SUMMARY
Chitin, a polysaccharide constituent of many allergens and parasites, initiates innate type 2 lung inflammation through incompletely defined pathways. We show that inhaled chitin induced expression of three epithelial cytokines, interleukin-25 (IL-25), IL-33 and thymic stromal lymphopoietin (TSLP), which non-redundantly activated resident innate lymphoid type 2 cells (ILC2) to express IL-5 and IL-13 necessary for accumulation of eosinophils and alternatively activated macrophages (AAMs). In the absence of all three epithelial cytokines, ILC2 normally populated the lung but failed to increase IL-5 and IL-13. Although eosinophils and AAMs were attenuated, neutrophil influx remained normal without these epithelial cytokines. Genetic ablation of ILC2, however, enhanced IL-1β, TNFα and IL-23 expression, increased activation of IL-17A-producing γδ T cells, and prolonged neutrophil influx. Thus, chitin elicited patterns of innate cytokines that targeted distinct populations of resident lymphoid cells, revealing divergent but interacting pathways underlying the tissue accumulation of specific types of inflammatory myeloid cells.
INTRODUCTION
Chitin, a polymer of β-1,4-N-actetylglucosamine and a widespread polysaccharide constituent of arthropods, parasites and fungi, is among the few molecular agents described to induce innate type 2 reactions accompanied by eosinophils and alternatively activated macrophages (AAMs; Reese et al., 2007). In fungi, chitin complexes with β-glucans, galactomannans and mannoproteins to form the hyphal cell wall (Fontaine et al., 2000), and in house dust mites and other insects chitin comprises both the exoskeleton and peritrophic matrix, forming a lattice with mucins and proteins containing chitin-binding peritrophin domains such as the allergen Der p 23 (Hegedus et al., 2009; Weghofer et al., 2013). The response to chitin is characterized by recombinase activating gene (RAG)-independent tissue accumulation of eosinophils, which is attenuated by the STAT6-inducible mammalian chitinase, AMCase (Reese et al., 2007; Van Dyken et al., 2011). Chitin also leads to Arg1 expression in mouse macrophages, a marker for IL-4- and IL-13-activated AAMs, which are associated with eosinophils in a variety of physiologic settings (Van Dyken and Locksley, 2013). The pathways underlying the induction of these cell types by chitin, however, remain incompletely defined.
Group 2 innate lymphoid cells (ILC2) are systemically dispersed tissue cells in humans and mice, and produce IL-5 and IL-13 in response to epithelial-associated cytokines such as thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 (reviewed in Walker et al., 2013). Exogenous administration or transgenic expression of any of these epithelial cytokines results in exaggerated type 2 immune pathology in the lung, suggesting roles in the initiation of allergic immunity (Fort et al., 2001; Schmitz et al., 2005; Zhou et al., 2005). Lung inflammation can be induced by the plant-derived proteinase papain by an unknown mechanism, but is attenuated in broadly immunodeficient mice that lack ILC2 (Halim et al., 2012). It remains to be established whether active papain resembles fungal-derived proteinases, which generate fibrinogen cleavage products to activate Toll-like receptor 4 (TLR4) in the airways (Millien et al., 2013). Nevertheless, chitin mediates eosinophilia in a TLR4-independent manner (Reese et al., 2007), and the relative contributions of IL-25, IL-33 and TSLP to ILC2 function in vivo remain unresolved. Here, we dissect the network of cytokines produced in response to chitin, a natural constituent of inhaled allergens, to reveal a non-redundant role for these three epithelial cytokines in activation of resident ILC2 to produce cytokines mediating eosinophil and AAM accumulation. Unexpectedly, chitin also generated a separate suite of cytokines implicated in the activation of resident γδ T cells that produce IL-17A and mediate neutrophil accumulation. Genetic ablation of ILC2 resulted in enhanced γδ T cell activation and prolonged neutrophil recruitment to tissues, revealing a previously unappreciated interaction between innate lymphoid cell activation and the control of specific types of infiltrating myeloid effector cells.
RESULTS
Chitin induces focal clustering of type 2 innate immune cells in lung tissue
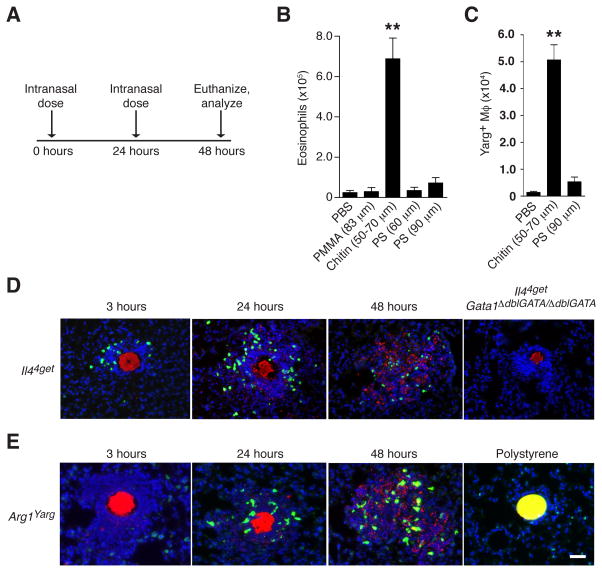
Chitin particles induce innate inflammatory responses in lung tissue characterized by eosinophils and Arg1-expressing AAMs (Reese et al., 2007); however, differences in particle size, concentration and source material impart variability to these responses (Da Silva et al., 2009). To dissect these mechanistic components, we size-fractionated endotoxin-free pure chitin beads to a range of 50–70 μm and administered a standard intranasal dose daily for 2 days before analysis the following day (Figure 1A). Treatment with chitin, but not similarly sized beads of synthetic polymers such as polystyrene (PS) or polymethylmethacrylate (PMMA), resulted in robust accumulation of lung eosinophils and Arg1-reporter (Yarg) positive AAMs (Figures 1B and 1C; Figure S1A), supporting earlier findings (Reese et al., 2007) and confirming specificity of the response to the polysaccharide chitin. Fluorescently labeled chitin was visualized in situ 1 hr after instillation using 2-photon live lung slice imaging (Thornton et al., 2012) and immunohistology, but no chitin-associated inflammation was apparent at this time point (Movie S1). Within 2–3 hrs, however, chitin beads were surrounded by focal inflammatory cell clusters that consisted of numerous motile c-fms+ myeloid cells (Movie S2) and cells that appeared to be eosinophils, as they expressed the Il44get/4get reporter allele (Mohrs et al., 2001) and were absent in eosinophil-deficient Il44get/4getGata1ΔdblGATA/ΔdblGATA mutant mice, which lack eosinophils due to a mutation in the high-affinity, palindromic “double” GATA protein binding site of the Gata1 promoter (Figure 1D; Yu et al., 2002). Subsequently, 12–24 hrs after chitin but not PS administration, Arg1-expressing AAMs were also present in inflammatory foci coincident with degradation of the chitin particles (Figure 1E; Movies S3–S5).
