Abstract
The in vivo elimination rate of dichloroacetate (DCA), an investigational drug; is determined by the rate of its biotransformation to glyoxylate, catalyzed by glutathione transferase ζ1 (GSTZ1). DCA is a mechanism-based inactivator of GSTZ1, thus elimination of DCA is slowed with repeated dosing. We observed that chloride, a physiologically important anion, attenuated DCA-induced GSTZ1 inactivation in human liver cytosol in a concentration and GSTZ1 haplotype-dependent way. In the absence of chloride, incubation with 0.5 mM DCA resulted in inactivation of GSTZ1 with a half-life of 0.4 h (samples with the KRT haplotype) to 0.5 h (EGT haplotype). At the hepatic physiological chloride concentration, 38 mM, samples with the EGT haplotype retained more activity (80%) following a 2-h incubation with 0.5 mM DCA than those possessing the KRT haplotype (55%). The chloride concentration that protected 50% of the GSTZ1 activity following 2-h incubation with 0.5 mM DCA (EC50) was 15.0 ± 3.1 mM (mean ± S.D., n=3) for EGT samples and 36.2 ± 2.2 mM for KRT samples. Bromide, iodide and sulfite also protected GSTZ1 from inactivation by DCA, however fluoride, sulfate, carbonate, acetate, cyanide did not. Protection by bromide varied by GSTZ1 haplotype: EC50 was 1.3 ± 0.3 mM for the EGT haplotype and 5.0 ± 0.60 mM for the KRT haplotype. The EC50 values for iodide and sulfite in liver cytosol samples with EGT haplotype were respectively 0.14 ± 0.06 mM and 9.6 ± 1.1 mM (mean ± S.D., n=3). Because the in vivo half-life of DCA is determined by the fraction of active GSTZ1 in the liver, identifying factors that regulate GSTZ1 activity is important in determining appropriate DCA dosing in humans.
Keywords: dichloroacetate, glutathione transferase zeta, DCA-induced inactivation, chloride, anion protection
1. Introduction
Dichloroacetate (DCA) is an investigational drug with therapeutic potential in the treatment of cancer, cardiovascular and metabolic disorders, such as pulmonary arterial hypertension (PAH), and lactic acidosis [1–3]. The therapeutic dose in people ranges from 12.5 mg/kg/day to 25 mg/kg/day and is usually given in two doses [1]. Although the first dose of DCA is cleared rapidly in adults and children, with respective elimination half-lives of 2.1 and 2.5 h, subsequent doses are cleared more slowly and after 6 months of DCA treatment, elimination half-lives rise to an average of 21 h in adults and 6.4 h in children [4].
Elimination of DCA depends on the activity of glutathione transferase ζ1 (GSTZ1), which catalyzes the glutathione (GSH)-dependent dechlorination of DCA to glyoxylate [5]. GSTZ1, also known as maleylacetoacetate isomerase (MAAI), is a member of the cytosolic GST superfamily [6]. GSTZ1 was first identified in the cytosol and later in the mitochondria (the principal dynamic site of action of DCA), where the expression level is lower [7]. Three non-synonymous single nucleotide polymorphisms (SNPs) have been reported to be functionally important in the coding region of the GSTZ1 gene: G94>A (rs7975) Glu→Lys at amino acid 32 (E32K), G124>A (rs7972) Gly→Arg at amino acid 42 (G42R) and C245>T (rs1046428) Thr→Met at amino acid 82 (T82M). Five major GSTZ1 haplotypes result from these three polymorphisms: KRT (Z1A), KGT (Z1B), EGT (Z1C), EGM (Z1D) and KGM (Z1F), with EGT the most frequent haplotype among the major racial and ethnic groups [8–10]. Subjects possessing at least one KRT allele generally showed faster DCA plasma clearance after the first dose than individuals who lack this allele [9]. In vitro studies were consistent with this, showing that human liver cytosol samples from individuals with the KRT haplotype had 3-fold higher specific activity with DCA than did other GSTZ1 variants [8].
In rats, as in humans, DCA elimination is slowed with repeated doses [4, 11]. Consistent with the role of GSTZ1 in DCA elimination, GSTZ1 activity and expression in rat liver were decreased in a dose-dependent and time-dependent manner by 1 to 5 days of DCA exposure [12, 13]. The slower elimination of DCA upon repeated dosing has been explained by demonstrating through in vitro studies that as well as being a substrate, DCA can be a mechanism-based inactivator of GSTZ1 [14]. One study showed that incubation with DCA and GSH resulted in inactivation of mouse, rat and human liver cytosolic GSTZ1 in a DCA-concentration and incubation time-dependent way, with respective half-lives of inactivation of 6.6, 5.4 and 22 min in the three species [14]. In contrast, another report showed no inactivation of GSTZ1 in human liver cytosol following a 30-min incubation with 0.5 mM DCA and 1 mM GSH, while 50% of the activity in rat liver cytosol was inactivated under these conditions [12]. In revisiting these discrepancies, we noted one difference was the presence of chloride in incubations in the study in which DCA failed to cause inactivation of human GSTZ1 [12]. Chloride is reported to be present at 38 mM in adult human liver [15], and its modulation of protein function in terms of enzyme activity, drug-receptor interaction and protein oligomerization has been documented [16–18]. Therefore, we tested the postulate that chloride and other anions might change the rate at which DCA inactivates GSTZ1 by conducting studies using human liver cytosols from donors with different GSTZ1 haplotypes.
