killeen GF, Ogoma S, et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE. 2010;5(1):e8951. doi: 10.1371/journal.pone.0008951. doi:10.1371/journal.pone.0008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, Van Loon JJA, et al. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Medical and Veterinary Entomology. 2006;20:280–287. doi: 10.1111/j.1365-2915.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Rudolfs W. Chemotrophism of mosquitoes. Bulletin of the New Jersey Agricultural Experiment Station. 1922;367:4–23. [Google Scholar]
- Smith CN, Smith N, Gouck HK, et al. L-lactic acid as a factor in the attraction of Aedes aegypti (Diptera: Culicidae) to human hosts. Annals of the Entomological Society of America. 1970;63:760–770. doi: 10.1093/aesa/63.3.760. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. Freeman; New York, New York: 1995. [Google Scholar]
- Spitzen J, Spoor CW, Grieco F, et al. A 3D analysis of flight behavior of Anopheles gambiae sensu stricto malaria mosquitoes in response to human odor and heat. PLoS ONE. 2013;8(5):e62995. doi: 10.1371/journal.pone.0062995. doi:10.1371/journal.pone.0062995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Dekker T, Wijnholds YG. Odor-mediated flight behavior of Anopheles gambiae Giles sensu stricto and An. stephensi Liston in response to CO2, acetone, and 1-octen-3-ol (Diptera: Culicidae). Journal of Insect Behavior. 1997;10:395–407. [Google Scholar]
- Tauxe GM, MacWilliam D, Boyle SM, et al. Targeting a dual receptor of skin and CO2 to modify mosquito host seeking. Cell. 2013;155 doi: 10.1016/j.cell.2013.11.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Turner SL, Li N, Guda T, et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474:87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ. The molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CR, Bergbauer R, Geier M, et al. Laboratory and field assessment of some kairomones blends for host-seeking Aedes aegypti. Journal of the American Mosquito Control Association. 2006;22:641–647. doi: 10.2987/8756-971X(2006)22[641:LAFAOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zöllner GE, Torr SJ, Ammann C, Meixner FX. Dispersion of carbon dioxide plumes in African woodland: implications for host-finding by tsetse flies. Physiological Entomology. 2004;29:381–394. [Google Scholar]