Abstract
Kisspeptin binding to its cognate G protein-coupled receptor (GPR54, aka Kiss1R) in gonadotropin-releasing hormone (GnRH) neurons stimulates peptide release and activation of the reproductive axis in mammals. Kisspeptin has pronounced pre- and postsynaptic effects, with the latter dominating the excitability of GnRH neurons. Presynaptically, kisspeptin increases the excitatory drive (both GABA-A and glutamate) to GnRH neurons and postsynaptically, kisspeptin inhibits an A-type and inwardly rectifying K + (Kir 6.2 and GIRK) currents and activates nonselective cation (TRPC) currents to cause long-lasting depolarization and increased action potential firing. The signaling cascades and the multiple intracellular targets of kisspeptin actions in native GnRH neurons are continuing to be elucidated. This review summarizes our current state of knowledge about kisspeptin signaling in GnRH neurons.
Relationship Between Kisspeptin and GnRH Secretion
Kisspeptin, encoded by the Kiss1 gene, is a key factor in the regulation of reproductive development and functions [1–6]. The Kiss1 gene encodes a 145 amino acid protein, which is proteolytically processed to produce a 54 amino acid peptide, called kisspeptin-54, and several other smaller peptide fragments [7]. Centrally administered kisspeptins stimulate GnRH and gonadotropin secretion in prepubertal and adult animals [1, 8–11]. The central application of kisspeptin induces cFos immunoreactivity within 1–2 h in more than 85% of GnRH neurons, further suggesting that direct activation of the neurons is responsible for the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [8]. As expected, kisspeptin is not able to stimulate LH or FSH release in GPR54 knockout animals [11]. Also, the kisspeptin-mediated release of LH is completely inhibited by the GnRH antagonist, acyline [1, 8]. Importantly, CNS administration of kisspeptin in the ewe has conclusively demonstrated a correlation between kisspeptin-induced GnRH and LH release [11]. Therefore, the stimulatory actions of kisspeptin appear to be primarily on GnRH neurons and not the pituitary. Kisspeptin, when applied to GnRH neurons in vitro, potently activates these neurons and causes increased neuronal firing [12–14].
Over the past several years, there have been many publications about the regulation of Kiss1 gene expression and the role of kisspeptins in regulating GnRH and LH secretion [1, 8, 11, 15–17]. Also, the distribution and regulation of kisspeptin mRNA ( Kiss1 ) expression by 17β-estradiol (E 2 ) has been extensively described in the mouse and rat brain [15, 17, 18]. In these rodent species, it is known that Kiss1 mRNA is expressed primarily in the anteroventral periventricular nucleus (AVPV) and adjacent periventricular (PeN) areas, as well as in the arcuate nucleus of the hypothalamus [19, 20]. Importantly, E 2 increases the mRNA expression of Kiss1 in the female AVPV, but decreases the expression in the arcuate nucleus [15, 17]. These findings are consistent with data showing that the AVPV is necessary for E2 positive feedback on GnRH and LH secretion in these species [21–23]. The number of AVPV kisspeptin neurons is significantly fewer in male rodents than in females, but the numbers are similar in the arcuate nucleus in adults of both sexes [24]. As in females, steroid treatment (testosterone or E 2 ) increases the number of Kiss1 neurons in the male AVPV and decreases the number of Kiss1 expressing cells in the arcuate nucleus [25]. The function of the AVPV kisspeptin neurons in the male rodent is not clear, but these neurons may be involved in generating the basal pulsa-tile release of LH via a stimulatory action on GnRH neurons.
In other species, such as the guinea pig, sheep, and rhesus monkey, the preoptic area (POA) appears not to be the main region responsible for E 2 positive feedback [26–30]. Thus, it appears that the basal hypothalamus may be sufficient for maintaining steroid-mediated positive feedback regulation of GnRH and LH secretion in these species. Consistent with these findings, kisspeptin neurons within the arcuate nucleus in guinea pig, sheep, and monkey appear to be involved in E 2 -mediated positive as well as negative feedback regulation of GnRH neurons [31–34]. The specific kisspeptin neurons within the arcuate nucleus that mediate positive feedback regulation of LH remains to be determined, although evidence suggests that a caudal arcuate population of neurons is involved [31–34]. However, irrespective of the role of the arcuate nucleus in mediating E 2 positive feedback on LH secretion in certain species, evidence suggests that the POA is also involved [31, 34, 35]. Importantly, regardless of differential regulation of Kiss1 neurons by E 2, in all instances, kisspeptin potently excites GnRH neurons via a phospholipase C (PLC) signaling pathway (see below).
To further study the role of kisspeptin in the regulation of GnRH neurons and LH release, kisspeptin analogs with mixed agonist/antagonist activities have been synthesized [36]. Of these, peptide 234 has primarily antagonist activities in Chinese Hamster Ovary K1 (CHO-K1) cells expressing Kiss1R and inhibits the kisspeptin response by 93%, with an IC50 of 7 nM [36]. This compound also has a binding affinity of 2.7 nM for Kiss1R stably expressed in CHO-K1 cells. Peptide 234 subsequently has been found to inhibit kisspeptin-induced GnRH neuronal firing in vitro, whereas in vivo treatment with the peptide attenuates kisspeptin-induced LH release in intact males [36]. Moreover, peptide 234 attenuates the castration rise in plasma LH levels in mouse and rat, and reduces pulsatile LH release in the ovariectomized ewe and rat [36, 37]. Also, in ovariectomized monkeys, peptide 234 attenuates pulsatile release of GnRH [36]. Collectively, these data support the concept that kisspeptin neurons (in the arcuate nucleus?) are involved in stimulating GnRH and LH release following gonadectomy.
Thus, the estrogen-mediated “negative” feedback inhibition of post-castration GnRH and LH release may be via the differential release of kisspeptin/opioid peptides since this group of arcuate neurons also co-localizes dynorphin [38, 39]. Although there is a robust μ-opioid receptor-mediated inhibition of GnRH neurons in guinea pig [40], a κ-opioid-mediated effect has not been demonstrated.
