Abstract
Clinical evidence indicates increased amyloid deposition in HIV-1-infected brains, which contributes to neurocognitive dysfunction in infected patients. Here we show that HIV-1 exposure stimulates amyloid beta (Aβ) nuclear entry in human brain endothelial cells (HBMEC), the main component of the blood-brain barrier (BBB). Treatment with HIV-1 and/or Aβ resulted in concurrent increase in early endosomal antigen-1 (EEA1), Smad, and phosphorylated Smad (pSmad) in nuclear fraction of HBMEC. A series of inhibition and silencing studies indicated that Smad and EEA1 closely interact by influencing their own nuclear entry; the effect that was attenuated by dynasore, a blocker of GTP-ase activity of dynamin. Importantly, inhibition of dynamin, EEA1, or TGF-β/Smad effectively attenuated HIV-1-induced Aβ accumulation in the nuclei of HBMEC. The present study indicates that nuclear uptake of Aβ involves the dynamin-dependent EEA1 and TGF-β/Smad signaling pathways. These results identify potential novel targets to protect against HIV-1-associated dysregulation of amyloid processes at the BBB level.
Keywords: HIV-1, blood-brain barrier, amyloid beta
INTRODUCTION
Combination antiretroviral therapy (cART) changed HIV-1 infection from acute to chronic disease, dramatically increasing the number of infected individuals who are 50 or older [1]. Neurocognitive impairments and dementia associated with HIV-1 infection are more prevalent in these patients [2, 3] . In addition, abundant amyloid beta (Aβ) deposition is a hallmark of HIV-1 infected brains [3, 4]. Although amyloid pathology is different in HIV-1 and Alzheimer's disease (AD) [5], HIV-associated neurocognitive disorders (HAND) in the aged population correlate with the early beta-amyloidosis [1, 6]. Several mechanisms have been proposed to explain elevated levels of amyloid in HIV-1 brains. For example, it was demonstrated that HIV-1 can increase brain Aβ levels by increasing amyloid precursor protein [7], stimulating Aβ production [8, 9], or inhibiting its degradation [10]. In addition, cART was shown to contribute to elevated brain amyloid by decreasing microglial Aβ phagocytosis [11].
Important observations from our laboratories indicate that exposure to HIV-1 results in enhanced Aβ levels in human brain endothelial cells (HBMEC) and increased its transendothelial transfer [12, 13], suggesting that brain endothelial cells contribute to accumulation of Aβ in HIV-1-infected brains. These observations are consistent with the evidence that brain vascular dysfunction and the blood brain barrier (BBB) are playing important roles in amyloid pathology observed in AD [14]. Importantly, amyloid and HIV-1 potentiate their cerebrovascular toxicity by disrupting the integrity of the brain endothelium and stimulation of inflammatory responses [15, 16].
The present study is based on a novel observation that Aβ not only accumulates in the cytoplasm of HIV-1 exposed cells but also enters the nuclei. We indicate that this process involves a complex interplay between dynamin-dependent stimulation of the early endosomal antigen-1 (EEA1) and TGF-β/Smad signaling pathway.
MATERIALS AND METHODS
Cell cultures, HIV-1 infection, and treatment factors
Human brain microvascular endothelial cells (HBMEC) used in the present study represent a stable, well characterized, and differentiated human brain endothelial cell line [17]. The cells were cultured on collagen type I (BD Biosciences Pharmingen, San Jose, CA) coated dishes in EBM-2 medium (Clonetics, East Rutherford, NJ) supplemented with VEGF, IGF-1, EGF, basic FGF, hydrocortisone, ascorbate, gentamycin, and 0.5% fetal bovine serum (FBS) [17].
HIV-1 stock was generated using human embryonic kidney (HEK) 293T cells (American Type Culture Collection, Manassas, VA) transfected with pYK-JRCSF plasmid containing full-length proviral DNA as described earlier [18]. Throughout the study, HBMEC were exposed to HIV-1 particles for 24 h at the p24 level of 30 ng/ml. This type of exposure was shown to be highly effective in stimulation of cellular Aβ levels [12]. Plasma levels of p24 were reported to reach ~5–6 log10 fg p24/ml in non-treated HIV-1-infected patients, which correspond to ~0.1-1 ng p24/ml [19]. HIV-1 infected monocytes from patients were shown to productively release live viruses in vitro [20] suggesting that HIV-1 may also directly affect the vascular endothelium and produce local p24 values that are higher than the average plasma levels.
Aβ (1–40) and Aβ (1–40) HiLyte were purchased from Anaspec (San Jose, CA). Aβ (1–40) was dissolved in sterile ultra-pure water (ELGA Purelab Classic, Lowell, MA). Freshly solubilized Aβ solutions without pre-aggregation were used for experiments. Such a form of Aβ was demonstrated to induce proinflammatory reactions in isolated rat brain microvessels [21]. Aβ (1–40) HiLyte was dissolved first in a basic buffer (0.1 M NH4OH) than diluted further in PBS as suggested by the manufacturer. Cells were treated with Aβ (1–40) or Aβ (1–40) HiLyte at the concentration of 1 µM for 10 min or 1 h in complete medium. Similar treatment was shown to result in an uptake by the BBB [22] and stimulation of pro-inflammatory responses [15].
Inhibitors SB431542 (Tocris Bioscience, Bristol, UK), dynasore hydrate (Sigma Aldrich, St. Louis, MO) were dissolved in DMSO and further diluted in the cell culture media. Cells were pretreated with 10 µM SB431542 for 2 h or 50 µM dynasore for 30 min prior to Aβ exposure.