2. Materials & Methods
2.1 Chemicals and reagents
[1-14C]-DCA (56 mCi/mmol, 99% purity) was purchased from American Radiolabeled Chemicals (St. Louis, MO), converted to the sodium salt by adding NaHCO3 then diluted with unlabeled clinical grade Na DCA (TCI America, Portland, OR) to make a 2 mM aqueous substrate solution of Na [1-14C]-DCA containing 10 to 20 μCi/ml. All other chemicals were of American Chemical Society grade or higher and were purchased from commercial suppliers.
2.2 Human liver cytosol
De-identified human liver samples from 4 female and 5 male donors, ages 10 to 74 years, were obtained from tissue banks, processed into subcellular fractions as described previously and stored at −80°C until use [7, 8]. GSTZ1 haplotypes were inferred following genotyping of DNA samples isolated from the nuclear pellets as previously published [8].
2.3 Influence of a physiological concentration of chloride on the half-life of GSTZ1 inactivation
Human liver cytosol samples were dialyzed against 0.1 M K-phosphate buffer (pH 7.4) to remove small molecules. The protein concentration of dialyzed samples was then determined by Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA). GSTZ1 inactivation by DCA was assayed by adapting the method of Tzeng et al [14]. In the absence or presence of 38 mM KCl, dialyzed cytosol (0.6 mg/ml) was incubated with 0.5 mM Na DCA, 5 mM GSH and 0.1 M K-phosphate buffer, (pH 7.4) in a volume of 500 μl at 37 °C with gentle shaking for 0–14 h; control incubations were those with 0 h incubation time. At each time point, small molecule assay components were removed by ultrafiltration through Amicon 10 kD MWCO filters (Millipore, Billerica, MA) following three concentration-dilution cycles. In every cycle, 0.1 M K-phosphate buffer (pH 7.4) was added in the filter up to 500 μl, which was then centrifuged at 4 °C at 14,000 g for 25 minutes. Recovered samples were assayed for protein with the BCA assay and for GSTZ1 activity with Na [1-14C]-DCA, as described previously [5]. In brief, recovered protein (0.25 – 0.3 mg) was incubated with 200 μM Na [1-14C]-DCA, 1 mM GSH and 0.1 M Hepes-NaOH (pH 7.6) in a total volume of 0.1 ml at 37 °C, with gentle shaking. The reaction was started by adding cytosolic protein and vortex-mixing, then stopped after 10 minutes by adding 100 μl ice-cold methanol to precipitate protein. Following centrifugation, the amount of [14C]-glyoxylate formed was determined by high-performance liquid chromatography with radiochemical detection [5]. All assays were performed in duplicate; substrate consumption did not exceed 15%. Enzyme activity was expressed as nmol glyoxylate/min/mg protein [7].
2.4: Effects of anions on DCA-induced inactivation of GSTZ1
Methods were similar to those described in section 2.3, except that a single time point, 2 h, was studied. Human liver cytosol was dialyzed against 0.1 M K-phosphate buffer, (pH 7.4) to remove small molecules. The protein concentration of dialyzed human liver cytosol was determined by BCA protein assay. In the absence or presence of the sodium or potassium salts of several anions, including fluoride, chloride, bromide, iodide, sulfite, sulfate, carbonate, cyanide and acetate at 38 mM, dialyzed cytosol (0.6 mg/ml) was incubated with 0.5 mM Na DCA, 5 mM GSH and 0.1 M K-phosphate buffer (pH 7.4) in a volume of 500 μl at 37 °C for 2 h with gentle shaking; control incubations lacked DCA and the salt of the anion under study. After incubation, unbound substrate and product were removed by ultrafiltration. Samples were assayed for protein with the BCA assay and 0.2 mg recovered protein was assayed for GSTZ1 activity with Na [1-14C]-DCA, as described above. Anions that protected GSTZ1 from inactivation under these conditions were further investigated for the concentration-dependence of the effect. Separate control assays determined the effect of direct addition of the salts on GSTZ1 activity with Na [1-14C]-DCA in human liver cytosol.
2.5 pH effect on GSTZ1 activity
Human liver cytosol samples of EGT/EGT or KRT/EGT haplotypes were dialyzed against 0.05 M K-phosphate buffer, (pH 7.4). Expression and purification of human recombinant GSTZ1 (hGSTZ1A, 1B and 1C) were as described previously [13]. After protein concentration was determined by BCA assay, GSTZ1 activity assays with Na [1-14C]-DCA were performed with dialyzed cytosol (0.1–0.2 mg protein) or hGSTZ1 (0.5–0.7 μg protein) under different pH conditions. Instead of 0.1M Hepes-NaOH (pH 7.6) as the standard assay buffer, we employed 0.1 M K-phosphate (pH 4.5–9.0), 0.1 M sodium acetate-acetic acid (pH 3.2–6.0) and 0.1 M glycine-NaOH (pH 8.0–10.5) and determined hGSTZ1 activity.
2.6 Data analysis
To determine the inactivation half-life (t1/2) of GSTZ1 in the presence and absence of KCl, the natural log of assay activity (A) divided by control activity (A0, activity at time 0) was plotted against time. Data were fitted to the equation with lines forced to go through (0, 0). The t1/2 was determined from the equation . Data analyses were performed with GraphPad Prism v5 (GraphPad Software Inc., San Diego, CA).