Kisspeptin Activation of Kiss1R
Kisspeptin-54 has been identified as the endogenous ligand of the orphan G protein-coupled receptor, GPR54 [7, 41], also known as Kiss1R. In addition to kisspeptin-54, the smaller peptide fragments derived from the precursor protein (e.g., kisspeptin 14, 13, and 10) all have biological activity at Kiss1R [7, 42]. These peptides bind with low nanomolar affinities to rat and human Kiss1R expressed in Chinese hamster ovary K1 cells and stimulate PIP2 hydrolysis, Ca 2+ mobilization, arachidonic acid release, extracellular signal-regulated protein kinase 1 (ERK1), ERK2, and p38 MAP kinase phosphorylation [7]. In mammals, Kiss1R is expressed both in the pituitary and in GnRH neurons [7, 8, 11, 12]. However, as stated above, evidence suggests that the stimulation of gonadotropin secretion by kisspeptin is via direct activation of GnRH neurons and not pituitary gonadotropes [1, 8–10, 43]. Although multiple actions of kisspeptin have been identified (see below), all of the signaling pathways have not been elucidated.
Kisspeptin Activation of Kiss1R in GnRH Neurons: Downstream Signaling Pathways
To date, kisspeptin is the most potent and efficacious neuropeptide/neurotransmitter to excite native GnRH neurons [44–49]. In most studies, kisspeptin is reported to depolarize and excite the vast majority (75–90%) of GnRH neurons (Fig. 6.1), which correlates with the expression of Kiss1R in the majority of GnRH neurons [8, 12, 14, 50]. However, Dumalska et al. found a lower percentage of GnRH neurons responding to kisspeptin and proposed that there are two physiologically distinct populations of GFP-GnRH neurons, one that responds to kisspeptin and the other that responds to the metabotropic glutamate receptor agonist, dihydroxyphenylglycine (DHPG) [51]. One explanation for these differences is that some of the recordings performed by Dumalska and coworkers were made from animals as young as 15 days of age [51], so the reduced response to kisspeptin could be age-related. Although the expression of Kiss1R is similar in juvenile as in adult male mice, and at both age levels Kiss1R can be detected in over 90% of GnRH neurons [12], the percent of GnRH neurons responding to kisspeptin is only about 27% in juvenile vs. 90% in adult males [12]. The reason for the reduced efficacy of kisspeptin in GnRH neurons from juvenile and prepubertal males is not fully understood but could be due to an immature Kiss1R signaling in the younger animals.
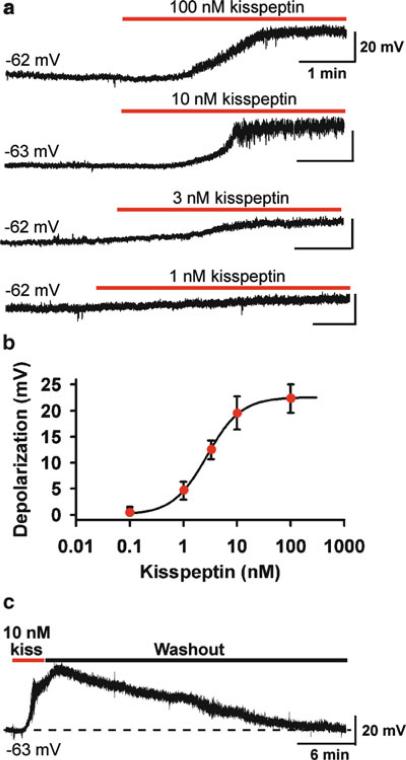
Fig. 6.1.
Kisspeptin depolarizes GnRH neurons in a concentration-dependent manner. ( a ) Representative traces showing that kisspeptin (1–100 nM) depolarized GnRH neurons in a concentration-dependent manner. The initial membrane potential for each trace is indicated. Only one cell was recorded from one slice. ( b ) Concentration–response curve of the kisspeptin-induced depolarization. Data are presented as mean ± SEM. The EC 50 for the kisspeptin-induced depolarization was 2.8 ± 0.2 nM ( n = 8–14) based on a logistic equation fit to the data points. ( c ) The kiss-peptin (10 nM)-induced depolarization was long lasting and typically took 30 min to recover. From Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin- releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 2008; 28: 4423– 4434. Reprinted with permission from The Society for Neuroscience
In the adult, kisspeptin depolarizes GnRH neurons via the coupling of Kiss1R to a phospholipase Cβ (PLCβ) signaling pathway that activates canonical transient receptor potential (TRPC) channels that allow influx of sodium and to a lesser extent calcium ions (Fig. 6.2) [14]. Besides activating TRPC channels in GnRH neurons, kisspeptin also attenuates resting and ligand-activated inwardly rectifying K + (Kir) channels and A-type potassium channels [13, 14, 50, 52]. The inhibition of Kir may be critical because Kir channels (e.g., K ATP and GIRK channels) are highly expressed in GnRH neurons and clamp the cells in a negative resting state of −63 mV [40, 53, 54]. This effect of kisspeptin is also vital for inhibiting GPCR- activated (μ-opioid, GABA B and perhaps melanin-concentrating hormone, MCH) GIRK (Kir) currents which are prominent in GnRH neurons [40, 54, 55]. Also, A-type K + currents are very prominent in GnRH neurons, and E 2 regulation of the A-current may play a role in negative feedback regulation of GnRH neurons [52, 56]. Therefore, kisspeptin inhibition of these K + currents would be of high functional significance. Moreover, kisspeptin increases calcium oscillations of mature as well as developing GnRH neurons, and these changes for the most part reflect the coupling of Kiss1R to a PLCβ signaling pathway [14, 50, 57, 58]. Therefore, by inhibiting potassium channels along with the pronounced activation of TRPC channels, kisspeptin depolarizes GnRH neurons to threshold (~45 mV) and induces sustained firing, which may be accompanied by a sustained calcium ion influx via calcium channels/TRPC channels and augmented GnRH release during positive feedback.
