Abstract
Strains of Klebsiella pneumoniae producing KPC-type beta-lactamases (KPC-Kp) are broadly disseminating worldwide and constitute a major healthcare threat given their extensively drug resistant phenotypes and ability to rapidly disseminate in healthcare settings. In this work we report on the characterization of two different capsular polysaccharide (CPS) gene clusters, named cps BO-4 and cps 207-2, from two KPC-Kp clinical strains from Italy belonging in sequence type (ST) 258, which is one of the most successful ST of KPC-Kp spreading worldwide. While cps BO-4 was different from known 78 K-types according to the recently proposed typing schemes based on the wzi or wzc gene sequences, cps 207-2 was classified as K41 by one of these methods. Bioinformatic analysis revealed that they were represented in the genomic sequences of KPC-Kp from strains of ST258 from different countries, and cps BO-4 was also detected in a KPC-Kp strain of ST442 from Brazil. Investigation of a collection of 46 ST258 and ST512 (a single locus variant of ST258) clinical strains representative of the recent Italian epidemic of KPC-Kp by means of a multiplex PCR typing approach revealed that cps BO-4 was the most prevalent type, being detected both in ST258 and ST512 strains with a countrywide distribution, while cps 207-2 was only detected in ST258 strains with a more restricted distribution.
Introduction
The capsular polysaccharide (CPS or K-antigen) is a recognized virulence factor of Klebsiella pneumoniae [1], [2]. This component exhibits a remarkable intra-specific structural diversity which translates into different antigenic properties that may be relevant to bacterial virulence [2]–[4].
CPS diversity has classically been detected by serotyping techniques [5], but genotyping systems have recently been developed, offering several advantages vs. the conventional serotyping approach [6]–[10]. Among systems that do not require a sequencing step, a PCR-based typing system has been proposed for the detection of isolates of the K1, K2, K5, K20, K54 and K57 capsular types, that are commonly associated with invasive diseases or having a prominent pathogenicity [6]. Conversely, two systems based on amplification and sequencing of the conserved wzi and wzc genes were recently proposed to determine the K-type of K. pneumoniae [9], [10].
During the last years, strains of K. pneumoniae producing KPC-type carbapenemases (KPC-Kp) belonging in sequence type (ST) 258 and related variants (e. g. ST512, ST437 and ST11) have undergone a global dissemination, with epidemic diffusion in some areas of North and South America, Europe and Asia [11]–[18]. Infections caused by these strains pose a major challenge due to their extended antibiotic resistance phenotypes and ability to rapidly disseminate in healthcare settings, and are associated with high mortality rates [19]–[20]. Detailed knowledge on the CPSs of these strains, however, is still limited. A ST258 KPC-Kp strain from Greece has recently been reported to express a K41 serotype CPS [21], while the chemical structure of the CPS of two representatives of an outbreak clone of ST258 KPC-Kp from USA has recently been described [22].
In this work we have characterized two different cps gene clusters from two KPC-Kp clinical strains of ST258 from Italy, and report on their distribution in a collection of KPC-Kp isolates of ST258 and ST512 representative of the recent Italian epidemic. We also propose a modification to a previously established PCR-based CPS typing system [6], to include recognition of these CPS types.
Results
Characterization of Two Different CPS Gene Clusters in ST258 KPC-Kp Strains of Clinical Origin
The CPS gene cluster of two KPC-Kp strains of clinical origin, KKBO-4 and KK207-2, were characterized by an HTGS approach. The two strains had been isolated in 2010 from bloodstream infections of inpatients in two different Italian hospitals and produced either KPC-2 (KK207-2) or KPC-3 (KKBO-4). They were both of ST258, and exhibited a related although not identical XbaI PFGE profile [12] (difference of two bands, data not shown).
Comparison of the draft genomes using GGDC 2.0 confirmed the close relatedness between the two strains at the genomic level (intergenomic distance of 0.0015). Despite this close relatedness, however, the cps gene clusters of the two strains were significantly different from each other.