FIGURE 1. Chitin induces focal clustering of type 2 innate immune cells.
(A) Intranasal chitin dosing regimen. (B) Total lung eosinophils and (C) Arg1+ macrophages from Yarg reporter mice (Arg1Yarg) after intranasal administration of equivalent numbers of indicated bead types. PS=polystyrene; PMMA=polymethylmethacrylate. Total live cell subset numbers were calculated from cell counts and flow cytometric percentages as described in Figure S1. (D) Lung histology from Il44get reporter and Il44getGata1ΔdblGATA/ΔdblGATA mice at indicated time points after intranasal chitin administration. Red=rhodamine-conjugated chitin-binding probe; Green=4get+ cells; Blue=DAPI+ cell nuclei. (E) Localization of Arg1+ cells (green) within chitin inflammatory clusters (red) in lung sections from Yarg reporter mice at indicated time points. Far right panel, yellow=PS bead (90 μm). Data are representative of at least two independent experiments, and results from similar treatment groups were pooled in (B) and (C) to represent mean±SEM, n=4/group; **p<0.0001 (unpaired t-test), compared to PBS-treated control. Scale bar=30 μm. See also Figure S1 and Movies S1–S5.
Type 2 cytokine production contributes to AAM and eosinophil accumulation
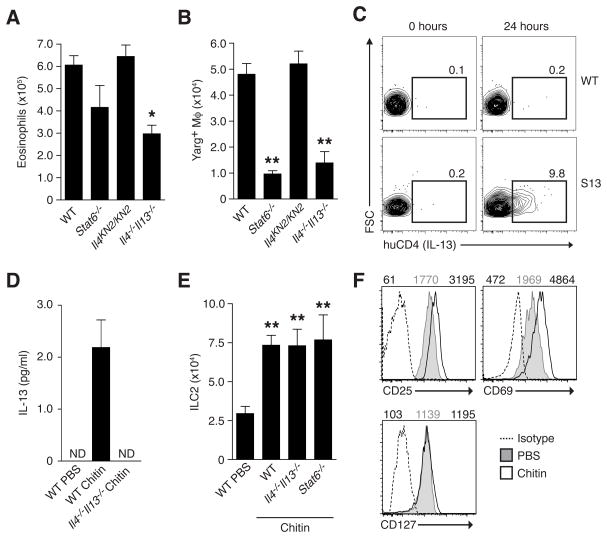
Eosinophils and AAMs are hallmarks of polarized type 2 tissue responses and their rapid recruitment to chitin inflammatory clusters suggested an innate contribution of the canonical type 2 cytokines IL-4, IL-5 and IL-13, which control tissue maintenance of these cells in concert with chemokines and lipid mediators (Reese et al., 2007; Rothenberg and Hogan, 2006; Van Dyken and Locksley, 2013). We tested the specific involvement of IL-4 and IL-13 by administering chitin to mice lacking IL-4 (homozygous Il4KN2/KN2 reporter mice, which contain a gene encoding a non-signaling human CD2 reporter element inserted at the Il4 start site thus preventing endogenous Il4 gene expression; Mohrs et al., 2005), both IL-4 and IL-13 (Il4−/−Il13−/−), or the shared signaling component STAT6 (Stat6−/−). The genetic absence of IL-4 alone did not affect eosinophil or AAM numbers, and we found no expression of the IL-4 KN2 reporter after chitin administration (data not shown). In contrast, we observed decreased numbers of lung eosinophils and AAMs in the combined absence of IL-4 and IL-13, implicating an IL-13-mediated contribution (Figures 2A and 2B). Chitin administration also led to a increase in lung expression of eotaxin-1 (CCL11), a chemokine previously linked to eosinophil recruitment via IL-13 activity, and mice lacking the eotaxin-1 receptor CCR3 exhibited a decreased eosinophil response (Figure S2). Although the absence of the transcription factor STAT6 did not impact eosinophil numbers, in agreement with prior findings (Reese et al., 2007), STAT6 was required for AAM induction (Figure 2B), suggesting that IL-13 signaling via STAT6 increases Arg1 expression in macrophages but also mediates STAT6-independent effects on eosinophil accumulation.
FIGURE 2. Induction of IL-13 from ILC2 in response to chitin contributes to AAM and eosinophil accumulation.
(A) Total eosinophil numbers and (B) Arg1+ macrophages in lungs of indicated mice (on a Yarg reporter background) treated with chitin as described in Figure 1. (C) Expression of IL-13 (huCD4) on lung ILC2 from wild-type (WT) and Il13Smart reporter (S13) mice 24 hours after intranasal chitin challenge. Number indicates percentage of ILC2 (Lin-CD25+Thy1+) positive for huCD4 marker. (D) IL-13 protein levels in culture supernatant from sorted ILC2 after in vivo PBS or chitin treatment. ND=none detected. (E) Total ILC2 numbers in the lungs of indicated mice and (F) cell surface expression of indicated markers among ILC2 48 hours after PBS or chitin treatment. MFI values are indicated above histograms for isotype (left), PBS- (middle), or chitin-treated populations (right). Flow cytometry results shown in (C) and (F) are representative of 3 independent experiments, and (A), (B), (D), and (E) represent mean±SEM, n=4–6/group; *p<0.001; **p<0.0001 (unpaired t-test), compared to PBS-treated control. See also Figures S2 and S3.
To identify cellular sources of IL-13 in lung tissue after chitin exposure, we used Il13Smart/Smart mice, whose cells contain a non-signaling huCD4 surface marker downstream of an IRES inserted in the 3′ region of the endogenous Il13 gene, thus sustaining normal expression (Liang et al., 2011). Lung cells from unchallenged mice expressed no detectable IL-13 reporter activity. After chitin, ILC2, but no other immune cells, were robustly positive for the huCD4 IL-13 reporter, consistent with their expression of IL-13 in situ (Figure 2C; Figure S3). IL-13 protein was also present in culture supernatants from ILC2 sorted from the lungs after chitin (Figure 2D); IL-13 was not detected in serum (data not shown). Increased IL-13 production by ILC2 correlated with their increased recovery that occurred even in the absence of IL-4/IL-13 or STAT6 (Figure 2E) and coincided with elevated expression of CD25 and CD69 (Figure 2F). Thus, chitin activates ILC2 to produce local IL-13 that contributes to the in vivo accumulation of eosinophils and AAMs.