To determine the concentration of anion that protected 50% of the GSTZ1 from inactivation by DCA (EC50), assay activity was normalized as a percent of control activity in the absence of DCA and plotted against the log of the salt concentration (KCl or other salts). Data were fitted to a variable slope sigmoidal curve with the bottoms of curves constrained to the activity that remained at 2 h in the absence of anion. The EC50 was calculated from the log[agonist] vs response equation provided by GraphPad Prism v5.
Means, standard deviations and statistical significance for the various assays were calculated and analyzed by Excel software (Microsoft, Redmond, WA) or GraphPad software. To compare EC50 values for chloride with other anions, one-way ANOVA was used with Bonferroni’s multiple comparison test. For comparing results from EGT/EGT and EGT/KRT haplotypes, two-tailed Student’s t test was used to analyze significance of data, assuming equal variance. For both analyses, a p value of <0.05 was considered to be statistically significant.
3. Results
3.1. Time course of GSTZ1 inactivation by DCA
In the absence of chloride, incubation with 0.5 mM DCA and 5 mM GSH resulted in rapid inactivation of human hepatic cytosolic GSTZ1, with a t1/2 of inactivation of 23 min for EGT/KRT samples and 31 min for EGT/EGT samples (Fig. 1, panel A). From these inactivation half-lives, we calculated that incubation for 2 h in the presence of 0.5 mM DCA would result in loss of 97.3% of activity of EGT/KRT samples and 93.2% of activity of EGT/EGT samples, and these values were consistent with the activity remaining after 2 hr in controls for our studies of the concentration-dependence of inactivation by anions (sections 3.2 and 3.3). At its reported physiological concentration in human liver (38 mM), chloride substantially prolonged the t1/2 of human hepatic cytosolic GSTZ1 inactivation in vitro (Fig. 1, panel B). Considering all haplotypes, the t1/2 determined in the presence of 38 mM chloride was 7 to 10 times longer than that determined without chloride, p<0.005. In the presence of 38 mM chloride, GSTZ1 activity in hepatic cytosol samples from EGT/KRT individuals was lost twice as quickly, t1/2 of 2.37–2.66 h, as activity in samples from EGT or EGM homozygotes, t1/2 of 5.02 to 5.73h (Fig. 1, panel B).
Figure 1.
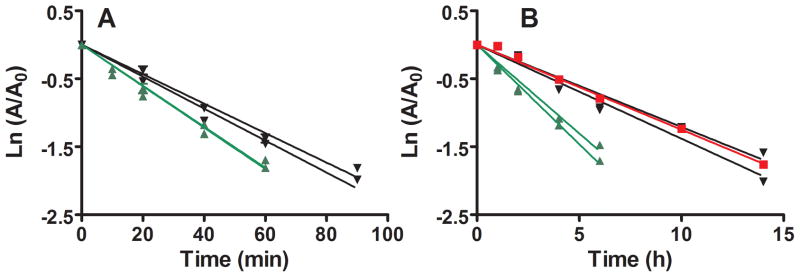
Effect of a physiological concentration of chloride on the time course of GSTZ1 inactivation by DCA in human liver cytosol of EGT/EGT (black circle), EGT/KRT (green triangle) and EGM/EGM (red square) samples. Each data point is the mean of duplicate determinations with individual cytosol samples; lines show the linear regression for each individual. Panel A shows inactivation in the absence of KCl: t½ values for EGT/EGT samples were 0.53 and 0.49 h, and were 0.38 h for both EGT/KRT samples. Panel B shows slower inactivation in the presence of 38 mM KCl: t½ values for EGT/EGT samples were 5.73 and 5.02 h, for EGM/EGM was 5.55 h and for EGT/KRT samples were 2.66 and 2.37 h.
3.2 Attenuation of DCA-induced GSTZ1 inactivation by chloride
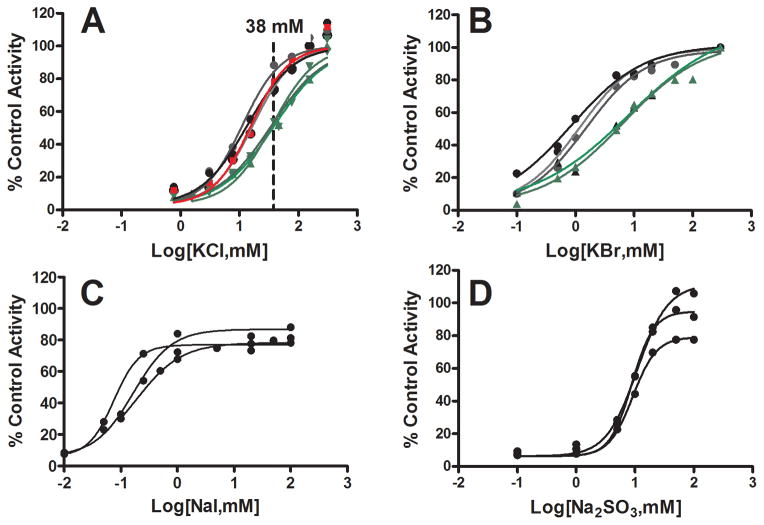
Chloride blunted GSTZ1 inactivation caused by incubation with 0.5 mM DCA for 2 h in a chloride concentration and GSTZ1 haplotype-dependent way (Fig. 2 panel A). When the concentration of KCl reached 300 mM, GSTZ1 activity was fully protected from inactivation caused by DCA in 2 h. Direct addition of KCl to incubations did not affect GSTZ1 activity (data not shown). The concentration of chloride that provided 50% protection against inactivation (EC50) from samples of EGT and EGM homozygotes were similar (Table 1). However, the EC50 concentration of chloride was about 2.5 times higher for the EGT/KRT haplotype than for the EGT/EGT and EGM/EGM haplotypes (p < 0.005; Table 1). At the physiological concentration of chloride (38 mM) in adult livers, GSTZ1 from EGT/KRT individuals was inactivated to a greater extent in 2 h than other variants (Fig. 2 panel A).