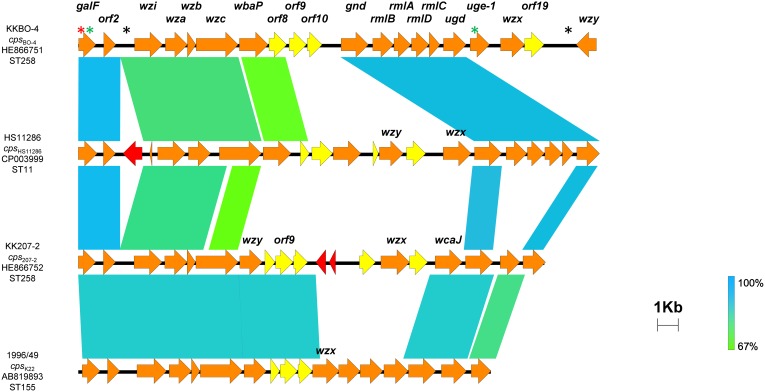
The CPS gene cluster of KKBO-4 (named cps BO-4) was found to be 26,587 bp-long, consisting of 20 ORFs (from galF to wzy), and was characterized by the presence of the K-antigen flippase- and polymerase-encoding genes (wzx and wzy, respectively) at the 3′-end, and by the presence of the rmlBADC operon for the synthesis of dTDP-L-rhamnose in the central region (Fig. 1).
Figure 1. Comparison of the CPS gene clusters from K. pneumoniae strains KKBO-4 (cps BO-4), HS11286 (cps HS11286), KK207-2 (cps 207-2), and 1996/49 (K-type 22, cps K22).
Sequence accession numbers and STs for the respective strains are also indicated (the ST of strain 1996/49 was deduced from ref. 30). The CPS gene cluster of strain 8238 (K-type 37) (accession number AB819894), differing from cps K22 by a single nucleotide deletion resulting in a frameshift mutation located in a putative acetyltransferase downstream gnd, is not included for simplicity. Homologous regions are connected by areas of different colors reflecting the degree of nucleotide identity (from 67% to 100%). Open reading frames encoding transposases are colored in red, while those encoding hypothetical glycosyltransferases are colored in yellow. The locations of synonymous, non-synonymous and intergenic single nucleotide variations (SNVs) occurring between the CPS gene clusters of KKBO-4 and Kp13 are indicated by green, red and black stars, respectively. The cps 207-2 gene cluster exhibited regions of similarity to cps BO-4 including the conserved galF-wzc region (83.2% of nucleotide identity), and the conserved gnd and ugd genes (95.5% and 96.8% nucleotide identity, respectively).
The cps BO-4 gene cluster was identical or very similar to those present in a number of ST258 K. pneumoniae strains from different countries, whose genome sequences are available in the public domain, and also very similar to that previously described in a ST442 KPC-Kp strain (Kp13) that caused an outbreak in Brazil [23] (Table 1 and Fig. 1). Compared to the CPS gene cluster of K. pneumoniae HS11286 (ST11, a single locus variant of ST258) [29], cps BO-4 exhibited significant similarities in some regions (e. g. from galF to orf8 and from gnd to uge-1, comprising the rmlBADC operon), but also substantial differences in the central and the 3′-region of the gene cluster (Fig. 1).
Table 1. Differences between the CPS gene clusters of strains KKBO-4 (cps BO-4, 26,587 bp) or KK207-2 (cps 207-2, 23,994 bp) and closely related CPS gene clusters detected in other sequenced genomes.