Cell fractionation
Cell fractionation was performed as described previously [23, 24] with minor modifications. Briefly, cells were solubilized in 1 ml of ice-cold MNE buffer (25 mM MES [morpholineethanesulfonic acid, pH 6.5]; 150 mM NaCl; 5 mM EDTA) with 1 mM sodium orthovanadate, protease inhibitors and 1% Triton X-100 on ice. The cell lysate was then homogenized with a loose-fitting Dounce homogenizer. The homogenate was centrifuged at 15000 ×g for 15 min, 4 °C, and mixed with 1 ml of 90% sucrose in MNE buffer. The resulting 45% sucrose cell lysate was overlaid with 5 ml of 35% sucrose and 5 ml of 5% sucrose (w/v in MNE buffer), followed by centrifugation for 20 h at 35,000 rpm at 4 °C using a Beckman SW41 rotor. Twelve 1 ml fractions were collected from top to bottom and aliquots of each fraction were subjected to SDS-PAGE and immunoblotting to assess EEA1 and Smad2.
Immunoblotting
Homogenates of cultured HBMEC were prepared in lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 0.1 mg/ml phenylmethylsulfonyl fluoride, 0.5% Nonidet P40, 1 mM EDTA, 2.5 µg/ml leupeptin, 1 µg/ml pepstatin A, and 1 µg/ml aprotinin). After centrifugation at 15,000×g for 15 min, supernatants were collected and protein concentrations were determined using the Bradford protein assay. For isolation of cytoplasmic and nuclear fractions, NucBuster nuclear protein extraction kit (Invitrogen) was used following the manufacturer’s protocol. Samples (8 µg protein/lane for both cytoplasmic and nuclear fractions) were separated on SDS-polyacrylamide gel, blotted onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), and incubated with the respective antibodies. Mouse monoclonal anti-EEA1 and mouse monoclonal anti-Nucleoporin p62 antibodies were from BD Transduction Laboratories (San Diego, CA). Rabbit monoclonal phospho-Smad2 and Smad2 antibodies were from Cell Signaling Technology (Boston, MA). Rabbit polyclonal anti-Rab5A and anti-GAPDH-HRP antibodies were from Santa Cruz Biotechnology. Secondary antibodies were purchased from Santa Cruz Biotechnology and Invitrogen (Carlsbad, CA). For visualization of detected proteins, immunoblots were analyzed using an ECL Western blot detection kit (Amersham Biosciences, Piscataway, NJ).
Confocal immunofluorescence microscopy
HIV-treated HBMEC cultured on collagen I coated chambered glass slides (BD Biosciences, San Jose, CA) were fixed with ethanol for 30 min at 4°C. After washing with PBS and blocking with 3% bovine serum albumin in PBS for 30 min, samples were incubated overnight at 4°C with the primary antibody. Then, the excess of primary antibody was removed, slides were washed with PBS, and incubated with Alexa Fluor568-conjugated secondary antibody for 2 h at room temperature. After washing with PBS, slides were mounted using ProLong Gold Antifade reagent containing 4',6-diamidino-2-phenylindole (DAPI, Invitrogen, Carlsbad, CA) to visualize the nuclei. Specimens were covered with coverslips and the immunofluorescent images were evaluated and captured using confocal microscopy.
The procedure was modified for nuclear and cytoplasmic Aβ measurements. HBMEC were exposed to HIV, followed by 10 min treatment with 1 µM fluorescently labeled Aβ (Aβ HiLyte-Alexa Fluor488). The cells were then fixed, washed, and mounted as described above. Green fluorescence originating from Aβ HiLyte-Alexa Fluor488 was acquired directly using confocal microscopy (Olympus, Fluoview 1200, dry lens UPLFLN 40x NA: 0.75, room temperature) and did not require the use of primary or secondary antibody. Fields were chosen at random and acquisition and quantification were performed using the FV10-ASW3.1 software. For the nuclear Aβ measurements, mean fluorescence intensity was measured in nuclear areas outlined by the DAPI staining. For the cytoplasmic Aβ measurements, cells were outlined by differential interference contrast (DIC) technique and the corresponding fluorescence was measured excluding the DAPI positive nuclear areas.
EEA1 silencing
Human EEA1 siRNA (a pool of 3 target-specific 20–25 nt siRNAs) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Cells were transfected with control siRNA or EEA1 siRNA-1 at a final concentration of 40 nM for 48 h using siPORT NeoFX transfection reagent from the Silencer siRNA Transfection II Kit (Ambion, Applied Biosystems, Foster City, CA) in OptiMEM I medium with GlutaMAX (Invitrogen, Carlsbad, CA).
Statistical Analysis
Data were analyzed using SigmaPlot for Windows 11.0 software (Systat Software Inc., San Jose, CA). One, two or three way ANOVA was used to compare responses among treatments. Treatment means were compared using All Pairwise Multiple Comparison Procedures and p<0.05 was considered significant.
RESULTS
Exposure to HIV-1 increases nuclear localization of Aβ
To explore a hypothesis that brain endothelial cells participate in HIV-1-induced amyloid accumulation in the brains of infected individuals, HBMEC were exposed to HIV-1 particles (p24, 30 ng/ml), followed by a 10 min treatment with fluorescently labeled Aβ HiLyte (1 µM). As illustrated in Figure 1, treatment with Aβ HiLyte alone resulted in weak Aβ fluorescence (Figure 1A). Importantly, a co-treatment with HIV-1 dramatically increased Aβ-specific fluorescence, which partly co-localized with DAPI positive staining, indicating a prominent nuclear localization (Figure 1B). Quantification of these results (Figure 1C) revealed that nuclear levels of Aβ increased more than 16 times in the presence of HIV-1. While Aβ fluorescence was also elevated in the cytoplasm of exposed cells, this effect was less prominent compared to nuclei.