Figure 2.
Concentration-dependent effects of chloride, bromide, iodide and sulfite on the extent of inactivation of GSTZ1 activity in human liver cytosol samples during incubation with 0.5 mM Na DCA for 2 h. In all panels, the data points shown are mean values of duplicate determinations in individual cytosol samples and lines are curve fits for each individual. Panel A: Effects of chloride on DCA-induced inactivation of GSTZ1 activity in human liver cytosol samples from three EGT/EGT donors (black circles), three EGT/KRT donors (green triangles) and one EGM/EGM (red square) donor. The concentration range of KCl was 0.77–300 mM; 38 mM, indicated with a dotted line, is the physiological concentration of chloride in liver of adults. Panel B: Bromide attenuation of DCA-induced inactivation of GSTZ1 in human liver cytosol of three EGT/EGT (black circles) and two EGT/KRT (green triangles) samples. The concentration range of KBr was 0.1–50 mM for EGT/EGT samples and 0.1–100 mM for EGT/KRT samples. Panel C: Iodide attenuation of DCA-induced inactivation of GSTZ1 in human liver cytosol of three EGT/EGT samples. The concentration range of NaI was 0.01–100 mM. Panel D: Sulfite attenuation of DCA-induced inactivation of GSTZ1 in human liver cytosol of three EGT/EGT samples. The concentration range of Na2SO3 was 0.1–100 mM.
Table 1.
EC50 (mM) values of various anions in protecting human cytosolic GSTZ1 from DCA-induced inactivation
| GSTZ1 | Anion | |||
|---|---|---|---|---|
| haplotype | Chloride | Bromide | Iodide | Sulfite |
|
|
|
|||
| EGT/EGT | 15.0 ± 3.1A | 1.3 ± 0.3B | 0.14 ± 0.06B | 9.6 ± 1.1C |
| EGT/KRT | 36.2 ± 2.2* | 5.0 ± 0.60* | ||
| EGM/EGM | 16.9 | |||
EC50 is the concentration of anion that protected half the cytosolic GSTZ1 from inactivation following incubation for 2 hours with Na DCA, 0.5 mM. Data are shown as mean ± S.D. (n=3) for EGT/EGT and EGT/KRT samples, and the mean of duplicate determinations for the EGM/EGM sample. Within the EGT/EGT samples, different superscript letters indicate significant differences in EC50 between anions, p<0.01, calculated by one-way ANOVA analysis of paired samples with Bonferroni’s multiplecomparison test.
Significant difference in EC50 values between EGT/EGT and EGT/KRT samples, p < 0.05. Data was analyzed by two-tailed t test assuming equal variances.
3.3 Modulation of DCA-induced GSTZ1 inactivation by other anions
At a concentration of 38 mM, bromide, iodide and sulfite protected GSTZ1 activity in a single human liver cytosol with EGT/EGT haplotype against inactivation from a 2 hour incubation with 0.5 mM Na DCA (Table 2). In contrast, fluoride, cyanide, sulfate, carbonate and acetate, did not prevent inactivation. Further studies showed that bromide, iodide and sulfite attenuated GSTZ1 inactivation in a concentration-dependent manner (Fig. 2 panels B to D). Compared with the mean EC50 of chloride in EGT/EGT samples, the mean EC50 of bromide was approximately 10 times lower; iodide was 100 times lower and sulfite was 1.6 times lower (Table 1). The EC50 values for bromide and iodide were significantly different from chloride (p<0.001), and sulfite was also significantly different from chloride (p<0.05). In studies with bromide, attenuation was also GSTZ1 haplotype-dependent. EGT/KRT samples showed ~4-fold higher EC50 than EGT/EGT samples (Fig. 2 panel B and Table 1). We did not determine haplotype differences in EC50 for iodide and sulfite.
Table 2.
Effects of one concentration, 38 mM, of several anions in modulating DCA-induced GSTZ1 inactivation in a single human liver cytosol
| Anion added | % activity lost |
|---|---|
| None | 92% |
| Fluoride | 87% |
| Chloride | 20% |
| Bromide | 7% |
| Iodide | 2% |
| Sulfite | 10% |
| Sulfate | 85% |
| Carbonate | 91% |
| Cyanide | 97% |
| Acetate | 98% |
The human liver cytosol used was an EGT/EGT sample; sodium or potassium salts at a concentration of 38 mM were used in duplicate 2 h incubations with 0.5 mM DCA. The reference cytosol sample was incubated for 2 h without DCA and its activity was used to calculate % activity lost.