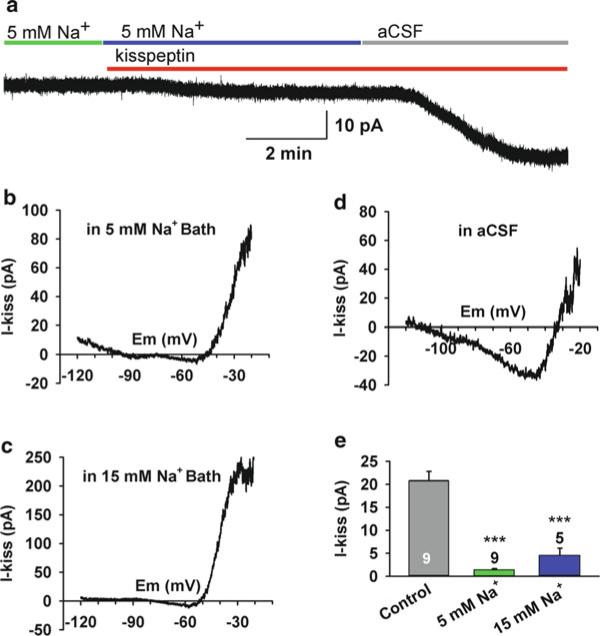
Fig. 6.2.
Kisspeptin predominantly activates a sodium-dependent, nonselective cationic (TRPC) channel. ( a ) The kisspeptin-induced inward current (at −60 mV) was greatly reduced in low Na + bath solution (5 mM Na + /140 mM N -methyl-d-glucamine [NMDG +]), and switching back to normal aCSF (control) solution revealed a kisspeptin sensitive inward current of 28 pA in this GnRH neuron. ( b, c ) The I–V relationships of the kisspeptin- evoked current in a low Na + bath solution (5 and 15 mM Na + ) between −20 and −120 mV showed a greatly reduced inward current. ( d ) A typical I–V relationship of the kisspeptin-induced inward current in normal aCSF (control) solution showed a larger inward current (−30 pA at −60 mV). ( e ) Summary of the effects of the extra-cellular sodium concentration on the kisspeptin-induced inward current at −60 mV. *** p < 0.001, significantly different from the effects of kisspeptin under control aCSF conditions. Cell numbers tested are indicated for each group. Error bars indicate SEM. From Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 2008; 28: 4423–4434. Reprinted with permission from The Society for Neuroscience
Kisspeptin Activation of TRPC Channels
The mammalian TRPC channel family consists of seven members, TRPC1–7, that appear to function as receptor-operated channels, analogous to the TRP channels involved in Drosophilia phototransduction [59]. With the exception of TRPC2, these channels are widely distributed in the mammalian brain [60]. The TRP channels are made of subunits with six membrane-spanning domains that co-assemble as tetrameric complexes similar to what has been described for K + channels [61, 62]. TRPC channels appear to co-assemble as heteromeric channels consisting of the TRPC1, 4, and 5 subfamily [63, 64], as well as TRPC3, 6, and 7 subfamily [65, 66]. It is well known that the current–voltage relationship and mechanisms of regulation of TRPC channels depend on the channel subunit composition [59]. However, the functional distinction between these channel subtypes in CNS neurons has been problematic because of a lack of selective pharmacological reagents. The main exception is the discriminatory effects of lathanides to augment TRPC4, 5 channel activity [62]. Whole-cell recording experiments, with K + channel blockers on board, have revealed that the current–voltage relationship for the kisspeptin-induced current in GnRH neurons resembles the current–voltage relationship of heteromeric complexes of TRPC 1 + 4 or TRPC 1 + 5 subunits expressed in HEK cells with the characteristic negative slope conductance and pronounced outward rectification (Fig. 6.2) [14, 59, 63]. Similar current–voltage relationships have been obtained for the leptin-induced currents in arcuate POMC and kisspeptin neurons and the mGluR1- and CCK2-induced currents in basolateral amygdala neurons [67–70]. All of these neurons have been found to express the same compliment of TRPC channels as GnRH neurons.
Interestingly, GnRH neurons express all of the “brain-type” TRPC channel subunits with the TRPC1, 4, and 5 family being the most prevalent in GnRH neurons [14]. Therefore, based on the current–voltage relationship, pharmacological profile and mRNA expression, TRPC1, 4, and 5 are key players in mediating the excitatory effects of kisspeptin in GnRH neurons (Figs. 6.2 and 6.3) [14]. Traditionally, these channels are known as “store operated calcium channels,” but this description is probably the result of poorly understood signaling mechanisms [59, 71]. Therefore, current research has focused on elucidating the signaling pathway(s) by which kisspeptin activates TRPC channels, and the sources of calcium mobilization following kisspeptin activation of GnRH neurons. Although the majority of findings seem to indicate that the initial calcium signal comes via plasma membrane channels [14, 57, 58], there is also evidence that kisspeptin induces the release of calcium from intracellular stores in GnRH neurons via inositol- 1,4,5-trisphosphate (IP3) receptors [50, 72]. However, intracellular dialysis with 2-APB, which abrogates the store release of calcium, does not inhibit the effects of kisspeptin [14]. Certainly, sustained calcium release is not required for kisspeptin's actions since calcium mobilization is transient [50].
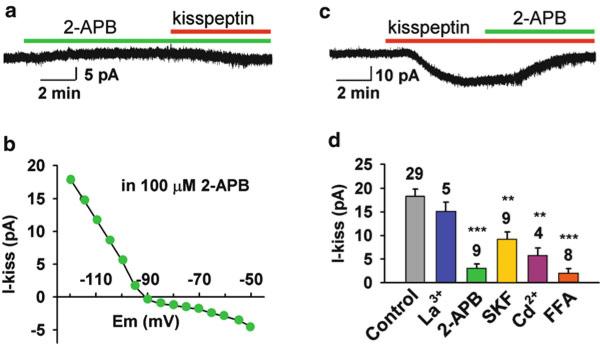
Fig. 6.3.