Figure 1. Exposure to HIV-1 increases nuclear localization of Aβ.
HBMEC were exposed to HIV-1 particles at p24 levels of 30 ng/ml for 24 h followed by co-treatment with 1 µM fluorescently labeled Aβ HiLyte for 10 min. Aβ HiLyte (green fluorescence) was assessed by confocal microscopy in cells treated with Aβ alone (A) or co-treated with HIV-1 (B). DAPI staining was performed to visualize the nuclei (blue staining). Representative images from three experiments. Scale bar: 10 µm. (C) Quantification of Aβ HiLyte nuclear and cytoplasmic levels. Values are mean ± SEM, n=13–17. ***Statistically significant at p<0.001.
Exposure to HIV and/or Aβ alters cellular distribution of EEA1 and Smad2
Early endosomes are involved in intracellular movement of proteins; therefore, we evaluated if they have impact on HIV-1 induced nuclear entry of Aβ. As indicated in Figure 2A, early endosomal markers EEA1 and Rab5A are present in both endothelial cell nuclear and cytoplasmic fractions. Quantification of these results demonstrated that nuclear levels of EEA1 were markedly altered cells exposed for 10 min to HIV-1 alone and HIV plus Aβ. Similar effects were observed with Aβ treatment for 1 h but the changes were statistically significant only as the result of HIV-1 exposure. Because expression of Rab5A was only minimally affected by the employed treatment factors, we focused on EEA1 in the remaining experiments.
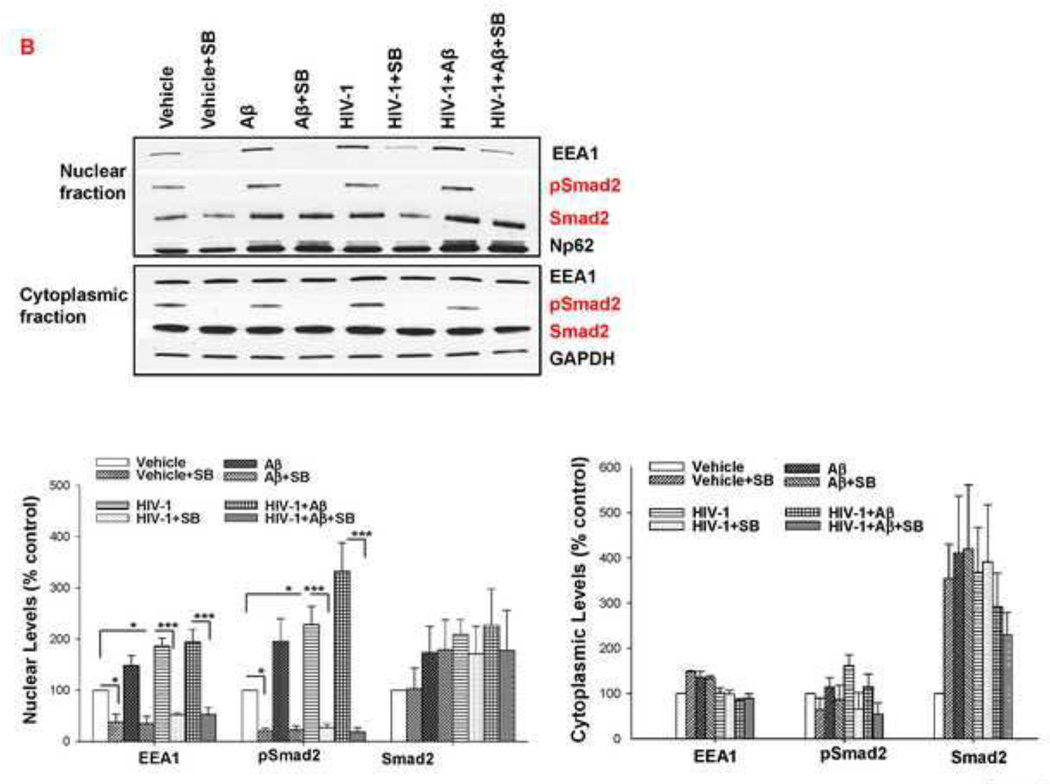
Figure 2. Exposure to HIV and/or Aβ alters cellular distribution of EEA1 and Smad2.
HBMEC were exposed to HIV-1 particles at p24 levels of 30 ng/ml for 24 h followed by co-treatment with 1 µM Aβ for 10 min or 1 h. (A) Early endosomal markers EEA1 and Rab5A were analyzed by immunoblotting in the cytoplasmic and nuclear fractions; n=4–12. (B) Cellular distribution of EEA1 by fractionation using sucrose density ultracentrifugation. (C) pSmad2 and Smad2 were analyzed by immunoblotting in the cytoplasmic and nuclear fractions after exposure to 1 µM Aβ for 10 min; n=8–12. (D) Cellular distribution of Smad2 by fractionation using sucrose density ultracentrifugation. The images show representative blots and the bar graphs in A and C reflect quantitative densitometric data. Values are mean ± SEM. *Statistically significant at p<0.05. GAPDH and nucleoporin p62 (Np62) were used as internal loading controls for the cytoplasmic and nuclear fractions in A and C.
The impact of HIV-1 and/or Aβ exposure on cellular distribution of EEA1 was also explored using fractionation by sucrose density ultracentrifugation. In control samples, EEA1 was distributed in fractions 6–12 (Figure 2B). Treatment with HIV-1 particles caused a visible shift to the right, with EEA1 being detected mainly in fractions 7–12, with only a weak band present in fraction 6. Treatment with Aβ and a co-treatment with HIV-1 and Aβ also resulted in a shift to the heavier cellular fractions as the first EEA1 band became present in fraction 7.