3.4 pH effect on GSTZ1 activity
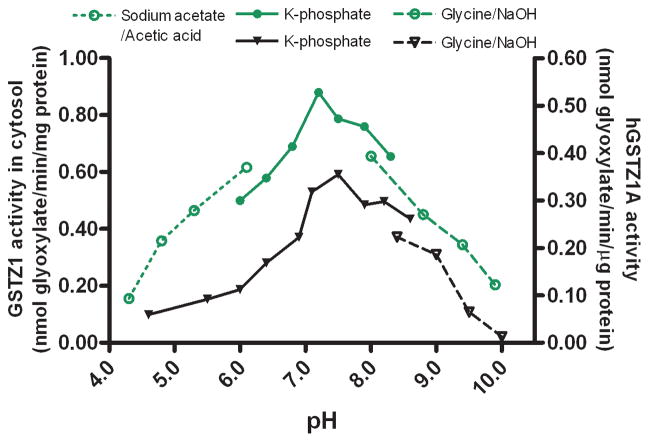
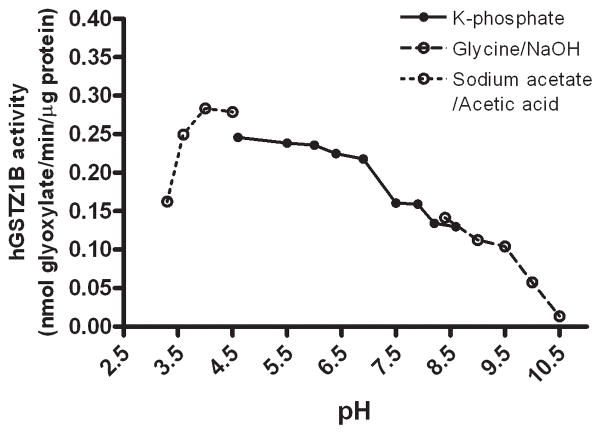
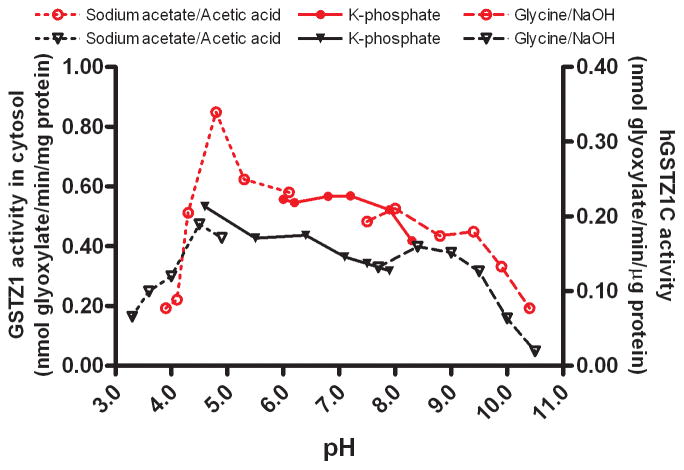
In human liver cytosol, the optimal pH for GSTZ1 activity was dependent on the haplotype of the sample (Figs. 3–5). The optimum pH was 7.2 for human liver cytosol samples with the EGT/KRT haplotype and 4.8 for those with the EGT/EGT haplotype. GSTZ1 activity in human liver cytosol from two samples with the EGT/EGT haplotype was not obviously affected by a change in incubation pH over the 6.0–8.3 range (Fig. 4), whereas the activity of samples with the EGT/KRT haplotype was sensitive to pH. Studies of human recombinant GSTZ1 confirmed that the response of enzyme activity to pH change was haplotype-dependent (Figs. 3–5). The optimal pH for hGSTZ1A (KRT) was 7.5, and activity at this pH was almost twice that of hGSTZ1B or 1C. The optimal pH values for hGSTZ1B (KGT) and 1C (EGT) were much lower, pH 4.0 and 4.5, respectively. However, the pH curve for GSTZ1C showed a broad shoulder between pH 5.5 and 9, where activity was only slightly lower than at the optimum pH.
Figure 3.
Activity of human recombinant GSTZ1A (KRT) [black triangle and right Y axis] and human liver cytosolic GSTZ1 of EGT/KRT haplotype [green circle and left Y axis] under different incubation pH conditions. The data points are mean values of duplicate determinations.
Figure 5.
Activity of human recombinant GSTZ1B (KGT) under different incubation pH conditions. The data points are mean values of duplicate determinations.
Figure 4.
Activity of human recombinant GSTZ1C (EGT) [black triangle and right Y axis] and human liver cytosolic GSTZ1 of EGT/EGT haplotype [red circle and left Y axis] under different incubation pH conditions. The data points are mean values of duplicate determinations.
4. Discussion
These data indicate that a physiological concentration of chloride prolongs the half-life of GSTZ1 inactivation by DCA, providing a reasonable explanation for the discrepancy between previously published results [12, 14]. In a concentration-dependent manner, chloride blunted the extent of inactivation of GSTZ1 in human liver cytosol following incubation with 0.5 mM DCA for two h. The protective effect of physiological concentrations of chloride was greatest in EGT/EGT homozygous samples and was least effective in EGT/KRT samples. GSTZ1 inactivation occurred more rapidly in cytosol fractions with the KRT variant, compared to those possessing the EGT variant in the absence or presence of chloride. This finding is in contrast to previous results with recombinant hGSTZ1 polymorphic variants studied in the absence of chloride, in which the KRT haplotype exhibited a 2-fold slower rate of DCA-induced inactivation vs. other variants [14]. The half-life of inactivation of the recombinant or cytosolic KRT variant in the absence of chloride was the same in both studies, 23 min, but the half-life of inactivation of the EGT variant found here was 31 min, compared to 10 min in the previous study [14]; the reason for this discrepancy is not clear.
Our results are consistent with those from clinical pharmacokinetic investigations, in which DCA plasma clearance after repeated doses decreased in subjects carrying the EGT/KRT or KRT/KRT haplotype to a greater extent than in subjects with the EGT/EGT haplotype [9]. Our finding that physiological concentrations of chloride were less effective in protecting the EGT/KRT haplotype from inactivation by DCA is consistent with the trend to slower clearance in KRT carriers after repeated DCA treatment.