Effects of TRPC channel blockers on the kisspeptin-induced inward currents at −60 mV. ( a ) A representative recording showing that 2-APB (100 μM), which had very little effect on basal holding current, potently blocked the kisspeptin (100 nM)-evoked inward current. ( b ) Mean I–V relationship of the kisspeptin-sensitive current in the presence of 2-APB reversed at −90 mV ( n = 4), clearly indicating that a Kir channel was inhibited by kisspeptin. ( c ) A representative recording showing that 2-APB (100 μM) applied after kisspeptin also strongly blocked the kisspeptin- evoked inward current. ( d ) Summary of the effects of different TRPC channel blockers (100 μM La 3+, 100 μM 2-APB, 30 μM SKF96365, 250 μM Cd 2+, 100 μM flufenamic acid) on the kisspeptin-induced inward currents at −60 mV. Blockers were applied 5–7 min before or after the application of kisspeptin (100 nM). The percent inhibition for the different blockers was as follows: 17.4% for 100 μM La 3+, 83.6% for 100 μM 2-APB, 50% for 30 μM SKF, 68.7% for 250 μM Cd 2+, and 89.6% for 100 μM FFA. ** p < 0.01 and *** p < 0.001, significantly different from the kisspeptin response under control aCSF conditions. Cell numbers tested are indicated. Error bars indicate SEM. From Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 2008; 28: 4423–4434. Reprinted with permission from The Society for Neuroscience
The mammalian TRPC channels can be activated by G protein-coupled receptors and receptor tyrosine kinases (see refs. [59, 73]). In a heterologous cell expression system (i.e., Chinese hamster ovary K1 cells expressing Kiss1R), kisspeptin is capable of activating multiple signaling pathway resulting in increased IP 3 formation, calcium mobilization, arachidonic acid release, and MAP kinase phosphorylation [7]. Although the kisspeptin induction of GnRH release in hypothalamic explants from immature animals incubated in vitro is reported to involve recruitment of ERK1/2 and p38 kinases, these actions of kisspeptin have not been confirmed in adult GnRH neurons [50, 72]. In native GnRH neurons, the PLC inhibitor U73122 inhibits the effects of kisspeptin [14, 50], and indeed, it is known that all mammalian TRPC channels require PLC for activation [60]. Therefore, it appears that Gq-coupled GPR54 activates PLCβ to signal downstream to open TRPC channels in GnRH neurons, thereby allowing the influx of sodium and calcium. Interestingly, in POMC neurons, PLCγ1 appears to be the isozyme coupled to TRPC channel activation by leptin [69].
Classically, the TRPC3, 6, and 7 subfamily is DAG sensitive [59, 73]. Although TRPC3 and 7, and to a lesser extent TRPC6, transcripts are expressed in GnRH neurons, the surrogate DAG signaling molecule 2-acetyl sn-glycerol (OAG) has only a small effect to activate an inward current (~25% of the kiss-peptin-induced current) in GnRH neurons [14]. A potential explanation is that both hydrolysis of PIP 2 by PLCβ and the calcium trigger that facilitates the TRPC channel opening (i.e., the influx of Ca 2+ through calcium channels [74]) might be missing when applying OAG alone to GnRH neurons. In addition, La 3+ at a 100 μM concentration, which potentiates TRPC4 and 5 and blocks TRPC3, 6, and 7 channels [75], did not attenuate or augment the kisspeptin-induced current, which indicates that an ensemble of these channel subunits must exist in GnRH neurons as revealed by single-cell RT-PCR [14]. Indeed, extracellular 2-APB (100 μM), which is a potent blocker of TRPC3, 4, 5, and 6 channels, and FFA, which is a potent blocker of TRPC4 and 5 channels, inhibit the effects of kisspeptin in GnRH neurons (Fig. 6.3). These blockers have a similar effects on the leptin activation of TRPC channels in arcuate POMC and kisspeptin neurons, although lanthanum clearly potentiates the leptin- induced activation of TRPC currents in POMC and kisspeptin neurons [69, 70], suggesting subtle differences between the GnRH neurons and the other two cell types. Collectively, these data suggest that, although all of the “brain” TRPC channels are expressed in GnRH neurons, the TRPC1, 4, and 5 family appear to be major (key) players in mediating the effects of kisspeptin in GnRH neurons [14].
Kisspeptin Inhibition of Kir Channels and Their Role in GnRH Neuronal Excitability
Kisspeptin augments the activity of GnRH neurons in part via inhibition of Kir potassium channels [13, 14, 50]. In this respect, the Kir blockers barium (0.3 mM) and tetraethylammonium (20 mM) robustly inhibit the kisspeptin-induced potassium currents in GnRH neurons [13, 14, 50]. The importance of kisspeptin inhibition of Kir is further substantiated by the ability of kisspeptin to attenuate the GABA B -induced hyperpolarization in GnRH neurons [54].
GABA is one of the most important neurotransmitters that regulate the excitability of GnRH neurons. Multiple studies have shown that GABA activates Cl − currents in GnRH neurons, and these effects are blocked by GABA A receptor antagonists [44, 46, 47, 76–78]. It is generally accepted that activation of GABA A receptors depolarizes and excites GnRH neurons [46, 78–80]. Several GABA A receptor subunits have been identified in GnRH neurons, including α1, α2, α3, α5, β1, β2, β3, γ1, γ2, and the rho 1 subunits [78, 81, 82]. The GABA B receptor subunits, R1 and R2, are also found in GnRH neurons [54, 83], and GABA activates GABA B - receptors in GnRH neurons [54, 84]. Moreover, as has been demonstrated in numerous other hypothalamic neurons [85–90], GABA B receptors are coupled (Gα i/o ) to activation of G protein-coupled inwardly rectifying K + (GIRK) channels, resulting in a robust hyperpolarization of GnRH neurons.