| Strains | STs | Countries | cps-types | NucleotideDifferences | Gene Mutations(AminoAcid Differences) | References/Accession numbers |
| KPNIH21a | 258 | USA | cps BO-4 | - | - | [24] |
| ST258–490 | 258 | Israel | cps BO-4 | - | - | [25] |
| ST258 K26BOb | 258 | Italy | cps BO-4 | - | - | [26] |
| MP14 | 258 | South Korea | cps BO-4 | - | - | [27] |
| ATCC BAA-1705 | 258 | USA | cps BO-4 | 1 | galF a191c (E64A) | [28] |
| UHKPC02c | 258 | USA | cps BO-4 | 1 | galF a191c (E64A) | ARSK00000000.1 |
| BIDMC 12C | 258 | USA | cps BO-4 | 2 | galF a191c (E64A) | AXLG00000000.1 |
| gnd t1220c (V407A) | ||||||
| galF a191c (E64A) | ||||||
| Kp13 | 442 | Brazil | cps BO-4 | 5d | galF t366a (silent) | [23] |
| uge-1 g150a (silent) | ||||||
| UHKPC06c | 258 | USA | cps 207-2 | 1 | wzc c1936a (P646T) | ARSJ00000000.1 |
The dash indicates 100% identity. The cut-off values used for the inclusion in the analysis were ≥99% nucleotide identity and ≥99% of query coverage, based on results from a BLAST search performed at the NCBI website (http://blast.ncbi.nlm.nih.gov/) using either nr or wgs databases, using default values but without the low complexity filter option.
KPNIH21 was chosen as a representative of the outbreak clone described in reference 24.
strains ST258 K26BO and ST258 K28BO, both described in reference 26, were characterized by identical CPS gene clusters.
strains UHKPC02 and UHKPC06 were representatives of those included in the Klebsiella pneumoniae Genome Sequencing Center Project (http://gsc.jcvi.org/projects/gsc/klebsiella_pneumoniae/index.php).
2 out of 5 SNVs are located in intergenic regions.
According to the cps-typing protocol based on sequencing of the wzi gene [9] cps BO-4 showed a single nucleotide difference with the wzi81-K81 reference amplicon. According to the cps-typing protocol based on sequencing of the wzc gene [10] cps BO-4 was <80% identical to that of any other reference sequence.
The CPS gene cluster of strain KK207-2 (named cps 207-2) was found to be 23,994 bp-long, consisting of 19 ORFs (from galF to ugd). It did not contain the rmlBADC operon but contained original genes, of which some encode putative glycosyltransferases, located between the wzy and wcaJ genes (Fig. 1). The cps 207-2 gene cluster was very similar to those present in ST258 K. pneumoniae strains from USA, whose genome sequences are available in the public domain (Table 1). It also exhibited regions of similarity with the CPS gene clusters of K. pneumoniae strains 1996/49 and 8238, producing CPS of the K22 and K37 serotype, respectively, and with both cps BO-4 and cps HS11286 (Fig. 1).
According to the cps-typing protocol based on sequencing of the wzi gene [9] cps 207-2 was identical to the wzi29-K41 reference amplicon. According to the cps-typing protocol based on sequencing of the wzc gene [10] cps 207-2 was 93% identical to the K22_ref and K37_ref reference sequences.
Taken together, these results suggested that the CPS composition of KK207-2 was different from that of KKBO-4, demonstrating that at least two different types of CPS gene clusters may be found in KPC-Kp of ST258, and that cps BO-4-like gene clusters can also be found in KPC-Kp of unrelated STs such as ST442.
Analysis of the cps Gene Clusters in a Contemporary Collection of ST258 and ST512 KPC-Kp Strains from the Italian Epidemic
A multiplex PCR protocol derived from that originally proposed by Turton et al. [6], modified to detect the cps BO-4 and cps 207-2 gene clusters, was used to analyze a collection of 46 nonreplicate KPC-Kp clinical strains of ST258 or ST512 isolated from 19 different centers (Fig. 2) during the first Italian countrywide survey on carbapenem-resistant Enterobacteriaceae [12] and selected as representatives of the recent Italian KPC-Kp epidemic. Nine additional carbapenem-resistant but KPC-negative K. pneumoniae strains with different carbapenem-resistance mechanisms (production of VIM-1, of OXA-48, or of an extended-spectrum beta-lactamase in presence of a permeability defect), isolated during the same survey, were also analyzed for comparison.