Because early endosomal markers were shown to be involved in nuclear signaling via Smad [25], this signaling pathway was also included in our experiments. Treatment with HIV-1 plus Aβ resulted in significantly increased nuclear levels of both Smad2 and phosphorylated Smad2 (pSmad2) while exposure to HIV-1 alone increased only pSmad2 nuclear levels (Figure 2C). Exposure to Aβ and/or HIV-1 also induced changes in cellular Smad2 allocation as determined in cellular fractions obtained by sucrose density ultracentrifugation. Smad2 immunoreactivity in control cells was observed mainly in fractions 6–11. Exposure to Aβ and/or HIV resulted in a shift of Smad2 to heavier fractions, with Smad2 immunoreactivity becoming present only in fraction 7 (Figure 2D), mirroring the effects of Aβ and/or HIV on cellular EEA1 localization. This synchronized shift of both EEA1 (Figure 2B) and Smad2 (Figure 2D) within the cellular fractions suggests a coordinated movement within the cells and involvement of both proteins in cellular responses induced by HIV-1 and/or Aβ.
Immunofluorescent quantification of HIV-induced EEA1 and pSmad2 nuclear localization is depicted in Figure 3. In control cultures (Figure 3A), EEA1-positive immunoreactivity exhibited a vesicular cytoplasmic staining pattern that appeared to be more abundant in the perinuclear area. A weaker nuclear staining pattern with different sized dot-like structures was also observed. In addition, Smad2 immunoreactivity showed a punctated cytoplasmic and nuclear staining pattern. Consistent with the immunoblotting results, HIV exposure enhanced both EEA1 and pSmad2 nuclear immunoreactivity (Figure 3B). Quantification of these results is illustrated in Figures 3C and 3D, respectively. Images in the upper parts of the bar graphs represent the immunoreactivity intensity profiles for EEA1 (Figure 3C) and pSmad2 (Figure 3D) in a representative nuclear area.
Figure 3. Exposure to HIV increases EEA1 and pSmad2 nuclear levels.
HBMEC were exposed to vehicle (A; control cultures) or HIV-1 particles (B) at p24 levels of 30 ng/ml for 24 h. Expression of EEA1 (red fluorescence) and pSmad2 (green fluorescence) was assessed by confocal microscopy. DAPI staining was performed to visualize the nuclei (blue staining). Representative images from three experiments. Scale bar: 10 µm. Quantification of EEA1 (C) and pSmad2 (D) levels in the nuclear area. Values are mean ± SEM, n=15. ***Statistically significant as compared to control at p<0.001. Images in the upper parts of the bar graphs depict the immunoreactivity intensity profiles for EEA1 or pSmad2 in a representative nuclear area.
Interplay between EEA1 and TGF-β/Smad signaling regulates their nuclear entry
In order to evaluate whether EEA1 can influence Smad signaling, EEA1 was silenced by a specific siRNA, followed by treatment with HIV-1 and/or Aβ. Figure 4A demonstrates the effectiveness of the procedure resulting in a marked decrease in nuclear and cytoplasmic levels of EEA1. While a combined exposure to HIV-1 plus Aβ elevated both pSmad2 and Smad2 in nuclear fractions, EEA1 silencing significantly attenuated these effects. In contrast, EEA1 silencing did not affect pSmad2 and Smad2 levels in the cytoplasm.
Figure 4. Interplay between EEA1 and TGF-β/Smad signaling regulates their nuclear entry.
(A) HBMEC were exposed to HIV-1 and/or Aβ as in Figure 1 with or without EEA1 silencing. pSmad2 and Smad2 levels were analyzed by immunoblotting in nuclear and cytoplasmic fractions; n=8–12. (B) HBMEC were exposed to HIV-1 and/or Aβ as in Figure 1, with the selected cultures pretreated with TGF-β/Smad inhibitor SB431542 (SB, 10 µM) for 2 h. Nuclear and cytoplasmic EEA1, pSmad2 and Smad2 levels were analyzed by immunoblotting; n=4–6. The images show representative blots from at least four experiments and the bar graphs reflect quantitative densitometric data. Values are mean ± SEM. Statistically significant at *p<0.05 or ***p<0.001. GAPDH and nucleoporin p62 (Np62) were detected as internal loading controls for the cytoplasmic and nuclear fractions, respectively.
To determine if the TGF-β/Smad pathway is involved in HIV-1-induced EEA1 nuclear entry, cells were pretreated with 10 µM SB431542, a specific TGF-β/Smad signaling inhibitor, for 2 h prior to exposure to HIV and/or Aβ (Figure 4B). SB431542 efficiently blocked Smad2 phosphorylation (positive control) in both nuclear and cytoplasmic fractions. Importantly, SB431542 markedly attenuated EEA1 levels in the nuclear fractions in all treatment groups. In contrast, EEA1 levels in the cytoplasmic fractions were barely affected by TGF-β/Smad signaling inhibition.
Dynamin provides a platform for HIV-1 and/or Aβ-induced EEA1 and Smad nuclear entry
EEA1 was reported to co-localize with dynamin in the perinuclear region of endothelial cells [26] and HIV-1 Tat-induced Aβ production was impaired in neurons transfected with dominant negative dynamin [9]. Therefore, we explored whether dynamin is involved in the HIV-1-induced nuclear EEA1 and Smad2 translocation. Control cells or cells exposed to HIV-1 for 24 h were pre-treated with dynamin inhibitor, dynasore (50 µM), for 30 min prior to exposure to HIV-1 and/or Aβ. Pretreatment with dynasore reduced the intensity of the nuclear EEA1 levels in all treatment groups, with cytoplasmic EEA1 only minimally affected (Figure 5A). Quantification of these results demonstrated that the impact of dynasore on nuclear EEA1 levels was statistically significant in the HIV plus Aβ group.