The importance of Kir channels in modulating GnRH neuronal excitability has been well documented [13, 14, 40, 53–55, 84]. Female GnRH neurons sit at a relatively negative resting membrane potential (−63 mV) that is due, in part, to the activity of Kir channels including GIRKs and K ATP channels [53, 54]. For example, blocking the K ATP channels with the sulfonylurea tolbutamide significantly depolarizes the cells by 4–6 mV, which puts the membrane potential in the range of most parvocellular hypothalamic neurons [53, 91]. In addition, GABA release is regulated by E 2 through presynaptic mechanisms [85, 87, 92–94] that affect GnRH neuronal activity [40, 95]. Augmented E 2 -induced GABA B receptor activity would further hyperpolarize the membrane through increased GIRK channel activity. However, this inhibitory tone must be attenuated during the excitatory (preovula-tory) phase of GnRH neurons. One possible mechanism is that kisspeptin signaling via KissR provides the stimulus to overcome this strong inhibitory tone (Fig. 6.4). Previous investigators have shown that there is a robust kisspeptin drive during E 2 “positive feedback” [13, 15, 96], and kisspeptin counters the hyperpolarizing effects of activation of GIRKs by the GABA B agonist baclofen, μ-opioid receptor agonists (Zhang et al., unpublished findings), and other Ba 2+ -sensitive inwardly rectifying K + channels in general [13, 14]. Moreover, Gαq/11 -coupled receptors are known to desensitize (i.e., heterologous desensitization) Gα i/o -coupled receptors through PIP 2 hydrolysis and attenuating the GIRK-mediated hyperpolarization (Fig. 6.4) [97–99]. In addition to attenuating Gα i/o -coupled receptor-mediated hyperpolarization, kisspeptin activates TRPC channels in GnRH neurons to cause further depolarization [14]. The Kiss1R-Gα q/11 -PLCβ signaling pathway would have a twofold effect to inhibit K + channels and activate TRPC channels, which underlies the pronounced excitatory effects of kisspeptin on GnRH neurons [12–14, 50, 52]. Interestingly MCH, although at higher concentrations, can block kisspeptin excitation of septal vesicular glutamate transporter 2 (vGluT2)-GnRH neurons by inhibiting Kir [55], which could be a mechanism by which GnRH neuronal excitability is reduced during certain physiological states.
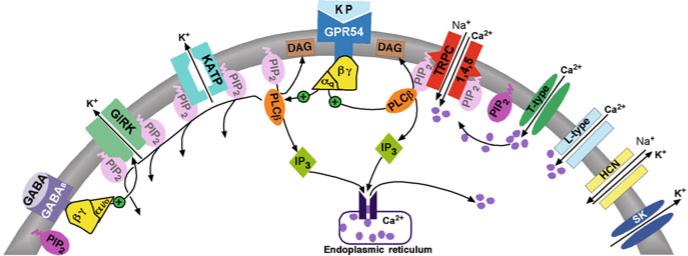
Fig. 6.4.
Model of kisspeptin's actions to depolarize GnRH neurons and facilitate burst firing. Kisspeptin binds to its cognate GPR54 receptor, which is Gq-coupled to activate phospholipase Cβ. PLCβ has multiple downstream actions resultant from cleaving phosphatidylinositol 4,5 bisphosphate (PIP 2 ) to inositol 1,4,5-triphosphate (IP 3 ) and diacylglycerol (DAG). Since PIP 2 facilitates Kir channel opening, cleavage of this fatty acid attenuates K-ATP and GIRK channel opening. Inhibition of GIRK channels renders a number of Gαi/o -coupled receptors ineffective to inhibit GnRH neurons (GABA B, μ-opioid, NPY, MCH, etc.). On the other hand, cleavage of PIP 2 facilitates TRPC 4 channel opening [126]. In addition the membrane-associated fatty acid DAG probably activates the TRPC1, 4, 5 channel complex [Note: The OAG (analogue of DAG) was only weakly effective to open the TRPC channels since PIP 2 still exerted a strong inhibition of the TRPC channel complex]. Ca 2+ potentiates the agonist-activated TRPC1, 4, 5 complex, and plasma membrane calcium channels appear to play a critical role. Intracellular 2-APB dialysis, which effectively blocks IP3 receptor-mediated release of Ca 2+, was ineffective, but extracellular Cd 2+ potently inhibited TRPC1, 4, 5 channel activity. Therefore, we propose that low voltage-activated T-type calcium channels are initially involved in facilitating TRPC channel opening. Once depolarized, Ca 2+ entry through high voltage-activated Ca 2+ channels can also contribute to facilitating TRPC channel opening. Also illustrated are other channels contributing to burst firing activity such as the hyperpolarization-activated, cyclic nucleotide-gated (HCN, pacemaker) channel, and the small conductance, Ca 2+ -activated K + channel (SK), which is involved in the repolarization of the membrane following a burst of action potentials
Presynaptic Effects of Kisspeptin on GnRH Neuronal Excitability
Based on extracellular recording in GnRH neurons in slices obtained from oil- and E 2 -treated mice, the kisspeptin-induced neuronal firing rate is potentiated in the E 2 -treated females [13, 96]. The E 2 -induced potentiation is reduced when GABA and glutamate inputs to GnRH neurons are blocked, suggesting the involvement of these fast synaptic transmitters in the E 2 effect. In addition, it is well known that kiss-peptin neurons in the AVPV are positively regulated by E 2 and are believed to contribute to positive feedback input to rodent GnRH neurons [15]. Therefore, E 2 may further augment the effects of kisspeptin in vivo via direct action on AVPV kiss-peptin neurons.