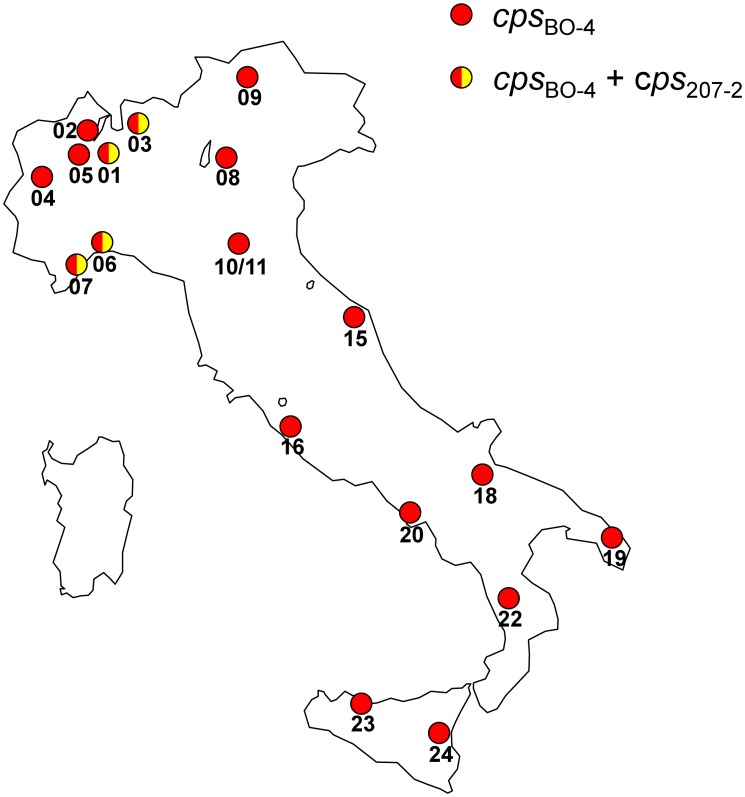
Figure 2. Map showing the distribution of Italian centers from which the 46 KPC-Kp strains of ST258 or ST512 investigated for CPS typing by the modified multiplex PCR were originated, and distribution of the different types of cps gene clusters.
Centers were as follows: 01, Milan; 02, Varese; 03, Lecco; 04, Torino; 05, Novara; 06, Genoa; 07, Sanremo; 08, Verona; 09, Bolzano; 10, Modena; 11, Modena; 15, Ancona; 16, Rome; 18, Foggia; 19, Lecce; 20, Naples; 22, Cosenza; 23, Palermo; 24, Catania.
Of the 46 KPC-Kp strains, 38 (82.6%) carried a cps BO-4-like cluster, while the remaining 8 (17.4%) carried a cps 207-2-like cluster (Table 2). The cps BO-4-like gene cluster was detected in both ST258 and ST512 strains from all 19 centers, while the cps 207-2-like gene cluster was only detected in ST258 strains from 4 centers (Table 2, Fig. 2). The 9 KPC-negative strains were not typeable by the modified multiplex PCR, with the exception of one isolate identified as K2, indicating that none of those strains carried cps BO-4-like or cps 207-2-like gene clusters. According to the wzi sequence-based typing method [9], the isolates were genotyped as K2 (n = 1), K9 (n = 1), K17 (n = 3), K38 (n = 1), K14/64 (n = 1), K15/17/50/51/52 (n = 2). Results were consistent with the fact that none of these K-types, except K2, could be detected by the modified multiplex PCR method.