Figure 5. Dynamin regulates HIV-induced EEA1 and pSmad2/Smad2 nuclear entry.
HBMEC were exposed to HIV-1 particles and/or Aβ as in Figure 1, with the selected cultures pretreated with dynamin inhibitor dynasore (Dy, 50 µM) for 30 min. Nuclear and cytoplasmic EEA1 (A), pSmad2, and Smad2 (B) levels were analyzed by immunoblotting. The images show representative blots from at least four experiments and the bar graphs reflect quantitative densitometric data. Values are mean ± SEM, n=4–6. *Statistically significant at p<0.05. GAPDH and nucleoporin p62 (Np62) were detected as internal loading controls for the cytoplasmic and nuclear fractions, respectively.
Taking into consideration a strong inter-relationship between EEA1 and TGF-β/Smad signaling, we also evaluated the impact of dynamin on nuclear levels of pSmad and Smad2. Inhibition of dynamin attenuated HIV and/or Aβ-induced changes in pSmad2 levels in the nuclear fraction. Interestingly, blocking dynamin also markedly diminished pSmad levels in the cytoplasm (Figure 5B).
EEA1, TGF-β/Smad, and dynamin control in concert HIV-1-induced nuclear entry of Aβ
In the final set of experiments, the impact of EEA1, TGF-β/Smad, and dynamin on HIV-induced nuclear uptake of Aβ was evaluated. Using the previously established conditions, EEA1 was silenced by transfection with specific siRNA, TGF-β/Smad signaling was inhibited by SB431542 (10 µM), and dynamin signaling by dynasore (50 µM). Cells were also treated with HIV-1 and/or Aβ HiLyte, followed by visualization of Aβ HiLyte-specific fluorescence by confocal microscopy and quantification for nuclear localization. EEA1 silencing, pretreatment with SB431542, or dynamin inhibition significantly reduced the intensity of nuclear fluorescence of Aβ HiLyte in HIV-1-treated cells (Figure 6A, 6B, 6C, respectively). Because of its apparent location upstream from EEA1 or Smad2, we suggest that dynamin may act as a master regulator to control nuclear levels of Aβ via the EEA1 and TGF-β/Smad pathways.
Figure 6. EEA1, TGF-β/Smad, and dynamin are involved in HIV-1-induced nuclear entry of Aβ.
(A) EEA1 was silenced by transfection with specific siRNA, (B) TGF-β/Smad signaling was inhibited by SB431542 (10 µM), and (C) dynamin signaling was blocked by dynasore (50 µM). Such pretreated cultures were then exposed to HIV-1 particles and/or Aβ as in Figure 1, followed by the assessment of Aβ HiLyte fluorescence in nuclear areas by confocal microscopy as in Figure 1. At least 5 images for every experimental condition from different samples were randomly acquired. Values are mean ± SEM, n=250. ***Statistically significant at p<0.001.
The effects of EEA1 silencing, pretreatment with SB431542, and dynamin inhibition on HIV-1-induced Aβ HiLyte accumulation HBMEC were also measured by in cell fluorescence (Supplementary Figures 1A, 1B, and 1C). This method allows for quantitative measurement of the total Aβ levels without discrimination between nuclear and cytosolic fractions. As illustrated in Supplementary Figures 1A and 1B, EEA1 silencing and pretreatment with SB431542 protected against HIV-1-induced Aβ accumulation when measured in the whole cells. Dynamin inhibition appears to be effective only on nuclear Aβ uptake because did not change the overall cellular Aβ accumulation in the presence of HIV-1 (Supplementary Figure 1C).
DISCUSSION
Although Aβ accumulation has been linked primarily to the pathology of AD, a moderate brain accumulation of this peptide is also characteristic for normal aging. Moreover, emerging clinical data provide evidence that amyloid levels are increased in HIV brains [1, 5, 27]. There appears to be prevalence for amyloid deposition in the hippocampus and frontal lobe with deposits in pyramidal neurons, and along axonal tracks in HIV-infected individuals [3]. Prominent Aβ localization is also observed in the perivascular space in HIV brains [1, 27] indicating the importance of cerebral microvasculature in amyloid pathology. HIV-associated neurocognitive disorders (HAND) in older patients correlate with early beta-amyloidosis [1, 6]. Amyloid accumulation in the brain may also affect the rate of HIV infection [28].
The BBB plays an important role both in HIV-1 [29] and amyloid pathology [30], regulating both entry of the blood-borne amyloid into the brain and clearance from the brain [31]. At the same time, Aβ is toxic for the brain vasculature as demonstrated by a loss of occludin, claudin-5, and ZO-1 in Aβ-laden capillaries. Treatment with Aβ disrupted endothelial permeability, impaired glucose transport, and resulted in apoptosis [32]. The underling mechanisms of Aβ toxicity in vascular system appear to be induction of reactive oxygen species, as Aβ cytotoxicity was reversed by administration of exogenous antioxidants and NOX-2 inhibitors. It was reported that HEK cells transfected with a construct of Aβ (1–43) exhibited nuclear Aβ accumulation [33]. A similar phenomenon was observed in CHO cells expressing Aβ in the endoplasmic reticulum [34]. However, to our knowledge, the present manuscript is the first report indicating enhanced exogenous Aβ accumulation in the nuclei of cells exposed to HIV-1.