IL-5 expression identifies lung ILC2 in vivo and mediates eosinophil, but not AAM, accumulation in response to chitin
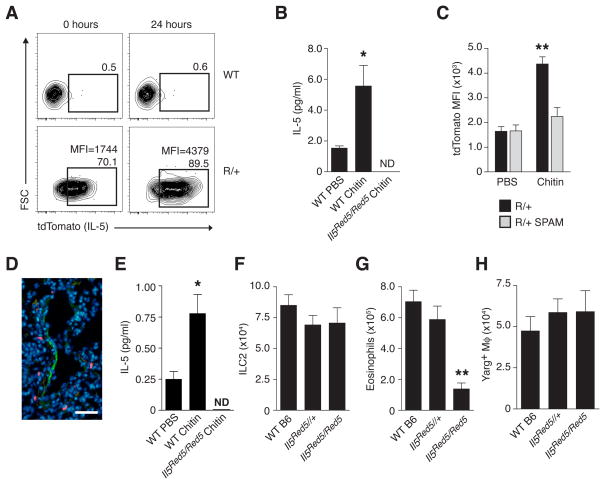
The activation of IL-13 expression by lung ILC2 in response to chitin led us to examine the involvement of IL-5, an ILC2-associated type 2 cytokine critical for eosinophilia in the context of allergen exposure and helminth infection. Using IL-5 reporter mice that express RFP (tdTomato) and Cre recombinase from the Il5 start site (Il5Red5/Red5; Molofsky et al., 2013), we noted that many lung ILC2 were RFP+ under resting conditions. After chitin administration, however, IL-5 expression increased (Figures 3A and 3B). Similar to IL-13, IL-5 expression was restricted to ILC2 and no other cells (Figure S4). We tested whether chitin-induced IL-5 expression by ILC2 could be modulated by acidic mammalian chitinase (AMCase), a STAT6-dependent enzyme involved in chitin degradation (Reese et al., 2007; Van Dyken et al., 2011). When administered to mice that expressed enhanced lung levels of AMCase (SPAM mice; Reese et al., 2007), chitin no longer induced IL-5 reporter expression in lung ILC2 (Figure 3C), thus genetically positioning intact chitin upstream of ILC2 activation and suggesting that AMCase induction limits the duration of the inflammatory response.
FIGURE 3. Chitin administration increases IL-5 expression among lung ILC2 in vivo and mediates eosinophil, but not AAM, accumulation.
(A) Expression of IL-5 (tdTomato) among lung ILC2 from wild-type (WT) and heterozygous Il5Red5/+ reporter (R/+) mice before and 24 hours after intranasal chitin challenge. Number indicates percentage of ILC2 positive for Red5 reporter, and mean fluorescence intensity (MFI) values, where indicated, represent entire ILC2 population. (B) IL-5 protein levels in culture supernatant from sorted ILC2 after in vivo PBS or chitin treatment. (C) Flow cytometric analysis of IL-5 (tdTomato) MFI among ILC2 populations from wild-type or SPAM transgenic mice on a heterozygous Il5Red5/+ (R/+) background 24 hours after in vivo PBS or chitin treatment. (D) Lung micrograph from chitin-challenged Il5Red5/Red5 mouse co-stained with VCAM-1 and DAPI. Green=VCAM-1; Blue=DAPI+ cell nuclei; Red=IL-5 (Red5)+ ILC2. (E) Serum IL-5 levels after in vivo PBS or chitin treatment in WT or homozygous Red5 (Il5Red5/Red5) mice. Total numbers of ILC2 (F), eosinophils (G), and Arg1+ macrophages (H) in lungs of indicated mice (on a Yarg reporter background) after chitin treatment as described in Figure 1. Results shown in (A) and (D) are representative of 3 independent experiments, and (B, C), and (E–H) represent mean±SEM, n=4–6/group; *p<0.001; **p<0.0001 (unpaired t-test), compared to PBS-treated (B, C, E) or WT control (F–H). ND=none detected. Scale bar=30 μm. See also Figures S4, S5 and Movies S6–S9.
Lung imaging during chitin challenge revealed IL-5-expressing ILC2 in close proximity with medium-to-large blood vessels containing VCAM1+ endothelial cells (Figure 3D) and with airways in which collagen was evident by second harmonic generation using 2-photon microscopy (Figure S5, Movies S6–S9). Consistent with the association between IL-5-expressing ILC2 and the vasculature, we observed increased serum IL-5 levels after chitin (Figure 3E), supporting a dual role for ILC2 in mediating both local and systemic effects on eosinophils. Indeed, IL-5 was critical for eosinophil accumulation after chitin challenge, as mice lacking IL-5 (homozygous Il5Red5/Red5) had normal numbers of lung ILC2 but diminished numbers of eosinophils after chitin challenge (Figures 3F and 3G). In contrast, IL-5 deficiency had no impact on chitin-induced AAM accumulation, since Il5Red5/Red5 x Arg1Yarg/Yarg mice had wild-type numbers of Arg1-expressing AAMs (Figure 3H), thereby dissociating the effects of IL-5 and eosinophils from that of IL-13 and AAMs. The impairment of eosinophils in the absence of either IL-5 or IL-13 implied that these two ILC2-derived cytokines cooperate to mediate non-redundant aspects of eosinophil production, recruitment and/or retention in response to chitin. Taken together, these results indicated that inhaled chitin provoked local accumulation of both eosinophils and AAMs by stimulating innate production of type 2 cytokines from ILC2, which could be attenuated by increased local chitinase activity.