Table 2. KPC-Kp strains of clinical origin from the Italian nationwide survey investigated for the nature of the cps gene cluster by the modified multiplex PCR developed in this work.
| Strain ID | Sample | PFGE | ST | cps-type |
| 01C03 | Urine | A6 | 258 | cps 207-2 |
| 01C06 | Urine | A3 | 258 | cps 207-2 |
| 01C08 | Wound exudate | A6 | 258 | cps 207-2 |
| 01C22 | Wound exudate | A0 | 512 | cps BO-4 |
| 02C01 | Urine | A5 | 512 | cps BO-4 |
| 02C06 | Wound exudate | A2 | 258 | cps BO-4 |
| 03C06 | Wound exudate | A4 | 512 | cps BO-4 |
| 03C08 | Urine | A3 | 258 | cps 207-2 |
| 03C12 | Urine | A1 | 258 | cps 207-2 |
| 04C35 | Urine | A0 | 512 | cps BO-4 |
| 04C38 | Bronchial aspirate | A4 | 512 | cps BO-4 |
| 04C49 | Bronchial aspirate | A2 | 258 | cps BO-4 |
| 05C15 | Blood | A4 | 512 | cps BO-4 |
| 06C02 | Urine | A3 | 258 | cps 207-2 |
| 06C04 | Urine | A4 | 512 | cps BO-4 |
| 06C05 | Urine | A2 | 258 | cps BO-4 |
| 06C07 | Bronchial aspirate | A3 | 258 | cps 207-2 |
| 06C19 | Blood | A0 | 512 | cps BO-4 |
| 07C06 | Urine | A3 | 258 | cps BO-4 |
| 07C07 | Bronchial aspirate | A2 | 258 | cps 207-2 |
| 08C02 | Urine | A0 | 512 | cps BO-4 |
| 08C04 | Urine | A2 | 258 | cps BO-4 |
| 09C06 | Urine | A1 | 258 | cps BO-4 |
| 10C04 | Urine | A5 | 512 | cps BO-4 |
| 10C09 | Wound exudate | A4 | 512 | cps BO-4 |
| 11C07 | Urine | A5 | 512 | cps BO-4 |
| 15C05 | Bronchial aspirate | A4 | 512 | cps BO-4 |
| 15C10 | Urine | A2 | 258 | cps BO-4 |
| 15C15 | Bronchial aspirate | A0 | 512 | cps BO-4 |
| 15C18 | Urine | A1 | 258 | cps BO-4 |
| 16C05 | Blood | A4 | 512 | cps BO-4 |
| 16C12 | Wound exudate | A2 | 258 | cps BO-4 |
| 18C01 | Bronchial aspirate | A4 | 512 | cps BO-4 |
| 18C22 | Abscess | A2 | 258 | cps BO-4 |
| 18C24 | Wound exudate | A1 | 258 | cps BO-4 |
| 19C09 | Blood | A5 | 512 | cps BO-4 |
| 19C11 | Blood | A2 | 258 | cps BO-4 |
| 20C14 | Blood | A6 | 258 | cps BO-4 |
| 22C06 | Bronchial aspirate | A2 | 258 | cps BO-4 |
| 22C09 | Urine | A1 | 258 | cps BO-4 |
| 22C24 | Wound exudate | A4 | 512 | cps BO-4 |
| 23C10 | Urine | A1 | 258 | cps BO-4 |
| 23C13 | Bile | A6 | 258 | cps BO-4 |
| 24C02 | Blood | A2 | 258 | cps BO-4 |
| 24C20 | Bronchial aspirate | A2 | 258 | cps BO-4 |
| 24C21 | Bronchial aspirate | A2 | 258 | cps BO-4 |
The first two characters of each strain ID identify the center from which the isolate was obtained. Identifiers are as reported in the legend to Fig. 2.
Discussion
Results of this work showed that KPC-Kp belonging to ST258, which have largely contributed to the epidemic dissemination of the KPC-type beta-lactamases in Italy and elsewhere [12], [20], can be equipped with at least two different types of CPS gene clusters, here named cps BO-4 and cps 207-2. The former type was more prevalent in a collection of representative isolates from the recent Italian epidemic, being also present in strains of ST512. The differences in the nature of these CPS gene clusters could be related with differences in the ability of spreading and virulence of different clones, which will deserve further investigation.