Apart from chloride, other cellular anions are physiologically important or are known to modulate the activity of enzymes [16, 19, 20]. Therefore, we first tested various anions at a concentration of 38 mM to see which were capable of protecting GSTZ1 from inhibition by DCA. The three anions other than chloride that protected against inactivation, bromide, iodide and sulfite, all exerted concentration-dependent inhibition of DCA’s effect on GSTZ1. Haplotype-dependent protection was confirmed for bromide. However, because the KRT haplotype occurs in only 5% of the general population [9], insufficient cytosol from KRT donors precluded similar studies with iodide and sulfite. Nevertheless, the greater protection afforded by both chloride and bromide against inactivation of GSTZ1 in samples from KRT carriers compared with the more common EGT carriers suggests that iodide and sulfite would also show haplotype-dependent effects. Bromide, iodide and sulfite had lower EC50 values, thus were more potent than chloride. The physiological concentrations of bromide, iodide and sulfite in human blood are reported to be about 26, 0.34 and 1.23 μM, respectively, i.e., much lower than the physiological concentration of chloride in blood of 100 mM [21–23]. We found no reports of concentrations of bromide, iodide or sulfite in human liver. However it is not likely that hepatic concentrations of these anions would be higher than blood concentrations. Therefore, it is reasonable to assume that chloride, rather than other anions investigated here, plays the most critical role in modulating GSTZ1 inactivation in vivo.
Although our observations have resolved a previous discrepancy in the literature regarding haplotype-dependent GSTZ1 inactivation by DCA, we have not proved the molecular mechanism(s) accounting for this protective effect. The elegant studies of Anders and Board [14, 24] proposed a plausible three-step mechanism that accounted for GSTZ1- and GSH-catalyzed conversion of DCA to glyoxylate as well as the mechanism-based inactivation of GSTZ1 through formation of a covalent adduct. They suggested that only the first step in this mechanism was enzyme-catalyzed, namely formation of S-(α-chloro-carboxymethyl)glutathione with elimination of HCl, followed by non-enzymatic elimination of the second chloride to give an unstable carbonium-sulfonium intermediate which could undergo a non-enzymatic SN1-type interaction with water, yielding glyoxylate and GSH, or which could react with nucleophilic groups in GSTZ1 such as cysteine 16 and form a covalent adduct [14, 25]. It was shown that addition of the alternative nucleophile N-acetyl-cysteine (10 mM) to incubations partially protected GSTZ1 from inactivation, supporting the hypothesis that an electrophilic intermediate was formed, however another nucleophile, cyanide (10 mM), did not protect GSTZ1 from inactivation [14]. Chloride and the other anions shown in this study to protect GSTZ1 from inactivation are nucleophiles, and their effectiveness in preventing adduct formation is in the order expected from their nucleophilic character, i.e. iodide > bromide > sulfite > chloride [26]. Fluoride, generally a very weak nucleophile in protic media, was ineffective at 38 mM. Although the observation that the effective anions are good nucleophiles fit the data and the mechanism of conversion of DCA to glyoxylate or an adduct to GSTZ1, it does not explain the observed haplotype differences in anion effect. An alternative or additional explanation is that the effective anions may bind either the active site or an allosteric site and cause a change in the GSTZ1 protein conformation such that the initial enzyme-catalyzed product can leave the active site more easily and be less likely to bind with C16 or other nucleophilic sites in GSTZ1. This scenario could perhaps explain the observed haplotype differences, if the KRT variant bound the effective anions differently than the other variants.
We speculated that the size and shape of the anions we studied could contribute to the observed differences in protection against DCA-induced inactivation because anion size is reported to affect the activity of other enzymes [27]. Halogens are spherical anions and sulfite is approximately spherical [28–30]. Other tested anions are reported to be linear (cyanide), planar (carbonate, acetate) or tetrahedral (sulfate). All the anions that were effective in blunting the inactivation of GSTZ1 by DCA have a spherical geometry. The radius of chloride, bromide and iodide is 181, 196 and 220 pm, respectively, while that of ineffective fluoride is 136 pm [28, 31]. Sulfite is reported to have a radius of 520 pm [30]. Our results show that to protect GSTZ1 from inactivation by DCA, anions must be larger than 136 pm, but we do not know the upper limit of size for efficacy. The potency of the effect was lower for the smallest and largest effective anions studied, chloride and sulfite respectively, than those with intermediate size, bromide and iodide, however we found too few effective anions to further analyze the effect of size on potency.
Because a high concentration of halogen caused a slight decrease in assay pH (from pH 7.4 to 7.2), while sulfite caused an increase in assay pH (from pH 7.4 to 7.7), we considered the possibility that their variable effects on GSTZ1 activity might be pH-dependent. However, data summarized in Figs. 3 to 5 using both human cytosolic GSTZ1 and human recombinant GSTZ1 ruled out this possibility. Although the pH profile of GSTZ1 activity in both cytosolic samples with the KRT haplotype and human recombinant GSTZ1A showed “bell” shapes, similar to many GSTs of other classes [32–34], cytosolic GSTZ1 activity in cytosolic fractions from EGT haplotype carriers was not sensitive to pH change in the range 6.0 to 8.3. The activities of recombinant hGSTZ1B and 1C also showed little variation over this pH range.