The precise localization of the kisspeptin inputs to GnRH neurons has not been identified. The AVPV is a complex nucleus that expresses other neurotransmitters in addition to kisspeptin, such as dopamine, GABA, and glutamate [100, 101]. Although projections from the AVPV to GnRH neurons have been described by a number of investigators, the functional interactions between the AVPV and GnRH neurons are just beginning to be elucidated [102]. Thus, stimulation of the AVPV and recording of responses in GnRH neurons reveals that low stimulation rates (<1 Hz) induce glutamate and GABA synaptic currents in GnRH neurons, whereas higher frequency stimulation (5–10 Hz) induces delayed excitation believed to be kisspeptin mediated since the response is absent in Kiss1r knockout animals and antagonized by the kisspeptin antagonist peptide 318 [102]. Therefore, the AVPV kisspeptin neurons may provide a critical excitatory input to GnRH neurons.
Kisspeptin neurons in the arcuate nucleus are negatively regulated by E 2 and are believed to be involved in negative feedback regulation of GnRH secretion [15, 37]. The mechanism by which arcuate neurons negatively regulate GnRH neurons is not completely understood, but has been proposed to also involve arcuate POMC neurons [40]. Interestingly, kisspeptin-immunoreactive fibers in the arcuate nucleus form close contacts onto POMC neurons, and kisspeptin excites POMC neurons via activation of a nonselective cation (TRPC?) channel and activation of a sodium/ calcium exchanger [103]. β-endorphin positive fibers, presumably from arcuate POMC neurons, are highly expressed in the POA, and β-endorphin synapses, as well as μ-opioid receptor expression, are found specifically on GnRH neurons [104–107]. Therefore, kisspeptin may influence GnRH neurons indirectly via actions on arcuate POMC neurons. While μ-opioid receptor activation would be inhibitory to GnRH neurons [40], recently it has been shown that an agonist of the melanocortin receptors 3 and 4 excite GnRH neurons [108]. This would suggest that POMC neurons may also excite GnRH neurons via release of αMSH, a POMC product. Clearly, further studies are needed to elucidate the role of arcuate kiss-peptin neurons in negative feedback regulation of GnRH neurons, as well as the role of these neurons in GnRH neuronal pulsatility.
Kisspeptin and Burst Firing in GnRH Neurons
It is well known that GnRH is released in a pulsatile manner, and the hypothalamic surge of GnRH and subsequent pituitary release of LH are required for triggering ovulation in the female. Although single action potential-induced calcium influx is enough to spark the release of classical transmitters, burst firing or tetanic stimulation is required for the release of neuropeptides such as vasopressin, oxytocin, substance P, and atrial natriuretic factor [109–111]. Experiments in vitro using perifused hypothalamic tissue, primary hypothalamic cultures, or a GT1 GnRH neuronal cell line have revealed that pulsatile GnRH release is evident in vitro [112–114]. Recordings in slices from genetically modified mice that express the calcium ratio-metric indicator Pericam in GnRH neurons have shown that intracellular calcium transients, generated through L-type calcium channels and amplified by calcium release from intracellular stores, are synchronized with burst firing in a subpopulation of GnRH neurons [115]. However, a recent publication suggests that kisspeptin inhibits high voltage-activated (HVA) Ca 2+ (e.g., L-type) channels, which would attenuate the calcium-activated afterhyperpolarization and thereby promote sustained firing [116]. Regardless of the role of the HVA Ca 2+ channels in kisspeptin's downstream signaling, T-type calcium channels and hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels and their respective currents are highly expressed in GnRH neurons [117–120]. Both the h-current and T-type calcium current contribute to burst firing [118, 121], and activation of these vital conductances is dependent on membrane (hyper) polarization [121, 122]. In fact, a hyperpolarizing stimulus removes the inactivation of T-type calcium channels and also activates the h-current. Both the GABA (via GABA B ) and opioids (via μ-opioid receptors) provide this hyperpolarizing stimulus to GnRH neurons [40, 54, 84]. The membrane hyperpolarization generated by Gα i/o -coupled receptors during E 2 negative feedback sets the stage for recruiting both the HCN and T-type calcium channels that are critical for phasic burst firing of GnRH neurons [40, 45, 121]. Ultimately, kisspeptin attenuates the hyperpolarized state of “negative feedback” by inhibiting K + channel activity and opening up TRPC channels to cause sustained depolarization and firing [13, 14, 50, 52]. Furthermore, the calcium-activated afterhyperpolarizing currents (e.g., small conductance, calcium-activated K +, SK) would serve to repolarize the cell membrane to allow the continued oscillation and burst firing [115, 123–125].
Summary
It is clear that kisspeptin has pronounced pre- and postsynaptic effects on GnRH neuronal excitability. Presynaptically, kisspeptin increases the excitatory drive (both GABA A and glutamate) to GnRH neurons, and postsynaptically kisspeptin binds to Kiss1R to activate a PLCβ signaling pathway that has multiple downstream effects to cause a robust and sustained depolarization of GnRH neurons. These downstream effects include inhibition of inwardly rectifying K + (K ATP and GIRK) channels and activation of TRPC1, 4, 5 channels (Fig. 6.4). Although all of the intermediary players (signaling molecules) have not been identified, it is clear that the sustained action potential firing in GnRH neurons is due to these membrane circumscribed actions of the Gαq-signaling pathway. In addition, T-type calcium channels are probably involved in the initial facilitation of TRPC channel opening (Fig. 6.4). However, future experiments need to address these nuances of Kiss1R signaling. In addition, the effects of kisspeptin on the presynaptic glutamatergic and GABAergic neurons also need to be elucidated. Regardless, it is clear that the highly potent neuromodulator, kisspeptin, robustly depolarizes GnRH neurons and promotes burst firing via multiple cellular actions.
Acknowledgments
The authors thank current and past members of their laboratories who contributed to the work described herein, especially Drs. Chunguang Zhang, Jian Qiu, Yuan Fang, Troy Roepke, and Ms. Martha A. Bosch; also, special thanks to Ms. Martha A. Bosch for her skilled assistance with the illustrations and manuscript preparations. The work from the authors’ laboratories was supported by NIH grants NS43330, NS38809, DK68098.