The detailed chemical structure of cps BO-4 was recently solved for two representatives of the outbreak clone of KPC-Kp found at the Clinical Center of the U.S. National Institutes of Health [22], [24]. The Authors demonstrated that this CPS type is structurally different from any other published K. pneumoniae CPS, even if similarity to K. pneumoniae K19 and K34 antigens was observed, possibly explaining the cross-reactivity of this CPS with the K34 antiserum [22]. These results corroborate the hypothesis that cps BO-4-like gene clusters belong to a novel capsular type, as also suggested by the results obtained for cps BO-4 using the typing methods based on wzi and wzc gene sequences.
On the other hand, results obtained for cps 207-2 using the above genotyping methods were not in agreement between each other. In fact, while according to the wzc-based method [10] cps 207-2 corresponded to a new K-type, according to the wzi-based method [9] this gene cluster corresponds to the known K41 K-type. The result obtained with the wzi-based method could be consistent with the finding that a strain of KPC-Kp of ST258, representative of the dominant clone circulating in Greece during 2009–2011, was serotyped as K41 [21]. This finding also suggests that this K-type has achieved a significant distribution in some settings, and it would therefore be interesting to further investigate the nature of the whole CPS gene cluster in KPC-Kp strains of K-type 41.
Data presented here also confirmed that the CPS gene cluster do not unambiguously correlate with any particular ST, confirming the notion that CPS gene clusters can be exchanged between different strains of Enterobacteriaceae species [30]–[32].
Materials and Methods
Bacterial Strains
Two KPC-Kp strains of ST258, KKBO-4 and KK207-2, isolated in 2010 from two different Italian hospitals and epidemiologically unrelated with each other, were used for high-throughput genome sequencing (HTGS) analysis and characterization of their cps gene clusters.
Forty-six additional KPC-Kp strains of ST258 or ST512 plus nine carbapenem-resistant but KPC-negative K. pneumoniae strains of different STs were investigated by the modified multiplex PCR for CPS genotyping developed in this work. These strains were selected as representative of the recent Italian epidemic of carbapenem-resistant K. pneumoniae from a collection of clinical isolates obtained during the first nationwide survey on carbapenem-resistant Enterobacteriaceae carried out in Italy in 2011 [12].
High-Throughput Genome Sequencing and Analysis of Sequence Data
HTGS was performed using a HiSeq 2000 Illumina platform and a paired-ends protocol with an average insert size of 300 bp. Reads were assembled using ABySS [33]. GGDC software was used to assess the genomic diversity of the investigated isolates [34]. HTGS for strain KKBO-4 has been described previously [35]. The web interface of BLAST available at the NCBI website was used to compare the CPS gene clusters of the two strains with homologues in the nr or wgs databases [36]. CPS gene clusters sequences were aligned with ClustalX [37]. Structural comparisons of KKBO-4, KK207-2 and other published cps gene clusters were performed with EasyFig. [38].
The nucleotide sequences of the cps gene clusters of KKBO-4 and KK207-2 were deposited in the DDBJ/EMBL/GenBank databases under accession numbers HE866751 and HE866752, respectively.
Multiplex PCR for CPS Typing
CPS gene clusters were genotyped using a multiplex PCR approach as previously described [6], modified by including two additional primer pairs designed to amplify specific targets in the cps gene clusters described in this paper: wziBO-4F (5′-CGGTTTCCTGATGCAGCGG-3′) and wziBO-4R (5′-ATCATGTGCTTCCAGGTACC-3′), targeting the wzi gene of the cps BO-4 gene cluster, and hgt207-2F (5′-GCAGCTGATTCCAGAAATATTG-3′) and hgt207-2R (5′-CATATGCTCTAATACCAAAGCC-3′), targeting a hypothetical glycosyltransferase gene of the cps 207-2 gene cluster (orf9 in Fig. 1). These two additional primer pairs yielded amplicons of 478 and 352 bp, respectively, being suitable for the inclusion in the multiplex PCR because of the unique band sizes. Primers K.pneumoniae Pf and K.pneumoniae Pr1, designed for the identification at the species level of K. pneumoniae and included in the original multiplex PCR protocol, were not included in the reaction mix.