The Smad signaling was linked to Aβ pathology [35]; therefore, this pathway was evaluated in the present study as a possible mediator of nuclear accumulation of Aβ. Exposure to HIV-1 and/or Aβ increased pSmad2 levels in the nuclear fractions, without affecting the total Smad2 levels in the cytoplasm. The mechanisms of these effects are not fully understood; however, HIV-1 gp120 [36] can signal via transforming growth factor-β1 (TGF-β), providing an activation pathway for Smad2 stimulation. Indeed, Smad is typically activated by stimulation of the TGF-β family receptor complex, which compromises the activin-like kinase 5 (ALK5)/TGF-β type I receptor (TβRI) and the TGF-β type II receptor (TβRII) subunits. Binding of a ligand (e.g., TGF-β) to TβRII induces the assembly of both types of receptors into a complex, followed by transphosphorylation. Activated TβRI phosphorylates receptor-activated Smads (Smad 2, 3, 5, and 8), which form a complex with Smad 4, translocate into the nucleus where they regulate the expression of various target genes. TGF-β/Smad pathway has been directly implicated in HIV-1 infection. For example, it was observed that HIV-1 and HIV-1 protein Tat increased TGF-β levels in the brain [37, 38]. In addition, TGF-β inhibited HIV-1 infection of mammary epithelial cells via downregulating the HIV-1 long terminal repeat (LTR) promoter; however, it increased infection of macrophages by HIV-1 by upregulation of the LTR promoter [39]. The p300-TGF-β/Smad binding was also shown to modulate HIV-1 gp120 induced tubular injury which may be relevant to HIV-associated nephropathy [36].
Important data from the present study indicate that inhibition of TGF-β/Smad protect against HIV-1-induced Aβ accumulation in the nuclei. These results are consistent with literature reports indicating that inhibition of this signaling pathway in mouse peripheral macrophages diminished Aβ deposits in the brain vessels [35]. Nevertheless, the role of Smad in Aβ pathology may be complex as it was also reported that AD brains are associated with deficiency of Smad [40, 41] and an aberrant localization of pSmad2/3 in the cytoplasm of the hippocampal neurons and in amyloid plaques [42]. In addition, inhibition of TGF-β/Smad increased vulnerability of hippocampal neurons to Aβ and enhanced neurodegeneration in an animal model [43]. The TGF-β/Smad signaling pathway was shown to be regulated by caveolin-1 in NIH-3T3 cells via interaction with the TβRI. While TβRI and Smad2 colocalize with caveolin-1 in caveolae, caveolin-1 can inhibit this pathway by blocking Smad2 phosphorylation [44]. In addition, it was demonstrated that HIV-1 gp41 has a caveolin-1 binding domain [45], suggesting that exposure of brain endothelial cells to HIV-1 may induce a direct binding of the virus to lipid rafts and/or caveolae, a hypothesis that is consistent with a possible raft-dependent endocytotic entry of HIV-1 to HBMEC [46]. These observations indicate a potential upstream gp41-caveolin-1-TGF-βRI signaling for the interactions of HIV-1 with brain endothelial cells.
Early endosomal proteins were suggested to regulate the Smad signaling pathway. For example, a dominant negative mutant of Rab5A caused amplification of Smad phosphorylation and its nuclear translocation, resulting in transcriptional activation of a Smad-dependent promoter in endothelial cells [25]. While expression of Rab5A was only slightly affected in the present study, exposure to HIV-1 and/or Aβ induced a prominent translocation of EEA1 into the nuclear fractions of HBMEC. This disconnection between these two early endosomal proteins was surprising because EEA1 was described as a Rab5 effector acting as an organelle-tethering molecule [47]. Nevertheless, their various nuclear entry in response to HIV-1 and/or Aβ treatment suggests a different role for EEA1 in the nuclear environment. Indeed, inhibition of Rab5A was shown to lead to increased Smad phosphorylation and nuclear translocation [25]. In contrast, the present study demonstrates that EEA1 silencing results in diminished Smad phosphorylation and nuclear entry with the concurrent attenuation of HIV-induced Aβ accumulation in HBMEC nuclei.
Dynamin-2 is a 100 kDa protein that co-localizes with several proteins, including EEA1, in the perinuclear region [26]. Dynamin has been implicated in vesicle fission and receptor endocytosis in endothelial cells [48–50] and may function as a signal transducing GTP-ase [51]. Importantly, it also plays a role in HIV-1 infection, as its inhibition blocked HIV-1 internalization, infection, and fusion with epithelial and lymphoid cells [52]. The results of the present study confirm the important role of dynamin in regulation of cellular processes. Pretreatment with dynasore affected nuclear localization of both EEA1 and Smad2/pSmad2 both in HIV-1 and/or Aβ treated and control cells, indicating the impact of dynamin on trafficking of intracellular proteins. Cytoplasmic levels of pSmad2 were also greatly affected by dynasore, further supporting a close interaction between dynamin and pSmad. Because dynamin appears to be upstream from Smad2 and EEA1, it may act as the master regulator of these signaling molecules and effectively control cellular uptake of Aβ. To support this statement, our important data indicate that inhibition of dynamin markedly attenuated HIV-1-induced nuclear uptake of Aβ. These results are in agreement with observations that transfection of HeLa cells with dominant negative dynamin diminished Aβ production [53]. In addition, dominant negative dynamin inhibited nuclear signaling of the intracellular domain (AICD) of the amyloid precursor protein (APP) [54]. Although Aβ has no overlapping sequence with AICD, these results support the role of dynamin in nuclear signaling.
