Abstract
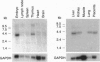
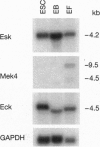
Eph and its homologues form the largest subfamily of receptor tyrosine kinases. Normal expression patterns of this subfamily indicate roles in differentiation and development, whereas their overexpression has been linked to oncogenesis. This study investigated the potential role of Eph-related molecules during very early embryonic development by examining their expression in embryonic stem (ES) cells and embryoid bodies differentiated from ES cells in vitro. By use of a strategy based on reverse transcriptase-mediated PCR, nine clones containing Eph-subfamily sequence were isolated from ES cells. Of these, eight were almost identical to one of four previously identified molecules (Sek, Nuk, Eck, and Mek4). However, one clone contained sequence from a novel Eph-subfamily member, which was termed embryonic stem-cell kinase or Esk. Northern analysis showed expression of Esk in ES cells, embryoid bodies, day 12 mouse embryos, and some tissues of the adult animal. Levels of expression were similar in ES cells and embryoid bodies. By comparison, Mek4 showed no significant transcription in the ES cell cultures by Northern analysis, whereas Eck displayed stronger signals in ES cells than in the embryoid bodies. These results suggest that Eph-subfamily molecules may play roles during the earliest phases of embryogenesis. Furthermore, the relative importance of different members of this subfamily appears to change as development proceeds.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbondanzo S. J., Gadi I., Stewart C. L. Derivation of embryonic stem cell lines. Methods Enzymol. 1993;225:803–823. doi: 10.1016/0076-6879(93)25052-4. [DOI] [PubMed] [Google Scholar]
- Anderson G., Jenkinson E. J. The role of the thymus during T-lymphocyte development in vitro. Semin Immunol. 1995 Jun;7(3):177–183. doi: 10.1016/1044-5323(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Andres A. C., Reid H. H., Zürcher G., Blaschke R. J., Albrecht D., Ziemiecki A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene. 1994 May;9(5):1461–1467. [PubMed] [Google Scholar]
- Bartley T. D., Hunt R. W., Welcher A. A., Boyle W. J., Parker V. P., Lindberg R. A., Lu H. S., Colombero A. M., Elliott R. L., Guthrie B. A. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994 Apr 7;368(6471):558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- Beckmann M. P., Cerretti D. P., Baum P., Vanden Bos T., James L., Farrah T., Kozlosky C., Hollingsworth T., Shilling H., Maraskovsky E. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. EMBO J. 1994 Aug 15;13(16):3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. D., Wang Z., Kuang W. J., Wang A., Groopman J. E., Goeddel D. V., Scadden D. T. Cloning and characterization of HTK, a novel transmembrane tyrosine kinase of the EPH subfamily. J Biol Chem. 1994 May 13;269(19):14211–14218. [PubMed] [Google Scholar]
- Boyd A. W., Ward L. D., Wicks I. P., Simpson R. J., Salvaris E., Wilks A., Welch K., Loudovaris M., Rockman S., Busmanis I. Isolation and characterization of a novel receptor-type protein tyrosine kinase (hek) from a human pre-B cell line. J Biol Chem. 1992 Feb 15;267(5):3262–3267. [PubMed] [Google Scholar]
- Böhme B., Holtrich U., Wolf G., Luzius H., Grzeschik K. H., Strebhardt K., Rübsamen-Waigmann H. PCR mediated detection of a new human receptor-tyrosine-kinase, HEK 2. Oncogene. 1993 Oct;8(10):2857–2862. [PubMed] [Google Scholar]
- Chan J., Watt V. M. eek and erk, new members of the eph subclass of receptor protein-tyrosine kinases. Oncogene. 1991 Jun;6(6):1057–1061. [PubMed] [Google Scholar]
- Cheng H. J., Flanagan J. G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994 Oct 7;79(1):157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ganju P., Shigemoto K., Brennan J., Entwistle A., Reith A. D. The Eck receptor tyrosine kinase is implicated in pattern formation during gastrulation, hindbrain segmentation and limb development. Oncogene. 1994 Jun;9(6):1613–1624. [PubMed] [Google Scholar]