Genetic deletion of ILC2 abolishes chitin-induced eosinophil and AAM accumulation but enhances innate-like T cell and inflammatory cytokine responses
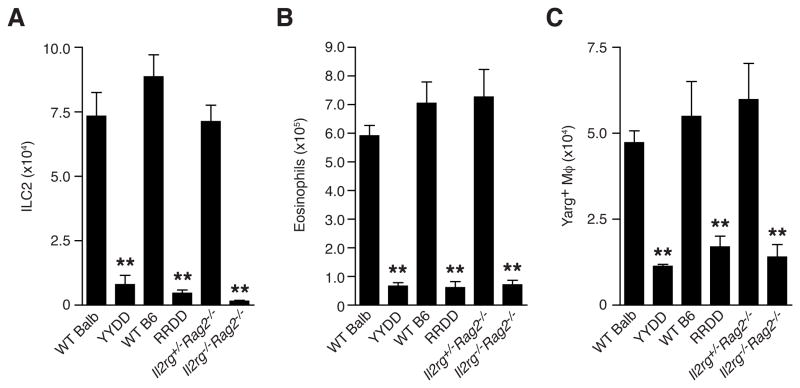
The expression of IL-5 and IL-13 solely by lung ILC2 in response to chitin enabled us to test the role of these cells in eosinophil and AAM accumulation by using cytokine-mediated cell deletion. To this end we crossed IL-5 (Il5Red5/Red5) and IL-13 (Il13YetCre/YetCre) reporter mice with mice bearing the Gt(Rosa)26DTA allele, which mediates cell deletion after IL-5 or IL-13 expression, respectively, via concurrent Cre-mediated activation and cytotoxic expression of endogenous diphtheria toxin α (Molofsky et al., 2013; Price et al., 2010; Voehringer et al., 2008). Consistent with the activation of these reporters in ILC2 after chitin challenge, both IL-5 (RRDD) and IL-13 (YYDD) deleter strains had reductions in lung ILC2 numbers that resembled levels in ILC2-deficient Il2rg−/−Rag2−/− mice after chitin, confirming that cytokine expression in these cells results in their specific elimination (Figure 4A). ILC2 deletion driven by either IL-5 or IL-13 resulted in severely diminished numbers of both eosinophils and AAMs in response to chitin, and resembled the reductions observed in Il2rg−/−Rag2−/− mice as compared to Il2rg+/−Rag2−/− littermate controls (Figures 4B and 4C). Together, these data demonstrate that IL-5/IL-13-producing ILC2 mediate the accumulation of eosinophils and AAMs in response to chitin. Notably, the ILC2 deletion efficiency was comparable in both cytokine deleter strains (Figure 4A), suggesting that a majority of ILC2 express both IL-5 and IL-13 in response to chitin challenge.
FIGURE 4. Genetic deletion of ILC2 ablates both eosinophil and AAM accumulation in lung tissue in response to chitin administration.
(A) Total ILC2, (B) eosinophil and (C) Arg1+ macrophages in lung tissue of indicated mice (on a Yarg reporter background) after treatment with chitin as described in Figure 1. WT=wild-type. YYDD=IL-13 deleter mice (Il13YetCre/YetCreGt(Rosa)26DTA/DTA). RRDD=IL-5 deleter mice (Il5Red5/Red5Gt(Rosa)26DTA/DTA). Data are presented as mean±SEM, n=4–6/group; **p<0.0001 (unpaired t-test), as compared to WT control.
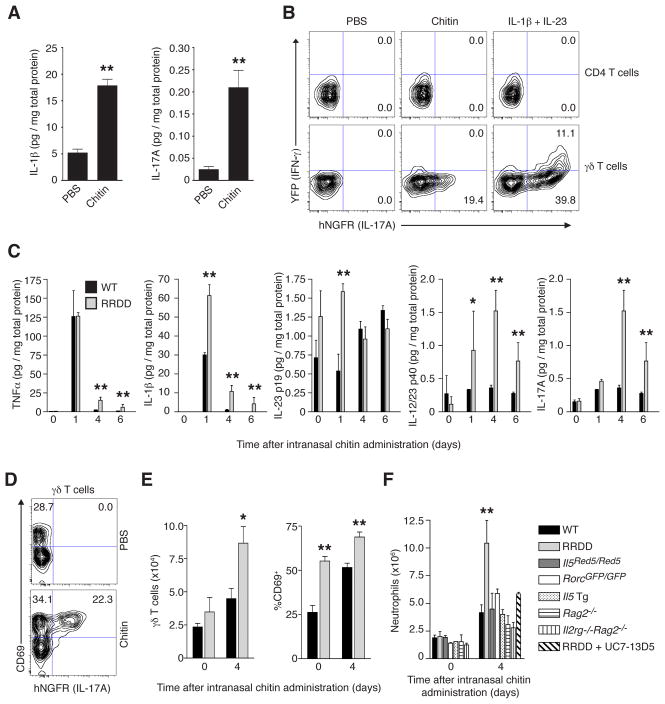
Although eosinophils and AAMs localized to chitin in lung tissue, we also noted the recruitment of additional cells (Figures 1D and 1E), suggesting the induction of additional inflammatory signals. Indeed, the inflammatory cytokines IL-1β and IL-17A were induced in lung tissue within 6 hrs of chitin treatment (Figure 5A). As lung resident innate-like γδ T cells rapidly produce IL-17A in response to IL-1β and/or IL-23 administration, we examined whether these cells were activated during chitin challenge using IL-17A, IFN-γ dual reporter mice (Price et al., 2012). In contrast to CD4+ T cells, a proportion of lung γδ T cells expressed IL-17A, but not IFN-γ, in response to intranasal chitin (Figure 5B).
FIGURE 5. ILC2 mediate suppression of inflammatory cytokine and innate-like T cell responses after chitin stimulation.
(A) IL-1β and IL-17A protein levels in whole lung lysates from wild-type (WT) mice 6 hours after intranasal treatment with PBS or chitin. (B) Expression of IFN-γ (Great; YFP) and IL17A (Smart17; hNGFR) reporters among CD3ε+CD4+ T cells and CD3ε+GL3+ γδ T cells from lungs of IfngGreat/GreatIl17aSmart/Smart dual reporter mice 18 hours after intranasal treatment with PBS, chitin, or IL-1β and IL-23 (10 ng each cytokine). (C) Cytokine levels in whole lung lysates from WT or IL-5 deleter (RRDD; Il5Red5/Red5Gt(Rosa)26DTA/DTA) mice at indicated time points after single intranasal challenge with chitin. (D) Expression of CD69 and IL17A (hNGFR) reporter among CD3ε+GL3+ γδ T cells in the lungs of Il17aSmart/Smart reporter mice 48 hours after single intranasal challenge with PBS (top) or chitin (bottom). (E) Total numbers of CD3ε+GL3+ γδ T cells (left) and percentage expressing CD69 (right), as well as total neutrophil numbers (F) in the lungs of indicated mice before (0) and 4 days after a single intranasal chitin dose. Numbers in each quadrant from flow cytometry plots in (B) and (D) indicate percentage of total T cell subset; data are representative of 3 independent experiments. (A), (C), (E), and (F) represent mean±SEM, n=3–6/group; *p<0.05; **p<0.01 (unpaired t-test), compared to PBS-treated (A) or wild-type control at corresponding time point (C, E, F).