References
- 1.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 2.Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 3.Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein- coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 4.Kuohung W, Kaiser UB. GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Rev Endocr Metab Disord. 2006;7:257–263. doi: 10.1007/s11154-006-9020-2. [DOI] [PubMed] [Google Scholar]
- 5.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K-I. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 6.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden J-M, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kiss-peptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 8.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuronendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 9.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K-I. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 11.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 16.Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of Kiss-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 17.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the fore-brain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57:288–298. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Endocrinol. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- 21.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat: alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 22.Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- 23.Ma YJ, Kelly MJ, Rønnekleiv OK. Pro-gonadotropin-releasing hormone (ProGnRH) and GnRH Content in the preoptic area and the basal hypothalamus of anterior medial preoptic nucleus/suprachiasmatic nucleus-lesioned persistent estrous rats. Endocrinology. 1990;127:2654–2664. doi: 10.1210/endo-127-6-2654. [DOI] [PubMed] [Google Scholar]
- 24.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 25.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 26.Terasawa E, Wiegand SJ. Effects of hypothalamic deafferentation on ovulation and estrous cyclicity in the female guinea pig. Neuroendocrinology. 1978;26:229–248. doi: 10.1159/000122830. [DOI] [PubMed] [Google Scholar]
- 27.King JC, Ronsheim PM, Liu E, Powers L, Slonimski M, Rubin BS. Fos expression in luteinizing hormone-releasing hormone neurons of guinea pigs, with knife cuts separating the preoptic area and the hypothalamus, demonstrating luteinizing hormone surges. Biol Reprod. 1998;58:323–329. doi: 10.1095/biolreprod58.2.323. [DOI] [PubMed] [Google Scholar]
- 28.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139:1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- 29.Plant TM, Krey LC, Moosy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female Rhesus monkey (Macaca mulatta). Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 30.Weick RF. Induction of the luteinizing hormone surge by intrahypothalamic application of estrogen in the rhesus monkey. Biol Reprod. 1981;24:415–422. doi: 10.1095/biolreprod24.2.415. [DOI] [PubMed] [Google Scholar]
- 31.Smith JT, Pereira LA, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Neuroendocrinology. 2009;150:5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 32.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JT, Shahab M, Pereira A, Pau K-YF, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83:568–577. doi: 10.1095/biolreprod.110.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: effects of 17β-estradiol. J Comp Neurol. 2012;520:2143–2162. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of Fos and in Vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Neuroendocrinology. 2011;152:214–222. doi: 10.1210/en.2010-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X-F, Kinsey-Jones JS, Cheng Y, Knox AMI, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Limmar RP, O'Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4:1–9. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 39.Lehman MN, Coolen LM, Goodman RL. Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 41.Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-couple receptor. Cancer Res. 2002;62:5399–5404. [PubMed] [Google Scholar]
- 42.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 43.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22:2313–2322. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–2891. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 47.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–1466. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- 48.Suter KJ. Control of firing by small (S)-a-amino-3-hydroxy-5methyl-isoxazolepropionic acid-like inputs in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Neuroscience. 2004;128:443–450. doi: 10.1016/j.neuroscience.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 49.Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010;1364:35–43. doi: 10.1016/j.brainres.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone (GnRH) neurons through a phosphilipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumalska I, Wu M, Morozova E, Liu R, Van den Pol AN, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pielecka-Fortuna J, DeFazio RA, Moenter SM. Voltage-gated potassium currents are targets of diurnal changes in estradiol feedback regulation and kisspeptin action on gonadotropin-releasing hormone neurons in mice. Biol Reprod. 2011;85:987–995. doi: 10.1095/biolreprod.111.093492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express KATP channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. GABAB receptor mediated inhibition of GnRH neurons is suppressed by kisspeptin-GPR54 signaling. Endocrinology. 2009;150:2388–2394. doi: 10.1210/en.2008-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu M, Dumalska I, Morozova E, Van den Pol AN, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106:17217–17222. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 57.Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150:1400–1412. doi: 10.1210/en.2008-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroll H, Bolsover S, Hsu J, Kim S-H, Bouloux P-M. Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin-releasing hormone neurones. Neuroendocrinology. 2011;93:114–120. doi: 10.1159/000321678. [DOI] [PubMed] [Google Scholar]
- 59.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 60.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 62.Clapham D, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 63.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 64.Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 65.Trebak M, Vazquez G, Bird GS, Putney JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 66.Berg AP, Sen N, Bayliss DA. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27:8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faber ESL, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience. 2006;137:781–794. doi: 10.1016/j.neuroscience.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 68.Meis S, Munsch T, Sosulina L, Pape H-C. Postsynaptic mechanisms underlying responsiveness of amygdloid neurons to cholecystrokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci. 2007;35:356–367. doi: 10.1016/j.mcn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 69.Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152:1503–1514. doi: 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 72.Castellano JM, Navarro VM, Fernández-Fernández R, Castaño JP, Malagón MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol. 2006;257–258:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Ambudkar IS, Ong HL. Organization and function of TRPC channelsomes. Pflügers Arch Eur J Physiol. 2007;455:187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- 74.Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist- activated TRPC5 channels. J Gen Physiol. 2009;133:525–546. doi: 10.1085/jgp.200810153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clapham DE. Snapshot: mammalian TRP channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 76.Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 77.Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- 78.Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M. Activation of A-type gamma-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol. 2008;20:566–575. doi: 10.1111/j.1365-2826.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe M, Sakuma Y, Kato M. GABA receptors mediate excitation in adult rat GnRH neurons. Biol Reprod. 2009;81:327–332. doi: 10.1095/biolreprod.108.074583. [DOI] [PubMed] [Google Scholar]