CONCLUSIONS
The present study demonstrates the role of EEA1, TGF-β/Smad, and dynamin signaling in HIV-1-induced nuclear entry of Aβ. While EEA1 and Smad closely interact regulating each other nuclear translocation, dynamin appears to be a master regulator controlling EEA1 and Smad nuclear entry and, ultimately, Aβ nuclear accumulation (Figure 7).
Figure 7. Schematic diagram of HIV-1-induced nuclear Aβ uptake in brain endothelial cells.
Our data indicate that HIV-1-induced nuclear Aβ accumulation in brain endothelial cells is dynamin-dependent, involving EEA1 and the TGF-β/Smad signaling pathway. Dynamin colocalizes with EEA1 in the perinuclear region of endothelial cells, appears to be upstream from the EEA1- and TGF-β/Smad signaling routes and may act as a master regulator of these signaling pathways and control nuclear uptake of Aβ. These events may contribute to HIV-1-induced brain deposition of amyloid and ultimately to the development of cognitive dysfunction in HIV-1-infected brains. Abbreviations: Aβ, amyloid beta; EEA1, early endosomal antigen 1; TGFβ-RI, transforming growth factor beta receptor type I.
Supplementary Material
HIGHLIGHTS.
-
□
HIV-1 induces nuclear accumulation of amyloid beta (Aβ) in brain endothelial cells.
-
□
EEA-1 and TGF-B/Smad act in concert to regulate nuclear entry of Aβ.
-
□
Dynamin appropriates the EEA-1 and TGF-B/Smad signaling.
-
□
Dynamin serves as a master regulator of HIV-1-induced nuclear accumulation of Aβ.
ACKNOWLEDGEMENT
pYK-JRCSF was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIH/NIAID. Supported by MH063022, MH098891, NS39254, DA027569. We acknowledge support from the Miami Center for AIDS Research (CFAR) at the University of Miami Miller School of Medicine funded by a grant (P30AI073961) from the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J. Neuroimmune. Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J. Neuroimmune. Pharmacol. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 4.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J. Neuroimmune. Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbrink F, Evers S, Buerke B, Young P, Arendt G, et al. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur. J. Neurol. 2013;20:420–428. doi: 10.1111/ene.12006. [DOI] [PubMed] [Google Scholar]
- 6.Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012;26:2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Liu J, Katafiasz B, Fox H, Xiong H. HIV-1 gp120-induced axonal injury detected by accumulation of beta-amyloid precursor protein in adult rat corpus callosum. J. Neuroimmune. Pharmacol. 2011;6:650–657. doi: 10.1007/s11481-011-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci. Lett. 2010;475:174–178. doi: 10.1016/j.neulet.2010.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol. Aging. 2013;34:2370–2378. doi: 10.1016/j.neurobiolaging.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 11.Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, et al. Shytle Antiretroviral medications disrupt microglial phagocytosis of beta-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol. Brain. 2011;4:23. doi: 10.1186/1756-6606-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andras IE, Eum SY, Huang W, Zhong Y, Hennig B, et al. HIV-1-induced amyloid beta accumulation in brain endothelial cells is attenuated by simvastatin. Mol. Cel.l Neurosci. 2010;43:232–243. doi: 10.1016/j.mcn.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andras IE, Eum SY, Toborek M. Lipid rafts and functional caveolae regulate HIV-induced amyloid beta accumulation in brain endothelial cells. Biochem. Biophys. Res. Commun. 2012;421:177–183. doi: 10.1016/j.bbrc.2012.03.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr. Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 15.Andras IE, Rha G, Huang W, Eum S, Couraud PO, et al. Simvastatin protects against amyloid beta and HIV-1 Tat-induced promoter activities of inflammatory genes in brain endothelial cells. Mol. Pharmacol. 2008;73:1424–1433. doi: 10.1124/mol.107.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Choi JJ, Choi YJ, Hennig B, Toborek M. HIV-1 Tat-induced cerebrovascular toxicity is enhanced in mice with amyloid deposits. Neurobiol. Aging. 2012;33:1579–1590. doi: 10.1016/j.neurobiolaging.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 18.Andras IE, Toborek M. HIV-1-induced alterations of claudin-5 expression at the bloodbrain barrier level. Methods Mol. Biol. 2011;762:355–370. doi: 10.1007/978-1-61779-185-7_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ondoa P, Dieye TN, Vereecken C, Camara M, Diallo AA, et al. Evaluation of HIV-1 p24 antigenemia and level of CD8+CD38+ T cells as surrogate markers of HIV-1 RNA viral load in HIV-1-infected patients in Dakar, Senegal. J. Acquir. Immune Defic. Syndr. 2006;41:416–424. doi: 10.1097/01.qai.0000209901.12750.d0. [DOI] [PubMed] [Google Scholar]
- 20.Wang T, Xu Y, Zhu H, Andrus T, Ivanov SB, et al. Successful isolation of infectious and high titer human monocyte-derived HIV-1 from two subjects with discontinued therapy. PLoS One. 2013;8:e65071. doi: 10.1371/journal.pone.0065071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paris D, Townsend KP, Obregon DF, Humphrey J, Mullan M. Pro-inflammatory effect of freshly solubilized beta-amyloid peptides in the brain. Prostaglandins Other Lipid. Mediat. 2002;70:1–12. doi: 10.1016/s0090-6980(02)00111-9. [DOI] [PubMed] [Google Scholar]