Unexpectedly, ILC2 deletion resulted in altered kinetics and elevated expression of several inflammatory cytokines in lung tissue after chitin challenge, including TNFα, IL-1β, IL-23, and IL-17A (Figure 5C), consistent with a heightened γδ T cell response. In support of this, lung γδ T cells that produced IL-17A in response to chitin co-expressed the activation marker CD69 (Figure 5D), and we detected increased total γδ T cell numbers in the lungs of RRDD mice after chitin challenge along with an elevated CD69+ proportion among these cells (Figure 5E). These alterations were accompanied by increased accumulation of neutrophils in ILC2-deficient RRDD mice as compared to wild-type and IL-5-deficient (Il5Red5/Red5) controls; however, this response was abrogated by treatment of RRDD mice with a γδ TCR antibody (UC7-13D5), resulting in a reduction of neutrophils to levels observed in mice deficient in either T cells (Rag2−/−) or IL-17A (RorcGFP/GFP) (Figure 5F), thereby implicating ILC2 in the suppression of exacerbated inflammatory cytokine signaling and γδ T cell activation in response to chitin. Intriguingly, γδ T cell and neutrophil numbers were unaltered as compared to control animals after chitin treatment in both Il4−/−Il13−/− and Il5 transgenic mice that contain excessive amounts of eosinophils (Figure 5F and data not shown). Thus, enhanced γδ T cell activation and neutrophil accumulation that occurred in the absence of chitin-activated ILC2 was not due to the loss of ILC2 IL-5 and IL-13 production or the absence of eosinophils and AAMs. These results indicated that chitin activated both ILC2 and innate-like γδ T cells to mediate downstream inflammatory cell recruitment but crosstalk between these two cell types, as revealed by specific in vivo deletion of ILC2, was independent of ILC2-derived type 2 cytokines.
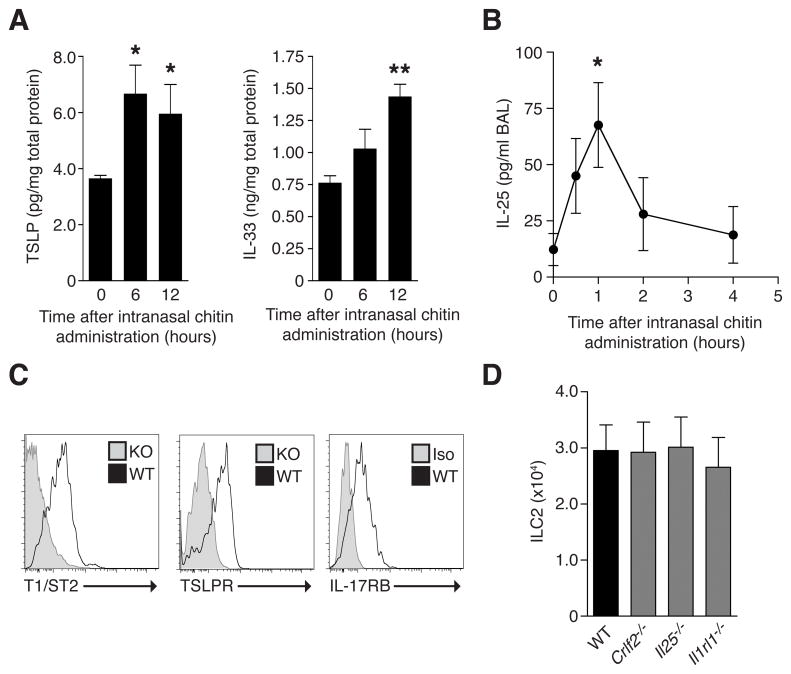
Chitin administration induces lung TSLP, IL-25 and IL-33 expression
The dual activation of innate ILC2 and innate-like γδ T cells by chitin suggested distinct modules of epithelial cytokines could account for the accumulation of different inflammatory myeloid cells. ILC2 produce type 2 cytokines following administration of IL-25, IL-33 and TSLP, which are epithelial-associated cytokines implicated in type 2 immunity (Walker et al., 2013). We assayed production of these cytokines in lung tissue to determine their roles in ILC2 stimulation in response to chitin. We detected increased amounts of lung IL-33 protein after intranasal chitin, in agreement with a previous study (Yasuda et al., 2012), but we also documented induction of TSLP in lung tissue and IL-25 in bronchoalveolar lavage (BAL) during overlapping time periods (Figures 6A and 6B). Lung ILC2 expressed cytokine receptor subunits specific for IL-25, IL-33 and TSLP under resting conditions (Figure 6C), but the steady-state numbers of lung ILC2 were unaltered in mice genetically deficient in TSLPR (Crlf2−/−) IL-25 (Il25−/−) or IL-33R (Il1rl1−/−; Figure 6D). Thus, multiple cytokines with potential ILC2-stimulating activity were produced in response to chitin and lung ILC2 were poised to respond to any or all of these cytokines.
FIGURE 6. Expression of type 2 epithelial cytokines and receptor components in response to chitin stimulation.
(A) IL-33 and TSLP protein levels in whole lung lysate and (B) IL-25 levels in bronchoalveolar lavage (BAL) fluid from wild-type mice at indicated times after intranasal chitin. (C) Cell surface expression of indicated markers on ILC2 from wild-type (WT) mice in comparison with cells from Il1rl1−/− mice (left panel), Crlf2−/− mice (middle), or cells stained with isotype control antibody (Iso; right). (D) Total ILC2 numbers in the lungs of indicated mice. Flow cytometry results shown in (C) are representative of 3 independent experiments, and (A, B, D) represent mean±SEM, n=3–5/group; *p<0.001; **p<0.0001 (unpaired t-test), compared to untreated control.