- 80.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABAA receptor activation on gonadotrophin-releasing hormone neurons: towards an emerging consensus. J Neuroendocrinol. 2011;23:557–569. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pape JP, Skynner MJ, Sim JA, Herbison AE. Profiling gamma-aminobutyric acid (GABAA) receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology. 2001;74:300–308. doi: 10.1159/000054697. [DOI] [PubMed] [Google Scholar]
- 82.Todman MG, Han S-K, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 83.Sliwowska JH, Billings HJ, Goodman RL, Lehman MN. Immunocytochemical colocalization of GABAB receptor subunits in gonadotropin-releasing hormone neurons of the sheep. Neuroscience. 2006;141:311–319. doi: 10.1016/j.neuroscience.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Herbison AE. Estrous cycle- and sex-dependent changes in pre- and postsynaptic GABAB control of GnRH neuron excitability. Endocrinology. 2011;152:1–9. doi: 10.1210/en.2011-1369. [DOI] [PubMed] [Google Scholar]
- 85.Kelly MJ, Loose MD, Rønnekleiv OK. Estrogen suppresses m-opioid and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lagrange AH, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- 87.Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci. 2001;21:2085–2093. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stern JE, Li Y, Richards DS. Postsynaptic GABA(B) receptors in supraoptic oxytocin and vasopressin neurons. Prog Brain Res. 2002;139:121–125. doi: 10.1016/s0079-6123(02)39012-5. [DOI] [PubMed] [Google Scholar]
- 89.Slugg RM, Zheng SX, Fang Y, Kelly MJ, Rønnekleiv OK. Baclofen inhibits guinea pig magnocellular neurones via activation of an inwardly-rectifying K+ conductance. J Physiol (Lond) 2003;551:295–308. doi: 10.1113/jphysiol.2003.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kelly MJ, Rønnekleiv OK. Electrophysiological analysis of neuroendocrine neuronal activity in hypothalamic slices. In: Levine JE, editor. Methods in neurosciences: pulsatility in neuroendocrine systems. Academic; San Diego: 1994. pp. 47–67. [Google Scholar]
- 92.Lagrange AH, Rønnekleiv OK, Kelly MJ. The potency of μ-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17β-estradiol. J Neurosci. 1994;14:6196–6204. doi: 10.1523/JNEUROSCI.14-10-06196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44:321–326. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 94.Jackson GL, Kuehl D. Gamma-aminobutyric acid (GABA) regulation of GnRH secretion in sheep. Reproduction. 2002;59:15–24. [PubMed] [Google Scholar]
- 95.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pielecka-Fortuna J, Moenter SM. Kisspeptin increases γ-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Neuroendocrinology. 2010;151:291–300. doi: 10.1210/en.2009-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Logothetis DE, Zhang H. Gating of G protein-sensitive inwardly rectifying K+ channels through phosphatidylinositol 4,5-bisphosphate. J Physiol. 1999;520:630. doi: 10.1111/j.1469-7793.1999.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nat Cell Biol. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- 99.Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharm. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- 100.Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- 101.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Porteous R, d'Anglemont de Tassigny X, Colledge WH, Millar R, Petersen SL, Herbison AE. Frequency-dependent recruitment of fast amino acid and slow neuro-peptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31:2421–2430. doi: 10.1523/JNEUROSCI.5759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu L-Y, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449:167–176. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]
- 105.Chen W-P, Witkin JW, Silverman AJ. β-endorphin and gonadotropin-releasing hormone synaptic input to gonadotropin-releasing hormone neurosecretory cells in the male rat. J Comp Neurol. 1989;286:85–95. doi: 10.1002/cne.902860106. [DOI] [PubMed] [Google Scholar]
- 106.Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- 107.Zheng SX, Bosch MA, Rønnekleiv OK. Mu-opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol. 2005;487:332–344. doi: 10.1002/cne.20557. [DOI] [PubMed] [Google Scholar]
- 108.Israel DD, Sheffer-Babila S, de Luca C, Jo Y-H, Lui SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC., Jr Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153:1–11. doi: 10.1210/en.2011-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- 110.Masterson SP, Li J, Bickford ME. Frequency-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus. J Neurophysiol. 2010;104:1758–1767. doi: 10.1152/jn.00010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- 112.Kelly MJ, Condon TP, Levine JE, Rønnekleiv OK. Combined electrophysiological, immunocytochemical and peptide release measurements in the hypothalamic slice. Brain Res. 1985;345:264–270. doi: 10.1016/0006-8993(85)91002-9. [DOI] [PubMed] [Google Scholar]
- 113.Krsmanovic LZ, Stojikovic SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci USA. 1992;89:8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Constantin S, Caraty A, Wray S, Duittoz AH. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology. 2009;150:3221–3227. doi: 10.1210/en.2008-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:6214–6224. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X-B, Spergel DJ. Kisspeptin inhibits high-voltage activated Ca2+ channels in GnRH neurons via multiple Ca2+ influx and release pathways. Neuroendocrinology. 2012;96:68–80. doi: 10.1159/000335985. [DOI] [PubMed] [Google Scholar]
- 117.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144:5118–5125. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 118.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormones (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30:13373–13383. doi: 10.1523/JNEUROSCI.1687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons subtype-specific regulation by 17beta-estradiol. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2012.12.021. DOI 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends Endocrinol Metab. 2002;13:409–410. doi: 10.1016/s1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- 122.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 123.Bosch MA, Kelly MJ, Rønnekleiv OK. Distribution, neuronal co-localization and 17β-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology. 2002;143:1097–1107. doi: 10.1210/endo.143.3.8708. [DOI] [PubMed] [Google Scholar]
- 124.Kato M, Tanaka N, Usui S, Sakuma Y. SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones. J Physiol. 2006;574(2):431–442. doi: 10.1113/jphysiol.2006.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149:3598–3604. doi: 10.1210/en.2007-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Otsuguro K-I, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2008;283:10026–10036. doi: 10.1074/jbc.M707306200. [DOI] [PMC free article] [PubMed] [Google Scholar]