Addendum in Proof
After the revised version of this manuscript had been submitted, two articles have been published reporting the occurrence of two distinct cps gene clusters in Klebsiella pneumoniae isolates belonging to the ST258 clonal lineage [39], and the development of a PCR-based assay for their detection [40]. The two gene clusters, named cps-1 and cps-2, correspond to cps 207-2 and cps BO-4 described here, respectively, while the PCR assay targets the different wzy genes of the two clusters. At the same time, an additional article has been published reporting that K. pneumoniae isolates of ST258 are characterized by cps gene clusters carrying a novel wzi allele (wzi-154) [41], that is identical to the wzi allelic variant of cps BO-4.
Funding Statement
This study was partially funded by the European Community projects EVOTAR and TEMPO test-QC contracts HEALTH-F3-2011-282004 and HEALTH-F3-2009-241742, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11: 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortés G, Borrell N, de Astorza B, Gómez C, Sauleda J, et al. (2002) Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 70: 2583–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shu HY, Fung CP, Liu YM, Wu KM, Chen YT, et al. (2009) Genetic diversity of capsular polysaccharide biosynthesis in Klebsiella pneumoniae clinical isolates. Microbiology 155: 4170–4183. [DOI] [PubMed] [Google Scholar]
- 4. Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, et al. (1983) Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun 40: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ørskov I, Ørskov F (1984) Serotyping of Klebsiella. In: “Methods in Microbiology” (T Bergan, ed.). Academic Press, London. 143–164.
- 6. Turton JF, Perry C, Elgohari S, Hampton CV (2010) PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J Med Microbiol 59: 541–547. [DOI] [PubMed] [Google Scholar]
- 7. Brisse S, Issenhuth-Jeanjean S, Grimont PA (2004) Molecular serotyping of Klebsiella species isolates by restriction of the amplified capsular antigen gene cluster. J Clin Microbiol 42: 3388–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Turton JF, Baklan H, Siu LK, Kaufmann ME, Pitt TL (2008) Evaluation of a multiplex PCR for detection of serotypes K1, K2 and K5 in Klebsiella sp. and comparison of isolates within these serotypes. FEMS Microbiol Lett 284: 247–252. [DOI] [PubMed] [Google Scholar]
- 9. Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, et al. (2013) wzi gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol 51: 4073–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan YJ, Lin TL, Chen YH, Hsu CR, Hsieh PF, et al. (2013) Capsular types of Klebsiella pneumoniae revisited by wzc Sequencing. PLoS ONE 8(12): e80670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordmann P, Naas T, Poirel L (2011) Global spread of carbapenemase-producing Enterobacteriaceae . Emerg Infect Dis 17: 1791–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giani T, Pini B, Arena F, Conte V, Bracco S, et al. (2013) Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: results of the first countrywide survey, 15 May to 30 June 2011. Euro Surveill 18. [PubMed]
- 13. Qi Y, Wei Z, Ji S, Du X, Shen P, et al. (2011) ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother 66: 307–312. [DOI] [PubMed] [Google Scholar]
- 14. Li JJ, Sheng ZK, Deng M, Bi S, Hu FS, et al. (2012) Epidemic of Klebsiella pneumoniae ST11 clone coproducing KPC-2 and 16S rRNA methylase RmtB in a Chinese University Hospital. BMC Infect Dis 12: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang J, Ye L, Guo L, Zhao Q, Chen R, et al. (2013) A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin Microbiol Infect 19: E509–E515. [DOI] [PubMed] [Google Scholar]
- 16. Lauderdale TL, Shi ZY, Lin CF, Lai JF, Tan MC, et al. (2012) KPC-2-producing sequence type 11 Klebsiella pneumoniae detected in Taiwan. Antimicrob Agents Chemother 56: 2207–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrade LN, Curiao T, Ferreira JC, Longo JM, Climaco EC, et al. (2011) Dissemination of bla KPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrob Agents Chemother 55: 3579–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomez SA, Pasteran FG, Faccone D, Tijet N, Rapoport M, et al. (2011) Clonal dissemination of Klebsiella pneumoniae ST258 harbouring KPC-2 in Argentina. Clin Microbiol Infect 17: 1520–1524. [DOI] [PubMed] [Google Scholar]