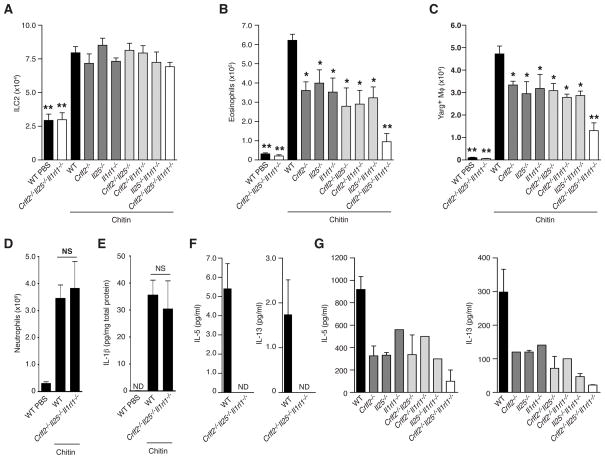
Lung ILC2 are present in the combined absence of TSLPR, IL-25 and IL-33R but fail to produce IL-5 and IL-13 in response to chitin
Combinations of TSLP, IL-25 and IL-33 may act together to regulate ILC2 (Halim et al., 2012; Hardman et al., 2013; Neill et al., 2010), but the in vivo contributions of these cytokines to ILC2 function have not been comprehensively explored. We generated an inbred cohort of mice with single or combined deficiencies in TSLPR, IL-25 and IL-33R to determine their contributions to ILC2 expansion and function during the innate chitin response. In the steady-state, ILC2 developed and populated the lung in normal numbers even in the total absence of TSLPR, IL-25 and IL-33R (Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice; Figure 7A). After chitin administration, however, mice lacking IL-33R showed significantly reduced numbers of lung eosinophils (Figure 7B), corroborating results obtained in IL-33-deficient mice (Yasuda et al., 2012) and this was accompanied by fewer AAMs. Unexpectedly, similar reductions in eosinophils and AAMs occurred in mice deficient in TSLPR or IL-25, as well as in mice with compound deficiencies of any two of these factors. The combined absence of all three factors in Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice, however, resulted in substantial attenuations of eosinophils and AAMs in response to chitin (Figures 7B and 7C). Thus, each of these cytokines has a non-redundant role in the innate response to chitin, yet each can play a compensatory role in the combined absence of the other two pathways.
FIGURE 7. Epithelial cytokines control chitin-induced eosinophil and AAM accumulation via regulation of IL-5 and IL-13 production from ILC2.
(A) Total ILC2, (B) eosinophil and (C) Arg1+ macrophage numbers in the lungs of indicated mice (on a Yarg reporter background) treated with PBS or chitin as described in Figure 1. WT=wild-type. (D) Total neutrophils (CD11b+Ly6G+ Siglec F−; also see Figure S1) in left lung lobe tissue and (E) IL-1β protein levels in lung tissue lysate 24 hours after intranasal treatment with PBS or chitin. (F) IL-5 and IL-13 protein levels in supernatant from sorted lung ILC2 from WT or Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice after in vivo chitin treatment, cultured in the presence of IL-7 or (G) ionomycin/PMA. NS=not statistically significant. ND=none detected. Data are presented as mean±SEM, n=3–5/group; *p<0.001; **p<0.0001 (unpaired t-test), compared to chitin-treated WT control.
Despite downstream effects on eosinophil and AAM accumulation, the absence of these epithelial activating signals alone or in combination had no effect on either the resting numbers of lung ILC2 or their enhanced recovery during the 48 hrs after chitin, including in Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice (Figure 7A). In contrast to the response observed in ILC2 deleter mice (Figure 5C–F), signals involved in the recruitment and maintenance of other inflammatory cell types were unaffected in the Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice, as neutrophil influx (Figure 7D), γδ T cell numbers, and induction of IL-1β and TNFα in the lung tissue and BAL were unaffected (Figure 7E; data not shown). Consistent with the deficiencies in eosinophils and AAMs, ILC2 sorted from chitin-exposed Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice produced no detectable IL-5 and IL-13 as compared with equal numbers of purified wild-type ILC2 (Figure 7F). Intriguingly, even after stimulation with ionomycin + PMA in vitro, ILC2 sorted from the various deficiency settings produced reduced amounts of IL-5 and IL-13 that corresponded to their respective in vivo impairments in mediating eosinophil and AAM accumulation (Figure 7G). Thus, these epithelial cytokines coordinately regulate ILC2 cytokine production and accumulation of innate type 2 myeloid cells in response to chitin through non-redundant yet partially overlapping pathways that are distinct from simultaneously induced mechanisms that mediate the recruitment and maintenance of other inflammatory cell types. In sum, these results indicate that innate and innate-like lymphoid cells operate in parallel and are poised to respond to multiple epithelial inputs that cooperate to influence their activation and function.
DISCUSSION
Our results define innate pathways of ILC2 and innate-like T cell activation induced by the polysaccharide chitin, a widespread environmental constituent derived from arthropods, fungi and helminth parasites. The primary initiators of the ILC2 response in vivo are the epithelial-associated cytokines IL-25, IL-33 and TSLP, which, as we show here, are each induced by chitin and are together required to stimulate ILC2 production of IL-5 and IL-13. The latter two cytokines, in turn, are required for accumulation of canonical innate cells, eosinophils and AAMs, that are associated with allergy. Because the production of these two cytokines is restricted to ILC2 during the chitin response, we utilized genetic markers and function-based deletion to demonstrate that IL-13 mediates AAM accumulation by a STAT6-mediated process, whereas both IL-13 and IL-5 mediate eosinophilia by a partially STAT6-independent process. The comparable effects achieved in the two cytokine-based deletion strains suggest that individually activated ILC2 produce both cytokines in response to chitin and distinguish ILC2 as essential regulators of lung eosinophil and AAM accumulation. Notably, this method of cytokine-mediated depletion of ILC2 achieved highly specific elimination of these cells with minimal in vivo manipulation, revealing unexpected effects on innate-like γδ T cells and indicating utility in future studies of ILC2 function, which heretofore has relied primarily on either non-specific and incomplete antibody-mediated or bone-marrow reconstitution approaches or the use of broadly immunodeficient (e.g., Il2rg−/−Rag2−/−) mouse strains (Halim et al., 2012; Monticelli et al., 2011; Wong et al., 2012; Roediger et al., 2013).
These findings demonstrate rigorously that ILC2 in the lung, and possibly in other organs, function as sentinel cells capable of processing multiple epithelial-derived signals to activate cytokines that mediate inflammatory cell recruitment. Whereas a close relationship between AAMs and eosinophils has been previously reported (Reese et al., 2007; Van Dyken and Locksley, 2013; Voehringer et al., 2007), the involvement of a common upstream regulator of both these cell types in the lung has not been established. Although lung ILC2 have been linked to the eosinophilic response to papain, their in vivo relationship to other innate cells and regulation by epithelial cytokines could not be resolved using lung explant cultures (Halim et al., 2012). Using in situ tissue imaging, our results indicate that lung ILC2 are positioned to relay signals emanating from epithelia to the endothelium, where recruitment, retention and/or survival signals would affect infiltrating inflammatory cells. Red5+ ILC2 were closely associated with vessels expressing VCAM-1, consistent with the role for eosinophil VLA-4 (α4β1 integrin)-mediated recruitment during allergic lung inflammation (Rothenberg and Hogan, 2006).