At this time, we cannot provide a single mechanism that would explain the differences in EC50 among different effective anions and explain the difference in effect by haplotype. Differences in either electrostatic interactions between anions and GSTZ1, altering flexibility in the binding site [35] or tertiary enzyme structure [36], remain testable alternative mechanisms.
In conclusion, we demonstrated that in vitro DCA-induced GSTZ1 inactivation in human liver cytosol is markedly attenuated by physiological concentrations of chloride in a haplotype-dependent manner, such that individuals with the KRT variant exhibit more rapid loss of GSTZ1 activity. Certain other nucleophiles, including bromide, iodide and sulfite, also protected GSTZ1 from DCA inactivation in human liver cytosol.
Supplementary Material
Highlights.
Inactivation of GSTZ1 by dichloroacetate (DCA) controls DCA pharmacokinetics.
This work reports factors affecting mechanism-based inactivation of GSTZ1-1 by DCA.
The physiologically important nucleophile, Cl− protected GSTZ1 from inactivation by DCA.
A lower concentration of Cl− was required to protect GSTZ1C than GSTZ1A.
Br−, I− and SO32− protected GSTZ1, but F−, SO42−, CO32−, CN− and CH3COO− did not.
Acknowledgments
This work was supported in part by a grant from the US Public Health Service RO1 GM 099871.
Abbreviations
- DCA
dichloroacetate
- Na DCA
sodium dichloroacetate
- PAH
pulmonary arterial hypertension
- GSH
glutathione
- GSTZ1
glutathione transferase ζ1
- h
hour
- MAAI
maleylacetoacetate isomerase
- SNPs
single nucleotide polymorphisms
- t1/2
half-life
- EC50
concentration that protected 50% of the GSTZ1 activity from inactivation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stacpoole PW. The Dichloroacetate Dilemma: Environmental Hazard versus Therapeutic Goldmine-Both or Neither? Environ Health Perspect. 2011;119:155–8. doi: 10.1289/ehp.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, et al. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223–8. doi: 10.1542/peds.2007-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1. 5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–8. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 4.Shroads AL, Guo X, Dixit V, Liu HP, James MO, Stacpoole PW. Age-dependent kinetics and metabolism of dichloroacetate: possible relevance to toxicity. J Pharmacol Exp Ther. 2008;324:1163–71. doi: 10.1124/jpet.107.134593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James MO, Cornett R, Yan Z, Henderson GN, Stacpoole PW. Glutathione-dependent conversion to glyoxylate, a major pathway of dichloroacetate biotransformation in hepatic cytosol from humans and rats, is reduced in dichloroacetate-treated rats. Drug Metab Dispos. 1997;25:1223–7. [PubMed] [Google Scholar]
- 6.Board PG, Anders MW. Glutathione transferase zeta: discovery, polymorphic variants, catalysis, inactivation, and properties of Gstz1−/− mice. Drug Metab Rev. 2011;43:215–25. doi: 10.3109/03602532.2010.549132. [DOI] [PubMed] [Google Scholar]
- 7.Li W, James MO, McKenzie SC, Calcutt NA, Liu C, Stacpoole PW. Mitochondrion as a novel site of dichloroacetate biotransformation by glutathione transferase zeta 1. J Pharmacol Exp Ther. 2011;336:87–94. doi: 10.1124/jpet.110.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Gu Y, James MO, Hines RN, Simpson P, Langaee T, et al. Prenatal and postnatal expression of glutathione transferase zeta 1 in human liver and the roles of haplotype and subject age in determining activity with dichloroacetate. Drug Metab Dispos. 2012;40:232–9. doi: 10.1124/dmd.111.041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroads AL, Langaee T, Coats BS, Kurtz TL, Bullock JR, Weithorn D, et al. Human Polymorphisms in the Glutathione Transferase Zeta 1/Maleylacetoacetate Isomerase Gene Influence the Toxicokinetics of Dichloroacetate. J Clin Pharmacol. 2012;52:837–49. doi: 10.1177/0091270011405664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn AC, Tzeng HF, Anders MW, Board PG. Discovery of a functional polymorphism in human glutathione transferase zeta by expressed sequence tag database analysis. Pharmacogenetics. 2000;10:49–57. doi: 10.1097/00008571-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 11.James MO, Yan Z, Cornett R, Jayanti VM, Henderson GN, Davydova N, et al. Pharmacokinetics and metabolism of [14C]dichloroacetate in male Sprague-Dawley rats. Identification of glycine conjugates, including hippurate, as urinary metabolites of dichloroacetate. Drug Metab Dispos. 1998;26:1134–43. [PubMed] [Google Scholar]
- 12.Cornett R, James MO, Henderson GN, Cheung J, Shroads AL, Stacpoole PW. Inhibition of glutathione S-transferase zeta and tyrosine metabolism by dichloroacetate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochem Biophys Res Commun. 1999;262:752–6. doi: 10.1006/bbrc.1999.1287. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Dixit V, Liu H, Shroads AL, Henderson GN, James MO, et al. Inhibition and recovery of rat hepatic glutathione S-transferase zeta and alteration of tyrosine metabolism following dichloroacetate exposure and withdrawal. Drug Metab Dispos. 2006;34:36–42. doi: 10.1124/dmd.105.003996. [DOI] [PubMed] [Google Scholar]