- Gilardi-Hebenstreit P., Nieto M. A., Frain M., Mattéi M. G., Chestier A., Wilkinson D. G., Charnay P. An Eph-related receptor protein tyrosine kinase gene segmentally expressed in the developing mouse hindbrain. Oncogene. 1992 Dec;7(12):2499–2506. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., Marengere L. E., McGlade J., Olivier J. P., Conlon R. A., Holmyard D. P., Letwin K., Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994 Apr;9(4):1001–1014. [PubMed] [Google Scholar]
- Hirai H., Maru Y., Hagiwara K., Nishida J., Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987 Dec 18;238(4834):1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Keller G., Kennedy M., Papayannopoulou T., Wiles M. V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993 Jan;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa E., Takai S., Tanaka M., Iwase T., Suzuki M., Xiang Y. Y., Naito Y., Yamada K., Sugimura H., Kino I. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994 Jul 15;54(14):3645–3650. [PubMed] [Google Scholar]
- Letwin K., Yee S. P., Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988 Dec;3(6):621–627. [PubMed] [Google Scholar]
- Lindberg R. A., Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990 Dec;10(12):6316–6324. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Barrezueta N. X., Yancopoulos G. D. Ehk-1 and Ehk-2: two novel members of the Eph receptor-like tyrosine kinase family with distinctive structures and neuronal expression. Oncogene. 1993 Dec;8(12):3277–3288. [PubMed] [Google Scholar]
- Maru Y., Hirai H., Takaku F. Overexpression confers an oncogenic potential upon the eph gene. Oncogene. 1990 Mar;5(3):445–447. [PubMed] [Google Scholar]
- Pasquale E. B. Identification of chicken embryo kinase 5, a developmentally regulated receptor-type tyrosine kinase of the Eph family. Cell Regul. 1991 Jul;2(7):523–534. doi: 10.1091/mbc.2.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. C., Robertson E. J. The expression of the receptor-protein tyrosine kinase gene, eck, is highly restricted during early mouse development. Mech Dev. 1994 May;46(2):87–100. doi: 10.1016/0925-4773(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Sajjadi F. G., Pasquale E. B. Five novel avian Eph-related tyrosine kinases are differentially expressed. Oncogene. 1993 Jul;8(7):1807–1813. [PubMed] [Google Scholar]
- Sajjadi F. G., Pasquale E. B., Subramani S. Identification of a new eph-related receptor tyrosine kinase gene from mouse and chicken that is developmentally regulated and encodes at least two forms of the receptor. New Biol. 1991 Aug;3(8):769–778. [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Shao H., Lou L., Pandey A., Pasquale E. B., Dixit V. M. cDNA cloning and characterization of a ligand for the Cek5 receptor protein-tyrosine kinase. J Biol Chem. 1994 Oct 28;269(43):26606–26609. [PubMed] [Google Scholar]
- Wicks I. P., Wilkinson D., Salvaris E., Boyd A. W. Molecular cloning of HEK, the gene encoding a receptor tyrosine kinase expressed by human lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1611–1615. doi: 10.1073/pnas.89.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning R. S., Sargent T. D. Pagliaccio, a member of the Eph family of receptor tyrosine kinase genes, has localized expression in a subset of neural crest and neural tissues in Xenopus laevis embryos. Mech Dev. 1994 Jun;46(3):219–229. doi: 10.1016/0925-4773(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Xu Q., Holder N., Patient R., Wilson S. W. Spatially regulated expression of three receptor tyrosine kinase genes during gastrulation in the zebrafish. Development. 1994 Feb;120(2):287–299. doi: 10.1242/dev.120.2.287. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zhou R., Copeland T. D., Kromer L. F., Schulz N. T. Isolation and characterization of Bsk, a growth factor receptor-like tyrosine kinase associated with the limbic system. J Neurosci Res. 1994 Jan;37(1):129–143. doi: 10.1002/jnr.490370117. [DOI] [PubMed] [Google Scholar]