Although numbers were drastically reduced, we did not observe complete elimination of chitin-induced AAMs and eosinophils even in the ILC2-deficient (Il2rg−/−Rag2−/−) mice. Thus, additional ILC2-independent mechanisms can mediate residual recruitment or retention of these cells, possibly via epithelial production of CCL2 (Roy et al., 2012), macrophage-derived LTB4 (Reese et al., 2007) or by STAT6-independent induction of Arg1 in the case of macrophages (Qualls et al., 2010). Although STAT6 expression has been linked with ILC2 proliferation in response to fungal challenge (Doherty et al., 2012), we found that neither STAT6 nor IL-4 and IL-13 were necessary for the expansion and activation of ILC2 in response to chitin, consistent with earlier findings made during nematode infection (Liang et al., 2011). Taken together, however, these data support a predominant role for ILC2-derived type 2 cytokines in mediating eosinophil and AAM accumulation following chitin exposure. Intriguingly, we observed a similar relationship among ILC2, eosinophils and AAMs in maintaining visceral adipose tissue homeostasis (Molofsky et al., 2013), suggesting that these innate type 2 immune circuits are also engaged in certain tissues under basal conditions. As previously reported (Nussbaum et al., 2013), some resident lung ILC2 spontaneously express IL-5 in the resting state, but were further induced by chitin to express IL-13 and additional IL-5.
We analyzed intercrossed lines of mice that lacked TSLPR, IL-25 or IL-33R alone or in all possible combinations, including Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice. Unexpectedly, each of the three pathways contributed in a non-redundant fashion to the optimal induction of ILC2 cytokines and the infiltration of lung tissues with eosinophils and AAMs in response to chitin. Absence of all three cytokines led to almost complete loss of ILC2 cytokine secretion and innate type 2 inflammatory cell accumulation. This impairment occurred independently of a parallel program mediating the tissue infiltration of other inflammatory cells, since IL-1β production and neutrophil recruitment in response to chitin were normal in Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice. In contrast, these other inflammatory responses were enhanced in the ILC2 deleter mice, suggesting that, although canonical type 2 cytokine signals can inhibit IL-17A-driven neutrophilia and acute lung damage in the context of parasite infection (Chen et al., 2012), activated ILC2 generate factors other than IL-5 and IL-13 that inhibit γδ T cell activation and neutrophil recruitment. ILC2 can produce mediators such as amphiregulin that have been implicated in lung epithelial repair after virus-induced injury (Monticelli et al., 2011), although we could not detect amphiregulin induction nor did exogenous treatment with amphiregulin have inhibitory effects on the enhanced chitin-mediated inflammatory response in ILC2 deleter mice (data not shown).
Although further experiments will be required to assess the cell-intrinsic nature of the epithelial cytokine receptor pathways on ILC2, we document that each of these receptors is expressed on lung ILC2. Synergistic effects of TSLP and IL-33 on cultured ILC2 have been reported (Halim et al., 2012) and combined IL-25 and IL-33 signals contributed to ILC2 expansion during helminth infection (Neill et al., 2010) and in response to fungal extracts (Hardman et al., 2013), but the in vivo absence of all three signaling pathways has not previously been explored. As assessed using this innate lung response, the numbers of ILC2 recovered from tissues before and after chitin were unaffected in Crlf2−/−Il25−/−Il1rl1−/− triple-deficient mice, suggesting that these cytokines are not required for the development of ILC2. ILC2 from triple-deficient mice, however, had impaired cytokine responses to PMA/ionomycin stimulation in vitro. As this challenge bypasses receptor signaling pathways, the possibility remains that terminal differentiation of ILC2 is dependent on epithelial cytokines produced in situ. These findings must be further addressed in light of the possible role of Th2 cells in overcoming the dependence on epithelial cytokines in adaptive type 2 immune responses, since important roles for CD4 T cells in ILC2 expansion and activation have also been described (Wilhelm et al., 2011).
Our data support a model in which an epistatic cascade of signals initiated at the epithelium converges on ILC2, which respond by altering the local microevironment to establish conditions conducive to specific immune cell retention and alleviation of the chitin stimulus through particle degradation. This multi-component signal is mainly comprised of TSLP, IL-25 and IL-33, but additional signals as well as the mechanisms governing the production and release of these cytokines remain under investigation. As such, innate sources aside from epithelial cells could possibly contribute or respond to TSLP, IL-25 and IL-33, including basophils (Kroeger et al., 2009; Sokol et al., 2008), macrophages and inflammatory dendritic cells (Wills-Karp et al., 2012) among others (reviewed in Bartemes and Kita, 2012). In response to chitin stimulation, however, expression of IL-5 or IL-13 was restricted to ILC2, while IL-4, which can be produced by basophils in vivo during helminth infection (Sullivan et al., 2011) and allergic skin inflammation (Egawa et al., 2013), was not required for the accumulation of eosinophils or AAMs. The kinetics of the response, along with the positioning of lung ILC2, is consistent with initiation at the subepithelial barrier, which may be compromised by intact chitin particles. In this respect, IL-33, a nuclear factor that can be biologically active in full-length form and lacks a signal peptide for secretion, has been suggested to act as an “alarmin” released during necrosis (Lüthi et al., 2009), as occurs in response to elevated extracellular ATP (Kouzaki et al., 2011), or by mechanical stress (Kakkar et al., 2012). Subsequent processing may also be relevant, as cleavage events can enhance the cytokine activity of both IL-25 (Goswami et al., 2009) and IL-33 (Lefrançais et al., 2012), while IL-33 is inactivated by pro-apoptotic caspases (Lüthi et al., 2009). Intriguingly, TSLP induces STAT5 and GATA3 in human ILC2 and mediates functional synergism with IL-33 (Mjösberg et al., 2012) in a manner echoing the antigen-independent activation of Th2 cells (Guo et al., 2009), suggesting similar mechanisms may be engaged during innate and adaptive type 2 inflammation. Activation of this innate pathway warrants further studies of its role in mediating allergic inflammatory responses to chitin-containing particles and organisms, particularly in regards to its effects on adaptive Th2 induction and the role of induced chitinase and chi-lectins in terminating the response.
EXPERIMENTAL PROCEDURES
Mice
Mice were maintained under specific pathogen-free conditions and all procedures were approved by the UCSF IACUC. Il1rl1−/− mice (Hoshino et al., 1999) were backcrossed 8 generations to BALB/c (Jackson Laboratories) before intercrossing with Il25−/− (Fallon et al., 2006) and Crlf2−/− mice (Carpino et al., 2004) on an Arg1Yarg/Yarg BALB/c reporter background (Reese et al., 2007) to generate triple-deficient Crlf2−/−Il25−/−Il1rl1−/− Yarg reporter mice, which were analyzed in comparison to wild-type control mice and mice with single and double deficiencies obtained from the same breeding scheme. Additional mice included SPAM (Reese et al., 2007), Il44get/4get (Mohrs et al., 2001), Il4KN2/KN2 (Mohrs et al., 2005), Gt(Rosa)26DTA/DTA, Il13YetCre/YetCre (Price et al., 2010), Il13Smart/Smart (Liang et al., 2011), IfngGreat/GreatIl17aSmart/Smart (Price et al., 2012), Il5Red5/Red5 (Molofsky et al., 2013) and others described in the Supplemental Experimental Procedures.