- 14.Tzeng HF, Blackburn AC, Board PG, Anders MW. Polymorphism- and species-dependent inactivation of glutathione transferase zeta by dichloroacetate. Chem Res Toxicol. 2000;13:231–6. doi: 10.1021/tx990175q. [DOI] [PubMed] [Google Scholar]
- 15.Widdowson EM, Dickerson JW. The effect of growth and function on the chemical composition of soft tissues. Biochem J. 1960;77:30–43. doi: 10.1042/bj0770030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Espana A, Alonso E, Rubio V. Influence of anions on the activation of carbamoyl phosphate synthetase (ammonia) by acetylglutamate: implications for the activation of the enzyme in the mitochondria. Arch Biochem Biophys. 1991;288:414–20. doi: 10.1016/0003-9861(91)90214-4. [DOI] [PubMed] [Google Scholar]
- 17.Tavoulari S, Forrest LR, Rudnick G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J Neurosci. 2009;29:9635–43. doi: 10.1523/JNEUROSCI.0440-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitani T, Fujisawa H. Influence of salts on the activity and the subunit structure of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1984;784:164–7. doi: 10.1016/0167-4838(84)90123-7. [DOI] [PubMed] [Google Scholar]
- 19.Maurus R, Begum A, Williams LK, Fredriksen JR, Zhang R, Withers SG, et al. Alternative catalytic anions differentially modulate human alpha-amylase activity and specificity. Biochemistry. 2008;47:3332–44. doi: 10.1021/bi701652t. [DOI] [PubMed] [Google Scholar]
- 20.Sinibaldi F, Howes BD, Smulevich G, Ciaccio C, Coletta M, Santucci R. Anion concentration modulates the conformation and stability of the molten globule of cytochrome c. J Biol Inorg Chem. 2003;8:663–70. doi: 10.1007/s00775-003-0462-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Wu Q, Sun HW, Rao J, Kannan K. Perchlorate and iodide in whole blood samples from infants, children, and adults in Nanchang, China. Environ Sci Technol. 2010;44:6947–53. doi: 10.1021/es101354g. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuhashi H, Ikeuchi H, Yamashita S, Kuroiwa T, Kaneko Y, Hiromura K, et al. Increased levels of serum sulfite in patients with acute pneumonia. Shock. 2004;21:99–102. doi: 10.1097/01.shk.0000105501.75189.85. [DOI] [PubMed] [Google Scholar]
- 23.Sharma V, Schwille PO. Sulfite inhibits oxalate production from glycolate and glyoxylate in vitro and from dichloroacetate infused i.v. into male rats. Biochem Med Metab Biol. 1993;49:265–9. doi: 10.1006/bmmb.1993.1028. [DOI] [PubMed] [Google Scholar]
- 24.Tong Z, Board PG, Anders MW. Glutathione transferase zeta-catalyzed biotransformation of dichloroacetic acid and other alpha-haloacids. Chem Res Toxicol. 1998;11:1332–8. doi: 10.1021/tx980144f. [DOI] [PubMed] [Google Scholar]
- 25.Anderson WB, Liebler DC, Board PG, Anders MW. Mass spectral characterization of dichloroacetic acid-modified human glutathione transferase zeta. Chem Res Toxicol. 2002;15:1387–97. doi: 10.1021/tx025553x. [DOI] [PubMed] [Google Scholar]
- 26.Parker AJ. The Effects of Solvation on the Properties of Anions in Dipolar Aprotic Solvents. Q Rev Chem Soc. 1962;16:163–87. [Google Scholar]
- 27.Santucci R, Bongiovanni C, Mei G, Ferri T, Polizio F, Desideri A. Anion size modulates the structure of the A state of cytochrome c. Biochemistry. 2000;39:12632–8. doi: 10.1021/bi000516v. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich B. Design of Anion Receptors - Applications. Pure Appl Chem. 1993;65:1457–64. [Google Scholar]
- 29.Dedzo GK, Letaief S, Detellier C. Kaolinite-ionic liquid nanohybrid materials as electrochemical sensors for size-selective detection anions. J Mater Chem. 2012;22:20593–601. [Google Scholar]
- 30.Fraenkel D. Electrolytic Nature of Aqueous Sulfuric Acid. 1. Activity. J Phys Chem B. 2012;116:11662–77. doi: 10.1021/jp3060334. [DOI] [PubMed] [Google Scholar]
- 31.Shannon RD. Revised Effective Ionic-Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr A. 1976;32:751–67. [Google Scholar]
- 32.Akkemik E, Taser P, Bayindir A, Budak H, Ciftci M. Purification and characterization of glutathione S-transferase from turkey liver and inhibition effects of some metal ions on enzyme activity. Environ Toxicol Pharmacol. 2012;34:888–94. doi: 10.1016/j.etap.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, Wang WN, Wang AL, He WY, Zhou QT, Liu Y, et al. Glutathione S-transferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under pH stress. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:224–30. doi: 10.1016/j.cbpc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Tang SS, Chang GG. Steady-state kinetics and chemical mechanism of octopus hepatopancreatic glutathione transferase. Biochem J. 1995;309 (Pt 1):347–53. doi: 10.1042/bj3090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoldak G, Sprinzl M, Sedlak E. Modulation of activity of NADH oxidase from Thermus thermophilus through change in flexibility in the enzyme active site induced by Hofmeister series anions. Eur J Biochem. 2004;271:48–57. doi: 10.1046/j.1432-1033.2003.03900.x. [DOI] [PubMed] [Google Scholar]
- 36.Uversky VN, Karnoup AS, Segel DJ, Seshadri S, Doniach S, Fink AL. Anion-induced folding of Staphylococcal nuclease: characterization of multiple equilibrium partially folded intermediates. J Mol Biol. 1998;278:879–94. doi: 10.1006/jmbi.1998.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.