- 22.Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, et al. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J. Biol. Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, et al. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 24.Lim EJ, Majkova Z, Xu S, Bachas L, Arzuaga X, et al. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem. Biol. Interact. 2008;176:71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panopoulou E, Gillooly DJ, Wrana JL, Zerial M, Stenmark H, et al. Early endosomal regulation of Smad-dependent signaling in endothelial cells. J. Biol. Chem. 2002;277:18046–18052. doi: 10.1074/jbc.M107983200. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya R, Kang-Decker N, Hughes DA, Mukherjee P, Shah V, et al. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J. 2005;19:1692–1694. doi: 10.1096/fj.05-3889fje. [DOI] [PubMed] [Google Scholar]
- 27.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 28.Wojtowicz WM, Farzan M, Joyal JL, Carter K, Babcock GJ, et al. Stimulation of enveloped virus infection by beta-amyloid fibrils. J. Biol. Chem. 2002;277:35019–35024. doi: 10.1074/jbc.M203518200. [DOI] [PubMed] [Google Scholar]
- 29.Toborek M, Lee YW, Flora G, Pu H, Andras IE, et al. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell. Mol. Neurobiol. 2005;25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 32.Blanc EM, Toborek M, Mark RJ, Hennig B, Mattson MP. Amyloid beta-peptide induces cell monolayer albumin permeability, impairs glucose transport, and induces apoptosis in vascular endothelial cells. J. Neurochem. 1997;68:1870–1881. doi: 10.1046/j.1471-4159.1997.68051870.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone EM, Babbey LE, Stephenson D, Paul DC, Santerre RF, et al. Nuclear and cytoplasmic localization of the beta-amyloid peptide (1–43) in transfected 293 cells. Biochem. Biophys. Res. Commun. 1996;220:710–771. doi: 10.1006/bbrc.1996.0469. [DOI] [PubMed] [Google Scholar]
- 34.Buckig A, Tikkanen R, Herzog V, Schmitz A. Cytosolic and nuclear aggregation of the amyloid beta-peptide following its expression in the endoplasmic reticulum. Histochem. Cell. Biol. 2002;118:353–360. doi: 10.1007/s00418-002-0459-2. [DOI] [PubMed] [Google Scholar]
- 35.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, et al. Blocking TGF-beta- Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapasi AA, Fan S, Singhal PC. p300 modulates HIV-1 gp120-induced apoptosis in human proximal tubular cells: associated with alteration of TGF-beta and Smad signaling. Nephron. Exp. Nephrol. 2006;102:e30–e38. doi: 10.1159/000088404. [DOI] [PubMed] [Google Scholar]
- 37.Rasty S, Thatikunta P, Gordon J, Khalili K, Amini S, et al. Human immunodeficiency virus tat gene transfer to the murine central nervous system using a replication-defective herpes simplex virus vector stimulates transforming growth factor beta 1 gene expression. Proc. Natl. Acad. Sci. U S A. 1996;93:6073–6078. doi: 10.1073/pnas.93.12.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawaya BE, Thatikunta P, Denisova L, Brady J, Khalili K, et al. Regulation of TNFalpha and TGFbeta-1 gene transcription by HIV-1 Tat in CNS cells. J. Neuroimmunol. 1998;87:33–42. doi: 10.1016/s0165-5728(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 39.Moriuchi M, Moriuchi H. Cell-type-dependent effect of transforming growth factor beta, a major cytokine in breast milk, on human immunodeficiency virus type 1 infection of mammary epithelial MCF-7 cells or macrophages. J. Virol. 2004;78:13046–13052. doi: 10.1128/JVI.78.23.13046-13052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HG, Ueda M, Zhu X, Perry G, Smith MA. Ectopic expression of phospho-Smad2 in Alzheimer's disease: uncoupling of the transforming growth factor-beta pathway? J. Neurosci. Res. 2006;84:1856–1861. doi: 10.1002/jnr.21072. [DOI] [PubMed] [Google Scholar]
- 41.Tesseur I, Zou K, Esposito L, Bard F, Berber E, et al. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer's pathology. J. Clin. Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueberham U, Ueberham E, Gruschka H, Arendt T. Altered subcellular location of phosphorylated Smads in Alzheimer's disease. Eur. J. Neurosci. 2006;24:2327–2334. doi: 10.1111/j.1460-9568.2006.05109.x. [DOI] [PubMed] [Google Scholar]
- 43.Caraci F, Battaglia G, Busceti C, Biagioni F, Mastroiacovo F, et al. TGF-beta 1 protects against Abeta-neurotoxicity via the phosphatidylinositol-3-kinase pathway. Neurobiol. Dis. 2008;30:234–242. doi: 10.1016/j.nbd.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 45.Benferhat R, Krust B, Rey-Cuille MA, Hovanessian AG. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 (CBD1) contains several overlapping neutralizing epitopes. Vaccine. 2009;27:3620–3630. doi: 10.1016/j.vaccine.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 46.Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 2002;76:6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 49.Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends. Cell. Biol. 2001;11:406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- 50.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, et al. Endothelial cellsurface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J. Cell. Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fish KN, Schmid SL, Damke H. Evidence that dynamin-2 functions as a signaltransducing GTPase. J. Cell. Biol. 2000;150:145–154. doi: 10.1083/jcb.150.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chyung JH, Selkoe DJ. Inhibition of receptor-mediated endocytosis demonstrates generation of amyloid beta-protein at the cell surface. J. Biol. Chem. 2003;278:51035–51043. doi: 10.1074/jbc.M304989200. [DOI] [PubMed] [Google Scholar]
- 54.Goodger ZV, Rajendran L, Trutzel A, Kohli BM, Nitsch RM, et al. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J. Cell. Sci. 2009;122:3703–3714. doi: 10.1242/jcs.048090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.