In vivo treatments
Pure chitin beads (New England Biolabs; NEB) ranging from 50–70 μm in diameter were prepared by size filtration through nylon mesh, washed and reconstituted in sterile PBS (Ca++, Mg++ free) at a final concentration of 105 chitin beads/ml. Mice were briefly anesthetized with isofluorane, and 50 μl of this suspension (5000 beads) was aspirated by intranasal administration, followed by euthanasia and analysis at various time points after instillation. Precision size standard 60 and 90 μm polystyrene beads (Polysciences, Inc.) as well as 83 μm polymethylmethacrylate beads (Bangs Laboratories, Inc.) were prepared and administered identically. For in vivo anti-γδ T cell treatment, purified UC7-13D5 γδ TCR antibody (0.25 mg) was administered to mice intraperitoneally 24 hrs prior to intranasal chitin.
Tissue preparation and ILC2 sorting
Whole lungs were perfused with 20 ml PBS via heart puncture before excising and preparing single-cell suspensions with an automated tissue dissociator (gentleMACS; Miltenyi Biotec), running program lung_01, followed by incubation for 35 min at 37° C in HBSS (Ca++, Mg++ free) containing 0.2 mg/ml Liberase Tm and 25 μg/ml DNase I (Roche), then program lung_02. The tissue was further dispersed by passing through 70-μm nylon filters, washed, and subjected to red blood cell lysis (PharmLyse; BD Biosciences) before final suspension in PBS/2% fetal calf serum. Cells were stained for flow cytometry and cultured as described in the Supplemental Experimental Procedures.
Protein analysis and bronchoalveolar lavage
Lungs were instilled with 1 ml PBS to collect bronchoalveolar lavage (BAL), which was centrifuged and supernatant was concentrated using Amicon Utra-4 filters as recommended (MWCO 10 kDa; EMD Millipore) prior to protein analysis by enzyme-linked immunoassay (ELISA) specific for IL-25 (eBioscience). Whole lung lysates were prepared in M tubes (Miltenyi Biotec) containing TNT lysis buffer and protease inhibitor tablets (Complete; Roche), using an automated tissue dissociator as recommended (gentleMACS; Miltenyi Biotec). Total protein content was determined by BCA (Pierce), and equal protein amounts were assayed for TSLP, IL-33, eotaxin-1, and IL-23p19 by ELISA (R&D Systems). Amounts of IL-5 and IL-13 in ILC2 culture supernatants, as well as TNFα, IL-1β, IL-12/23 p40, and IL-17A levels in total lung lysates were quantified by cytometric bead array (CBA) using an LSRII flow cytometer and FCAP Array analysis software (BD Biosciences).
Immunohistology
Lungs were fixed in 4% paraformaldehyde, immersed in 30% sucrose, then embedded in OCT compound (Sakura Finetek) prior to frozen sectioning at 6 μm using a Leica CM 3050S cryomicrotome (Leica Microsystems). Amplification and detection of YFP and GFP signals were performed as described (Reese et al., 2007), while tdTomato fluorescence in Il5Red5/Red5 mice was directly visualized without amplification. Chitin was detected using FITC- and rhodamine-conjugated chitin-binding domain probes (NEB; (Van Dyken et al., 2011) and images were acquired with an AxioCam HRm camera and AxioImager M2 upright microscope (Carl Zeiss).
Statistical analysis
Statistical analysis was performed as indicated in figure legends using Prism software (GraphPad, Inc.).
Supplementary Material
HIGHLIGHTS.
Chitin-induced cytokine production mediates distinct inflammatory cell accumulation
Genetic deletion of ILC2 abolishes eosinophil and AAM responses
Innate-like γδ T cell activity and neutrophilia is enhanced in the absence of ILC2
ILC2 develop in the combined absence of TSLPR, IL-25 and IL-33R but are dysfunctional
Acknowledgments
We thank S. Akira (Osaka University), J. Ihle (St. Jude) and M. Steinhoff (UCSF) for mice, D. Sheppard, C. Lowell and D. Erle for helpful comments on the manuscript, and Z. Wang, N. Flores and M. Consengco for expert technical assistance. This work was supported by National Institute of Health grants AI026918, AI030663, HL107202, Howard Hughes Medical Institute, and the Sandler Asthma Basic Research Center at the University of California, San Francisco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, Ihle JN. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for Th2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009;182:3573–3582. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, Broide DH. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H. Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine, Vorgias CE, Diaquin M, Latge JP. Molecular Organization of the Alkali-insoluble Fraction of Aspergillus fumigatus Cell Wall. Journal of Biological Chemistry. 2000;275:27594–27607. doi: 10.1074/jbc.M909975199. [DOI] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Krauß RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, Akira S. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, Song LZ, Knight JM, Creighton CJ, Luong A, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. The Journal of Immunology. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. Journal of Leukocyte Biology. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Mjösberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Reinhardt RL, Liang HE, Locksley RM. Marking and quantifying IL-17A-producing cells in vivo. PLoS One. 2012;7:e29750. doi: 10.1371/journal.pone.0039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal. 2010;3:ra62. doi: 10.1126/scisignal.2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Roy RM, Wüthrich M, Klein BS. Chitin elicits CCL2 from airway epithelial cells and induces CCR2-dependent innate allergic inflammation in the lung. J Immunol. 2012;189:2545–2552. doi: 10.4049/jimmunol.1200689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Locksley RM. Interleukin-4- and Interleukin-13-Mediated Alternatively Activated Macrophages: Roles in Homeostasis and Disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol. 2008;180:4742–4753. doi: 10.4049/jimmunol.180.7.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434–1444. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, Thomas WR, Fernandez-Caldas E, Kabesch M, Ferrara R, et al. Identification of Der p 23, a Peritrophin-like Protein, as a New Major Dermatophagoides pteronyssinus Allergen Associated with the Peritrophic Matrix of Mite Fecal Pellets. J Immunol. 2013;190:3059–3067. doi: 10.4049/jimmunol.1202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209:607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, Taki Y, Futatsugi-Yumikura S, Tsutsui H, Ishii KJ, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.