- 19. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL (2012) Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25: 682–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, et al. (2013) Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tzouvelekis LS, Miriagou V, Kotsakis SD, Spyridopoulou K, Athanasiou E, et al. (2013) KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob Agents Chemother 57: 5144–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubler-Kielb J, Vinogradov E, Ng WI, Maczynska B, Junka A, et al. (2013) The capsular polysaccharide and lipopolysaccharide structures of two carbapenem resistant Klebsiella pneumoniae outbreak isolates. Carbohydr Res 369: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos PI, Picao RC, Almeida LG, Lima NC, Girardello R, et al. (2014) Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genomics 15: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, et al. (2012) Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4: 148ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chmelnitsky I, Doniger T, Shklyar M, Naparstek L, Banin E, et al. (2012) Draft genome sequence of an extremely drug-resistant KPC-producing Klebsiella pneumoniae ST258 epidemic strain. J Bacteriol 194: 6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comandatore F, Sassera D, Ambretti S, Landini MP, Daffonchio D, et al. (2013) Draft genome sequences of two multidrug resistant Klebsiella pneumoniae ST258 isolates resistant to colistin. Genome Announc 1. 10.1128/genomeA. [DOI] [PMC free article] [PubMed]
- 27. Hong SK, Yong D, Kim K, Hong SS, Hong SG, et al. (2013) First outbreak of KPC-2-producing Klebsiella pneumoniae sequence type 258 in a hospital in South Korea. J Clin Microbiol 51: 3877–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broberg CA, Palacios M, Miller VL (2013) Whole-genome draft sequences of three multidrug-resistant Klebsiella pneumoniae strains available from the American Type Culture Collection. Genome Announc 1. 1/3/e00312–13 [pii];10.1128/genomeA. [DOI] [PMC free article] [PubMed]
- 29. Liu P, Li P, Jiang X, Bi D, Xie Y, et al. (2012) Complete genome sequence of Klebsiella pneumoniae subsp. pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J Bacteriol 194: 1841–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, et al. (2009) Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 4: e4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nelson K, Selander RK (1994) Intergeneric transfer and recombination of the 6-phosphogluconate dehydrogenase gene (gnd) in enteric bacteria. Proc Natl Acad Sci U S A 91: 10227–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rahn A, Drummelsmith J, Whitfield C (1999) Conserved organization in the cps gene clusters for expression of Escherichia coli group 1 K antigens: relationship to the colanic acid biosynthesis locus and the cps genes from Klebsiella pneumoniae . J Bacteriol 181: 2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, et al. (2009) ABySS: a parallel assembler for short read sequence data. Genome Res 19: 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Auch AF, Klenk HP, Goker M (2010) Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand Genomic Sci 2: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cannatelli A, D’Andrea MM, Giani T, Di Pilato V, Arena F, et al. (2013) In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother 57: 5521–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, et al. (2008) NCBI BLAST: a better web interface. Nucleic Acids Res 36: W5–W9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 38. Sullivan MJ, Petty NK, Beatson SA (2011) Easyfig: a genome comparison visualizer. Bioinformatics 27: 1009–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, et al. (2014) Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae . Proc Natl Acad Sci U S A 111: 4988–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Chavda KD, Findlay J, Peirano G, Hopkins K, et al. (2014) Multiplex PCR for identification of two capsular types in epidemic KPC-producing Klebsiella pneumoniae ST258 strains. Antimicrob Agents Chemother Apr 14. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]